Abstract
Cyclomodulins are virulence factors that modulate cellular differentiation, apoptosis, and proliferation. These include colibactin (pks), cytotoxic necrotizing factor (cnf), and cytolethal distending toxin (cdt). Pathogenic pks+, cnf+, and cdt+ E. coli strains are associated with inflammatory bowel disease (IBD) and colorectal cancer in humans and animals. Captive marmosets are frequently afflicted with IBD-like disease, and its association with cyclomodulins is unknown. Cyclomodulin-encoding E. coli rectal isolates were characterized using PCR-based assays in healthy and clinically affected marmosets originating from three different captive sources. 139 E. coli isolates were cultured from 122 of 143 marmosets. The pks gene was detected in 56 isolates (40%), cnf in 47 isolates (34%), and cdt in 1 isolate (0.7%). The prevalences of pks+ and cnf+ E. coli isolates were significantly different between the three marmoset colonies. 98% of cyclomodulin-positive E. coli belonged to phylogenetic group B2. Representative isolates demonstrated cyclomodulin cytotoxicity, and serotyping and whole genome sequencing were consistent with pathogenic E. coli strains. However, the presence of pks+, cnf+, or cdt+ E. coli did not correlate with clinical gastrointestinal disease in marmosets. Cyclomodulin-encoding E. coli colonize laboratory common marmosets in a manner dependent on the source, potentially impacting reproducibility in marmoset models.
Similar content being viewed by others
Introduction
Escherichia coli is a ubiquitous and diverse species of Gram-negative, facultatively anaerobic bacilli in the family Enterobacteriaceae. Most strains of E. coli are commensals within the gastrointestinal tract of animals. However, pathogenic E. coli strains cause a variety of intestinal and extraintestinal infections ranging in severity from self-limiting to lethal1. These strains are classically categorized into pathotypes, including enteropathogenic E. coli (EPEC), uropathogenic E. coli (UPEC), meningitis-associated E. coli (MNEC), and others. Each pathotype represents a collection of strains with similar virulence factors conferring the ability to cause similar pathology2.
Cyclomodulins are a heterogeneous class of virulence factors that alter the eukaryotic cell cycle to promote bacterial invasion and host colonization. These actions induce genetic instability that may lead to the development of cancer. Cyclomodulins that are found in E. coli include colibactin, cytotoxic necrotizing factor (CNF), cytolethal distending toxin (CDT), cycle inhibiting factor, shiga toxin, and subtilase toxin3.
Colibactin is a genotoxic secondary metabolite produced by Enterobacteriaceae species harboring the polyketide synthase (pks) genomic island. Colibactin from E. coli alkylates adenine, causing DNA interstrand crosslinks and double strand breaks, leading to cell cycle arrest and senescence in vitro, as well as increased virulence in extraintestinal infections and tumor development in vivo4,5,6. The presence of pks+ E. coli is associated with inflammatory bowel disease (IBD) and colorectal cancer, and was recently shown to cause a unique mutational signature that is enriched within a subset of human cancers7,8,9. The highly reactive and unstable cyclopropane warheads of colibactin are activated during secretion to prevent autotoxicity to the bacteria; therefore, toxicity in vitro requires direct contact of cells with live bacteria. Effects on cells in vitro include megalocytosis, phosphorylated γ-H2AX foci, and G2 cell-cycle arrest10,11.
Cytotoxic necrotizing factor (CNF) is a family of AB toxins produced by UPEC strains that cause cytoskeletal changes resulting in macropinocytosis of bacteria into the host cell as well as G2 cell cycle arrest. These actions impair epithelial turnover and favor E. coli colonization. Consistent with these mechanisms, many cell lines treated with sonicates of cnf+ E. coli exhibit multinucleation and ruffled cell borders. CNF1 can also induce epithelial to mesenchymal transition in vitro, and thus may increase the risk of cancer12. Three CNF types are described in E. coli, however, CNF2 and CNF3 are not frequently detected13,14.
