Abstract
Marital status has long been recognized as an important prognostic factor for many cancers, however its’ prognostic effect for patients with laryngeal cancer has not been fully examined. We retrospectively analyzed 8834 laryngeal cancer patients in the Surveillance Epidemiology and End Results database from 2004 to 2010. Patients were divided into four groups: married, widowed, single, and divorced/separated. The difference in overall survival (OS) and cancer-specific survival (CSS) of the various marital subgroups were calculated using the Kaplan–Meier curve. Multivariate Cox regression analysis screened for independent prognostic factors. Propensity score matching (PSM) was also conducted to minimize selection bias. We included 8834 eligible patients (4817 married, 894 widowed, 1732 single and 1391 divorced/separated) with laryngeal cancer. The 5-year OS and CSS of married, widowed, single, and separated/divorced patients were examined. Univariate and multivariate analyses found marital status to be an independent predictor of survival. Subgroup survival analysis showed that the OS and CSS rates in widowed patients were always the lowest in the various American Joint Committee on Cancer stages, irrespective of sex. Widowed patients demonstrated worse OS and CSS in the 1:1 matched group analysis. Among patients with laryngeal cancer, widowed patients represented the highest-risk group, with the lowest OS and CSS.
Similar content being viewed by others
Introduction
Laryngeal cancer has the highest incidence in head and neck cancer, accounting for approximately 1–5% of global cancer incidence1. The majority of the patients with laryngeal cancer are middle-aged and elderly men over 40 years of age, with a slightly younger trend2. Surgery remains the primary treatment for laryngeal cancer, although the status of radiotherapy, chemotherapy, and a new targeted therapy is gradually rising3. For example, a recent study showed that definite radiotherapy is the preferred treatment regardless of the characteristics of the tumor4. However, the survival rate was unsatisfactory. Therefore, it is crucial to explore factors affecting the prognosis of patients with laryngeal cancer.
In the past, most cancer research focused on biology, and social or psychological factors were easily ignored. Fortunately, studies on the influence of marital status on the prognosis of cancer have gradually attracted widespread attention. Many studies have confirmed that marital status may affect the prognosis of various types of cancer, including endometrial cancer5, ovarian cancer6, glioblastoma multiforme7, chondrosarcoma8 and male breast cancer9. Nevertheless, currently there has been limited research on the relationship between laryngeal cancer prognosis and marital status. This study explored the effect of marital status on the survival rate of patients with laryngeal cancer by analyzing data from the Surveillance Epidemiology and End Results (SEER) database.
Results
Demographic characteristics
The cohort included a total of 8,834 eligible cases of laryngeal cancer diagnosed between 2004 and 2010. The exact screening process is shown in Fig. 1. The baseline characteristics of eligible patients and the relationship between marital status and variables are shown in Table 1. Among these, 54.5% (n = 4817) patients were married, 10.1% (n = 894) were widowed, 19.6% (n = 1732) were single, and 15.8% (n = 1391) were divorce/separated. The differences in demographic and pathological characteristics between the married group and the other three groups were statistically significant in terms of sex, age, race, grade, surgery, American Joint Committee on Cancer (AJCC) stage, radiotherapy, and chemotherapy (P < 0.001). However, there were no significant differences in histological types between the married group and the other three groups (P = 0.950).
The widowed group had the highest proportion of women, the highest number of elderly patients (≥ 65 years), the highest number of AJCC II/III tumors, and the highest proportion of untreated patients (surgery, radiotherapy, and chemotherapy), which was significantly different from the other marital status groups (P < 0.001). Compared with patients who were widowed (33.0%), single (39.1%), or divorced/separated (37.1%), married (41.5%) patients were more likely to have surgery (P < 0.001).
Influence of marriage status on overall survival (OS) of laryngeal cancer in SEER database
Kaplan–Meier survival analysis showed significant differences in OS results between the various marital status (P < 0.001, Fig. 2A). The married group had the highest 5-year OS (58.6%) compared to the other groups. The 5-year OS of patients with laryngeal cancer in the widowed group was the lowest (32.3%). After the univariate log-rank test, all differences were significant except for sex (P < 0.001), Table 2. Multivariate Cox regression indicated that age, race, grade, histological type, surgery, AJCC stage, chemotherapy, and marital status were independent prognostic factors affecting survival. However, radiotherapy was not an independent prognostic factor affecting survival (Table 2). Cox regression analysis showed that, compared with married patients, the risk of widowed, single (HR: 1.34, 95% CI: 1.24–1.44), divorced/separated (HR: 1.36, 95% CI: 1.27–1.47) was higher, and widowed patients (HR: 1.62, 95% CI: 1.49–1.77) had the highest risk of death.
Effect of marital status on cancer-specific survival (CSS) survival
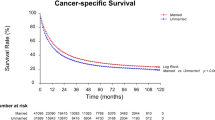
To explore the correlation between marital status and CSS, we performed Kaplan–Meier survival analysis on patients in the dataset. Figure 2B shows a significant difference in CSS among laryngeal cancer patients with various marital statuses. Male sex (P = 0.004), age < 65 years (P < 0.001), race (P < 0.001), grade I (P < 0.001), squamous cell carcinoma (P < 0.001), yes for surgery (P < 0.001), AJCC Stage I (P < 0.001), yes for radiotherapy (P = 0.001), no or unknown for chemotherapy (P = 0.001), and married state (P < 0.001) were associated with a higher 5-year CSS. (Table 3). To prevent possible interference between the variables, we used multivariate Cox regression analysis. As with the OS results, radiotherapy was not an independent predictor of CSS in patients with laryngeal cancer. In terms of marital status, married state remains a protective factor for the prognosis of laryngeal cancer.
Subgroup analysis by AJCC stage
We further evaluated the impact of marital status on OS and CSS of each AJCC stage. Interestingly, we obtained similar results in the various AJCC stage subgroups (Tables 4, 5, Fig. 3). First, marital status was an independent factor affecting the OS and CSS of each AJCC stage in univariate and multivariate analyses (P < 0.001). Second, the 5-year OS and CSS of patients in the widowed group were consistently lower than those of the other marital groups (Fig. 3). In terms of OS, among all AJCC stages, the survival rate of widowed patients in the AJCC stage II group was significantly lower than that of married patients (32.6% vs. 63.7%, P < 0.001; HR 2.06, 95% CI 1.70. 2.50, P < 0.001, Table 4). In the analysis of CSS, the most significant difference was between widowed patients and married patients in the AJCC stage III subgroup (32.3% vs. 58.6%, P < 0.001; HR 1.28, 95% CI 1.00.1.64, P = 0.042, Table 5). This phenomenon also occurred in the AJCC stage III subgroup. The risk of death was higher in the divorced/separated group than in the married group (HR 1.19; 95% CI, 0.97. 1.46), although the difference was not significant (P = 0.092, Table 5).
Subgroup analysis by sex
Subsequently, we analyzed the influence of marital status on OS and CSS rates for each sex. Figures 4 shows the Kaplan–Meier curve of OS and CSS rates among the sexes. Regardless of the sex, the OS and CSS of the widowed group were lower than those of the other groups. Compared with married patients in the male group, the 5-year OS and CSS of widowed patients were reduced by 26.9% and 19.5%, respectively (58.50% vs. 31.60%, P < 0.001, 70.80% vs. 51.30%, P < 0.001, Tables 6, 7). Compared with married patients in the subgroup, the reduction was 25.9% and 23.0%, respectively (59.00% vs. 33.10%, P < 0.001; 68.60% vs. 45.60%, P < 0.001, Tables 6 and 7). In line with the previous results, widowed patients were at the highest risk for death when comparing the OS and CSS among all the groups. (Tables 6 and 7).
Survival analysis in 1:1 matched group
To minimize the effect of possible confounding factors on the baseline features of the marriage subgroups and to verify the reliability of our results, we implemented a 1:1 matching cohort utilizing propensity score-matching (PSM) methods. We obtained three 1:1 matched cohorts, including a single and married cohort, a widowed and married cohort, and a divorced/separated and married cohort. The demographic and clinicopathological features of the matched cohort are presented in Table 8. As expected, the clinicopathological parameters were well balanced between the groups after PSM. Widowed patients showed worse OS and CSS in the divorce/separated-married cohort (Fig. 5A,B), the single-married cohort (Fig. 5C,D), and the widowed-married cohort (Fig. 5E,F).