UPEC strains also produce hemolysin, a membrane pore-forming toxin. The cnf1 gene, when present, is always encoded on the hlyCABD operon and is co-transcribed with hemolysin. However, hlyCABD can exist without cnf115. Hemolysin is produced as either a free form or an outer membrane vesicle (OMV)-associated form. The free form irreversibly inserts into cell membranes of erythrocytes and epithelial cells, where depending on concentration it causes ion imbalance, structural changes, and cell lysis. The OMV-associated form is internalized by epithelial cells, where it targets mitochondria and triggers caspase-9-mediated apoptosis16,17,18.
Cytolethal distending toxin (CDT) is an AB2-type toxin produced by several pathogenic Gram-negative bacteria which causes DNA damage and cell cycle arrest. The catalytic subunit CdtB is highly conserved between species and causes single and double-strand DNA breaks via DNase I-like activity. This triggers the DNA damage response via ATM kinase, leading to both G2/M and G1/S cell cycle arrest. CDT increases bacterial gut colonization, promotes pro-inflammatory responses, and dysregulates the immune response. Treatment of HeLa cells with CDT in vitro causes cellular distension and multinucleation3,19.
The common marmoset (Callithrix jacchus) is a nonhuman primate species that is increasingly used in a variety of biomedical research fields including neuroscience, toxicology, infectious disease, immunology, reproduction, obesity, and aging20. Common marmosets in captivity are frequently afflicted with IBD-like disease, which is often diagnosed post-mortem as chronic lymphocytic enteritis (CLE)21, the etiology and pathogenesis of which are presently unknown. The potential role of cyclomodulin-encoding enteropathogenic E. coli in the pathogenesis of IBD-like disease of marmosets has not previously been investigated, although E. coli has previously been associated with GI disease in marmosets22,23,24.
Most captive marmoset colonies are not specific pathogen free (SPF), and none exclude E. coli. As such, the prevalence of cyclomodulin-encoding E. coli in laboratory marmosets is currently unknown. We hypothesized that common marmosets, similar to laboratory rodents and other laboratory nonhuman primates25,26,27,28,29, are colonized by cyclomodulin-encoding E. coli, and the prevalence of pks+, cnf+, and cdt+ E. coli in our population would vary significantly depending on the colony of origin, as has been demonstrated in laboratory rats28. In addition, we hypothesized that pks+, cnf+, and cdt+ E. coli are present in animals afflicted with gastrointestinal disease with greater frequency than in clinically normal animals. Additionally, colonization of marmosets by cyclomodulin-encoding E. coli might be expected to induce physiological variability between animals in a study, potentially impacting reproducibility.
Results
Microbiologic characterization of marmoset E. coli isolates
In total, 139 E. coli strains were isolated from 122 of the 143 marmosets sampled in all three colonies (Supplementary Table S1). In Colony A, 31 strains of E. coli were isolated from 26 of the 33 animals. In Colony B, 33 strains of E. coli were isolated from 32 of the 33 animals. In Colony C, 75 strains of E. coli were isolated from 64 of the 77 animals. Eight E. coli isolates originating from Colony C demonstrated hemolytic activity when cultured on blood agar plates. Some animals harbored multiple E. coli isolates as determined by distinct colony morphology and biochemical characterization by API testing. Of the 139 total E. coli isolates, 54 were API code 5144572, and 51 isolates were API code 5144552, constituting the majority of isolates present in all three marmoset colonies. The major metabolic difference between these codes is that strains with API code 5144572 ferment sucrose, whereas those with 5144552 did not. Other API codes represented were 5044552, 5144172, 7144552, 7145552, 5044572, 5144573, 1044552, 4144512, and 7144572 (Supplementary Figure S1). There were significant associations between marmoset colony of origin and E. coli strain-linked biochemical characteristics, including the presence of ornithine decarboxylase (p < 0.001), butylene glycol pathway (p = 0.01), sucrose fermentation (p < 0.001), and amygdalin fermentation (p < 0.05) (Supplementary Figure S1).