Discussion
For the first time, a population analysis based on the SEER database was performed to assess the prognostic impact of marital status on the survival rate of patients with laryngeal cancer. This study found that marital status is an independent factor affecting the prognosis of laryngeal cancer. More specifically, married patients have the lowest risk of death, while widowed patients have the highest risk of death. This is like many previous research results10,11,12. We further confirmed after PSM that widowed patients had better OS and CSS than divorced, single, or married patients.
In 1977, Engel proposed a new model of biological psychological medicine13. He believed that biological factors, psychological elements, and social factors influenced disease progression and outcome14. Since then, extensive research has been conducted on the relationship between biological psychological factors and disease15,16,17. The role of biopsychosocial factors in cancer patients has also gradually gained attention18,19. A study of women's marital status and mortality rates showed that single patients had higher mortality rates than divorced or widowed patients20. Another large-scale survey found that married patients with oral and laryngeal cancer are less likely to have metastases21. A Swedish study found that divorce and bereavement are risk factors for esophageal and gastric cancer22. The relationship between marital status and prognosis may be influenced by tumor stage, proportion of patients receiving treatment, and social support23,24,25,26. A higher percentage of married laryngeal cancer patients receive timely treatment, including surgery and adjuvant treatment, which may explain the high survival rate. However, it emphasizes the interrelationship between marital status and survival rather than causality. Therefore, it is necessary to explore how marital status affects the potential mechanism of survival to improve the outcome of patients with laryngeal cancer.
This study showed that marital status was associated with survival in patients with laryngeal cancer. We hypothesize the following reasons for the beneficial effect of married state on survival in patients with laryngeal cancer. First, a happy marriage may result in a well-balanced emotional state, and a wholesome family environment may ease work and social pressures. Second, married patients that have stable marriages usually are accompanied by appropriate family finances. A commonly accepted explanation of why married people have lower cancer mortality, was that it was related to better socioeconomic status. This was believed to buffer the effects of stressful events27,28. Chronic stress may cause long-term secretion of cortisol29, which leads to reverse regulation of leukocytes by down-regulating the cortisol receptor of leukocytes. This downregulation, in turn, reduces the ability of cells to respond to anti-inflammatory signals and leads to the vigorous development of cytokine-mediated inflammatory processes30, which has been proven to be a poor prognostic factor for cancer31,32. Third, married patients have a wider social range than unmarried patients. They have a broader information base regarding medical equipment, experts, and treatment methods. This can help improve treatment outcomes33. Social networks influence patient compliance, and good compliance ultimately affects a patients' health outcomes34.
In addition, our results raise another intriguing question as to why widowed patients exhibit worse clinical outcomes. There is evidence to suggest that a widows' health is a problem before they are diagnosed with cancer35. Studies have shown that the recent death of a husband results in a significant decrease in the level of natural killer (NK) cells in the widows’ body36,37. More importantly, NK cells are known to play an important role in the fight against cancer38. Compared with married patients, widowed patients had more psychological stress and less psychological support. This can cause disorders of the immune system and promote cancer progression39. Such an alteration affects the release of glucocorticoids and catecholamines, further affecting the tumor microenvironment40,41. An enhanced development of tumors results in a shortened survival time for widowed patients.
Although this study is both instructive and relevant to clinical practice, it has some limitations. First, the marital status information provided in the SEER database is incomplete. It provides only marital status at the time of diagnosis, and some patients' marital status may change during follow-up. In the same way, it does not reflect whether marital status changes after diagnosis, which may also affect survival outcomes. In addition, patients aged 65 and above account for approximately 44% of the total. This means that it is possible to change from a married status to a widowed status during the follow-up period. Second, in addition to marital status, various other social factors are included in the bio-psycho-social medical model, such as education, income, and insurance. The SEER database lacks information regarding other societal factors. Our analysis cannot correct these factors, which may affect the survival outcomes. Third, the SEER database does not provide information on whether married patients are happy or not. Even in an equivalent married group of patients, the happiness of marriage may be questionable. For instance, prolonged marital discord can damage the immune system and have a negative effect on health42. Fourth, personal history such as smoking, drinking, and human papilloma virus (HPV) infections is not reflected in the SEER database. Some studies have shown that unmarried people may be more likely to engage in the bad habits of smoking and/or drinking43,44. Fifth, some patients may live with a partner without being married, despite the fact that the proportion of people in this circumstance may be small. In addition, we classify these patients as single patients, however their survival outcomes are expected to be better than those of other single people, which confounds our results. Sixth, the SEER database does not contain the number of patients with alternate sexual orientation. Finally, the AJCC stage provided in the SEER database is the sixth edition, and now the eighth edition is used in the clinic. The subgroup results of our AJCC stage need to be verified in further clinical practice.
Despite these limitations, our findings are credible for the following reasons. First, our data is from the SEER database, which is composed of data from multiple centers across the country. Second, we chose the two outcomes of OS and CSS, which adds to the accuracy of our results. Last but not least, we also performed a stratified analysis on the AJCC stage and obtained high survival rates for married patients and low survival rates for the widowed patients. Due to a lack of social and psychological support, widowed patients should be advised to obtain psychological therapy during cancer treatment to improve their prognosis.
Conclusion
Marital status was associated with survival (OS and CSS) in patients with laryngeal cancer. It is clear that married patients had a better survival outcome, while widowed patients had a worse prognosis. If we are able to fully understand the impact of marital status on cancer patients, we will be able to provide individualized treatment and improve survival outcomes.
Material and methods
Patients
We utilized the SEER database access online. The reference number 16606-nov2018 was approved for use for accessing the database. From the SEER database (http://seer.cancer.gov), we screened information on 8,834 patients who met the diagnostic criteria for primary laryngeal cancer between 2004 and 2010. The patient inclusion criteria consisted of: (1) age ≥ 18 years age at diagnosis; (2) solitary primary tumor; and (3) histologic type: ICD-O-3 morphology code (8000/3, 8010/3, 8012/3, 8013/3, 8020/3, 8021/3, 8032/3, 8033/3, 8041/3 , 8045/3, 8046/3, 8050/3, 8051/3, 8052/3, , 8070/3, 8071/3, 8072/3, 8073/3, 8074/3, 8075/3, 8076/3, 8078/3, 8082/3, 8083/3, 8123/3, 8140/3, 8141/3, 8200/3, 8246/3, 8340/3, 8430/3, 8560/3, 8571/3, 8574/3, 8941/3). Patients were excluded if: (1) the marital status was unclear; (2) there was a lack of some crucial clinicopathological information, such as AJCC stage, grade, race, and surgical style; (3) they were only diagnosed clinically; and (4) there was an absence of prognostic information.
Description of covariates
The study variables included the following: sex (male, female), age (< 65 or ≥ 65 years), race (White, Black, or other), Grade (Grade I, Grade II ,Grade III/IV), histological type (squamous cell carcinoma, other), surgery (yes or no), AJCC stage (I, II, III, IV), radiotherapy (yes or no/unknown), chemotherapy (yes or no/unknown), marital status (married, widowed, single, divorced/separated). The laryngeal specific surgical code 10–90 was defined as invasive treatment and code 00 as non-surgical treatment. The primary outcome indicators were laryngeal cancer OS and CSS.
Statistical analysis
The clinical and pathological characteristics of patients with various marital statuses were compared using the chi-square test (χ2). Kaplan–Meier estimates and log-rank tests were used to estimate and compare survival functions between the different variables. Multivariate Cox proportional risk regression was used to estimate the risk ratio (HR) and 95% confidence interval (CI) for OS and CSS in the various marital statuses. To reduce potential baseline confounding factors, we employed propensity score matching (PSM) to re-examine the impact of marital status45. One- to- one PSM was conducted using the nearest-neighbor algorithm with a caliper width of 0.2. Data were analyzed using SPSS version 25 (IBM Statistics, New York, NY), and the survival curves were generated by R project version 3.6.1. P < 0.05 (2-sided) was considered significant.
Data availability
The data used in this study were provided by the SEER database.
References
Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2016. CA Cancer J. Clin. 66, 7–30 (2016).
Shin, J. Y. & Truong, M. T. Racial disparities in laryngeal cancer treatment and outcome: a population-based analysis of 24,069 patients. Laryngoscope 125, 1667–1674 (2015).
Steuer, C. E., El-Deiry, M., Parks, J. R., Higgins, K. A. & Saba, N. F. An update on larynx cancer. CA Cancer J Clin 67, 31–50 (2017).
Lee, K. C. & Chuang, S. K. The nonsurgical management of early stage (T1/2 N0 M0) laryngeal cancer: a population analysis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 130, 18–24 (2020).