Phylogenetic distribution of marmoset E. coli isolates
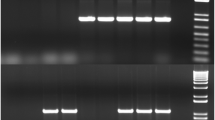
Phylogenetic group was determined according to the specific gel electrophoresis banding pattern resulting from multiplex PCR amplification of genes svg, chuA, yjaA, uidA, and TspE4.C2 (Fig. 1a). Isolates in phylogroup A were positive for yjaA and uidA, phylogroup B1 were positive for uidA, and phylogroup B2 were positive for chuA, yjaA, uidA, and TspE4.C2. Out of 135 total isolates tested, 21 were in phylogroup A (16%), 54 were in phylogroup B1 (40%), and 60 were in phylogroup B2 (44%) (Fig. 1b). In marmoset colony A, 1 of the 31 isolates was phylogroup A, 12 were phylogroup B1, and 18 were phylogroup B2. In marmoset colony B, 20 of the 33 isolates were in phylogroup A, 5 were in phylogroup B1, and 8 were in phylogroup B2. In marmoset colony C, 37 of the 71 isolates were in phylogroup B1, 34 were in phylogroup B2, whereas none of the isolates in marmoset colony C were in phylogroup A. The distribution of E. coli phylogroups was significantly different in marmoset colony B compared to E. coli isolates from the other two colonies (p < 0.001). Phylogenetic group correlated strongly with genotype, in that all phylogroup A isolates, and all but one of the phylogroup B1 isolates, were negative for pks, cnf, and hlyA. Of the 60 phylogroup B2 isolates, 54 were cyclomodulin-positive. All 6 phylogroup B2 isolates that were pks-/cnf- were cultured from marmoset colony B.
Phylogenetic analysis of E. coli isolates from marmosets. (a) Sets of primers for svg, chuA, yjaA, uidA, and TspE4.C2 genes were used in multiplex PCR assays to determine the phylogroup of each isolate. The phylogenetic groups were determined according to the PCR gel pattern, with the presence of 3 or more bands indicating membership in phylogroup B2. Phylogroup multiplex PCR gel: lane 1,1-kb ladder; lane 2, negative control; lane 3, phylogroup B2 positive control (NC101); lanes 4–16, E. coli isolates from marmosets (lanes 3–9 and 11–13, phylogroup B2; lanes 10 and 14–16, phylogroup B1). (b) Distribution of phylogenetic groups of E. coli isolated from marmosets according to colony. Colony B had a significantly different distribution than either of the other two colonies. *** p < 0.001, Fisher’s Exact Test. Unedited images of gels are shown in Supplementary Figure S6.
Distribution of marmoset E. coli isolates encoding cyclomodulins
All E. coli isolates were tested by PCR for cyclomodulin genes with primers for the clbQ gene of the pks island (Fig. 2a), with multiplex primers to amplify both chromosomal and plasmid cnf genes (Fig. 2b), and with multiplex primers to amplify all known variants of cdtB genes (Fig. 2c). Of the 139 E. coli isolates, 56 were positive for pks (40.3%), 47 were positive for cnf (33.8%), and 1 was positive for cdt (0.7%) (Supplementary Table S2). All 47 cnf+ isolates were also pks+. Of the 9 pks+/cnf- isolates, one was cdt+. (Fig. 2d) The single animal with cdt+ E. coli had a similar pks+/cnf−/cdt+ E. coli isolate simultaneously cultured from a nasal swab taken as a diagnostic sample, and the PCR bands from both isolates are depicted in Fig. 2c. The cdtB amplicon sequences of both E. coli isolates from this animal aligned with 98% identity to the gene for CDT Type IV subunit B found in E. coli strain NCTC 8196. The remaining 83 isolates were negative for all three cyclomodulin genes tested. From marmoset colony A, 17 of the 31 E. coli isolates were pks+/cnf+/cdt−, one isolate was pks+/cnf−/cdt+, and the remaining 13 isolates were pks-/cnf-/cdt-. In marmoset colony B, 2 of the 33 E. coli isolates were pks+, and all of the 33 isolates were cnf-. In marmoset colony C, 36 of the 75 E. coli isolates were pks+, and 6 of those were also cnf-. The remaining 39 E. coli isolates from marmoset colony C were pks-/cnf-. The prevalence of both pks and cnf were significantly lower in colony B compared to the other two marmoset colonies (p < 0.001).