Dong, J., Dai, Q. & Zhang, F. The effect of marital status on endometrial cancer-related diagnosis and prognosis: a Surveillance Epidemiology and End Results database analysis. Future Oncol. (London, England) 15, 3963–3976 (2019).
Luo, P., Zhou, J. G., Jin, S. H., Qing, M. S. & Ma, H. Influence of marital status on overall survival in patients with ovarian serous carcinoma: finding from the surveillance epidemiology and end results (SEER) database. J. Ovarian Res. 12, 126 (2019).
Xie, J. C., Yang, S., Liu, X. Y. & Zhao, Y. X. Effect of marital status on survival in glioblastoma multiforme by demographics, education, economic factors, and insurance status. Cancer Med. 7, 3722–3742 (2018).
Gao, Z. et al. Marital status and survival of patients with chondrosarcoma: a population-based analysis. Med. Sci. Monitor 24, 6638–6648 (2018).
Liu, L., Chi, Y. Y., Wang, A. A. & Luo, Y. Marital status and survival of patients with hormone receptor-positive male breast cancer: a surveillance, epidemiology, and end results (SEER) population-based study. Med. Sci. Monitor 24, 3425–3441 (2018).
Qiu, M., Yang, D. & Xu, R. Impact of marital status on survival of gastric adenocarcinoma patients: results from the Surveillance Epidemiology and End Results (SEER) Database. Sci. Rep. 6, 21098 (2016).
Zhou, H. et al. Marital status is an independent prognostic factor for pancreatic neuroendocrine tumors patients: An analysis of the Surveillance, Epidemiology, and End Results (SEER) database. Clin. Res. Hepatol. Gastroenterol. 41, 476–486 (2017).
Xu, C. et al. Impact of marital status at diagnosis on survival and its change over time between 1973 and 2012 in patients with nasopharyngeal carcinoma: a propensity score-matched analysis. Cancer Med. 6, 3040–3051 (2017).
Engel, G. L. The need for a new medical model: a challenge for biomedicine. Science (New York NY) 196, 129–136 (1977).
Engel, G. L. The clinical application of the biopsychosocial model. J. Med. Philos. 6, 101–123 (1981).
Calobrisi, A. Biopsychosocial study of diabetes mellitus. Psychother. Psychosom. 39, 193–200 (1983).
Pierin, A. M. et al. Biopsychosocial variables and attitudes towards treatment influence complicated hypertension. Arq. Bras. Cardiol. 95, 648–654 (2010).
Liossi, C. & Howard, R.F. Pediatric Chronic Pain: Biopsychosocial Assessment and Formulation. Pediatrics 138 (2016).
Shi, R. L. et al. The impact of marital status at diagnosis on cancer survival in patients with differentiated thyroid cancer. Cancer Med. 5, 2145–2154 (2016).
Zhang, W. et al. Prognostic value of marital status on stage at diagnosis in hepatocellular carcinoma. Sci. Rep. 7, 41695 (2017).
Cheung, Y. B. Marital status and mortality in British women: a longitudinal study. Int. J. Epidemiol. 29, 93–99 (2000).
Inverso, G. et al. Marital status and head and neck cancer outcomes. Cancer 121, 1273–1278 (2015).
Lagergren, J. et al. Marital status, education, and income in relation to the risk of esophageal and gastric cancer by histological type and site. Cancer 122, 207–212 (2016).
Aizer, A. A. et al. Marital status and survival in patients with cancer. J. Clin. Oncol. 31, 3869–3876 (2013).
Adekolujo, O. S. et al. Impact of marital status on tumor stage at diagnosis and on survival in male breast cancer. Am. J. Men’s Health 11, 1190–1199 (2017).
Hinyard, L., Wirth, L. S., Clancy, J. M. & Schwartz, T. The effect of marital status on breast cancer-related outcomes in women under 65: A SEER database analysis. Breast (Edinburgh, Scotland) 32, 13–17 (2017).
Wang, X., Cao, W., Zheng, C., Hu, W. & Liu, C. Marital status and survival in patients with rectal cancer: an analysis of the Surveillance, Epidemiology and End Results (SEER) database. Cancer Epidemiol. 54, 119–124 (2018).
Du, K. L. et al. Impact of marital status and race on outcomes of patients enrolled in radiation therapy oncology group prostate cancer trials. Support. Care Cancer 20, 1317–1325 (2012).
Giese-Davis, J. et al. Screening for distress, the 6th vital sign: common problems in cancer outpatients over one year in usual care: associations with marital status, sex, and age. BMC Cancer 12, 441 (2012).
McEwen, B. S. Physiology and neurobiology of stress and adaptation: central role of the brain. Physiol. Rev. 87, 873–904 (2007).
Miller, G. E., Cohen, S. & Ritchey, A. K. Chronic psychological stress and the regulation of pro-inflammatory cytokines: a glucocorticoid-resistance model. Health Psychol. 21, 531–541 (2002).
Formica, V. et al. Systemic inflammation, as measured by the neutrophil/lymphocyte ratio, may have differential prognostic impact before and during treatment with fluorouracil, irinotecan and bevacizumab in metastatic colorectal cancer patients. Med Oncol (Northwood, London, England) 31, 166 (2014).
Hamilton, T. D. et al. Identification of prognostic inflammatory factors in colorectal liver metastases. BMC Cancer 14, 542 (2014).
Iwashyna, T. J. & Christakis, N. A. Marriage, widowhood, and health-care use. Soc. Sci. Med. 1982(57), 2137–2147 (2003).
DiMatteo, M. R., Haskard, K. B. & Williams, S. L. Health beliefs, disease severity, and patient adherence: a meta-analysis. Med. Care 45, 521–528 (2007).
Miller, A. H. & Raison, C. L. The role of inflammation in depression: from evolutionary imperative to modern treatment target. Nat. Rev. Immunol. 16, 22–34 (2016).
Zhang, N. et al. Multiple exposure to environmental factors and variations in CYP27B1 and the microRNA-binding site of IL-13 are associated with breast cancer risk. Cancer Med. 8, 3237–3249 (2019).
Irwin, M., Daniels, M., Risch, S. C., Bloom, E. & Weiner, H. Plasma cortisol and natural killer cell activity during bereavement. Biol. Psychiat. 24, 173–178 (1988).
Chiossone, L., Dumas, P. Y., Vienne, M. & Vivier, E. Natural killer cells and other innate lymphoid cells in cancer. Nat. Rev. Immunol. 18, 671–688 (2018).
Sood, A. K. et al. Stress hormone-mediated invasion of ovarian cancer cells. Clin Cancer Res 12, 369–375 (2006).
Zhang, X. et al. Chronic stress promotes gastric cancer progression and metastasis: an essential role for ADRB2. Cell Death Disease 10, 788 (2019).
Antoni, M. H. et al. The influence of bio-behavioural factors on tumour biology: pathways and mechanisms. Nat. Rev. Cancer 6, 240–248 (2006).
Jaremka, L. M., Glaser, R., Malarkey, W. B. & Kiecolt-Glaser, J. K. Marital distress prospectively predicts poorer cellular immune function. Psychoneuroendocrinology 38, 2713–2719 (2013).
Power, C., Rodgers, B. & Hope, S. Heavy alcohol consumption and marital status: disentangling the relationship in a national study of young adults. Addiction (Abingdon, England) 94, 1477–1487 (1999).
Lindstrom, M. Social capital, economic conditions, marital status and daily smoking: a population-based study. Public Health 124, 71–77 (2010).
Zhao, E., Zhou, C. & Chen, S. Prognostic nomogram based on log odds of positive lymph nodes for gastric carcinoma patients after surgical resection. Future Oncol (London, England) 15, 4207–4222 (2019).
Acknowledgements
The authors are grateful for the help of the SEER database.
Author information
Authors and Affiliations
Contributions
Z.D. and D.Y. planned the study. Z.D. and H.L. statistical analysis of data. Z.D. wrote this manuscript. Y.D. supervise the whole project. All the authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ding, Z., Yu, D., Li, H. et al. Effects of marital status on overall and cancer-specific survival in laryngeal cancer patients: a population-based study. Sci Rep 11, 723 (2021). https://doi.org/10.1038/s41598-020-80698-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-80698-z
- Springer Nature Limited
This article is cited by
-
Studying the impact of marital status on diagnosis and survival prediction in pancreatic ductal carcinoma using machine learning methods
Scientific Reports (2024)
-
Epidemiology and prognostic nomogram for chronic eosinophilic leukemia: a population-based study using the SEER database
Scientific Reports (2024)
-
Depression, anxiety and stress among metastatic breast cancer patients on chemotherapy in China
BMC Nursing (2023)