PCR amplification of cyclomodulin genes in representative E. coli isolates from marmosets. (a) Amplification of clbQ gene from pks island. (b) Multiplex PCR amplification was used to detect both chromosomal and plasmid cnf genes. (c) Multiplex PCR amplification was used to detect all known variants of the cdtB gene. PCR gels: lane 1,1-kb ladder; lane 2, blank; lane 3, negative control; lane 4, positive control; lanes 5 through 16, representative E. coli isolates from marmosets. Both cdt-positive isolates shown (lanes 13 and 14) are from the same animal. (d) Distribution of cyclomodulin genotypes of E. coli isolated from marmosets according to colony. Colony B had a significantly different distribution than either of the other two colonies. *** p < 0.001, Fisher’s Exact Test. Unedited images of gels are shown in Supplementary Figure S6.
Captive marmosets are maintained in cohoused family units, which may influence transmission of E. coli within colonies. Therefore, we evaluated if E. coli strains differed between family lines in each colony. The two largest extended family lines (Family 1 and Family 2) in Colony C consisted of cohoused parents, adult offspring that were previously cohoused with the parents, and unrelated mates cohoused with the offspring. These two families were found to have a significantly higher proportion of pks+/cnf− E. coli isolates (24% prevalence) than the rest of Colony C (0% prevalence) (p < 0.001) (Supplementary Figure S2). Similar pks+/cnf− E. coli isolates had been cultured from these animals over the previous two years (Supplementary Figure S2). The cyclomodulin prevalence in E. coli isolates from other large families were not significantly different from E. coli isolates in their colonies of origin.
To determine if marmosets are stably colonized over time with cyclomodulin-encoding E. coli, we analyzed data from animals which had been sampled multiple times over the course of two years. Interestingly, of the 51 animals that had been sampled 12 and 24 months previously, 5 consistently had pks+/cnf+/cdt− E. coli, 6 consistently had pks+/cnf−/cdt− E. coli, and an additional 20 marmosets consistently had pks−/cnf−/cdt− E. coli isolated from rectal swabs (Supplementary Figure S2).
Identification of mutant hemolysin gene
Cytotoxic necrotizing factor, when present, is always encoded on the same operon with hemolysin, though it is common for an isolate to encode hemolysin without CNF15. Therefore, it was surprising when 45 of the 47 cnf+ E. coli isolates did not demonstrate hemolytic activity when cultured on blood agar. The presence of hemolysin was tested by PCR in 136 E. coli isolates by amplification of the gene hlyA (Supplementary Figure S3). The hlyA PCR product size was 584 bp, which was produced from all eight hemolytic isolates. The sequence of this amplicon demonstrated 100% identity with the hemolysin A gene from 16 different E. coli reference isolates, including UTI89. Forty-two out of 136 marmoset E. coli isolates produced a 1665 bp amplicon, all of which were non-hemolytic and cnf+ . The hlyA gene in these longer amplicons was disrupted by an IS481-like element ISKpn28 family transposase, an insertional sequence previously found in Klebsiella pneumoniae isolates30. The mutant hemolysin was thus designated hlyA::ISKpn28. All three marmoset colonies had significantly different prevalence of wildtype and mutant hemolysin genes, with Colony A having proportionally more E. coli isolates with hlyA::ISKpn28, all Colony B isolates being negative for both wildtype and mutant hemolysin, and all wildtype hlyA+ isolates originating from Colony C (p < 0.01) (Supplementary Figure S3).
Clinical correlates
Medical records were evaluated for temporal correlations of GI clinical signs from animals which had cultured pks+, cnf+, or cdt+ E. coli isolates. Of 122 animals from which E. coli was cultured, 96 had no history of GI disease, 18 had a history of clinical GI disease but were asymptomatic at the time of sample collection, 5 had IBD-like disease, and 3 were previously diagnosed with duodenal ulcers. There were no significant associations of gastrointestinal disease with the presence or absence of cyclomodulin-encoding E. coli overall, or when sorted by colony (Fig. 3).
(a) Health status of captive marmosets was not significantly different between animals that were or were not colonized by cyclomodulin-encoding E. coli isolates (as determined by PCR for pks, cnf, or cdt), nor was there a significant colony effect. (b) Health status of captive marmosets was significantly different in Colony A from the other two colonies. * p < 0.05, Fisher’s Exact Test.
In vitro cytotoxicity of E. coli isolates from marmosets
Cell culture assays were performed to demonstrate cytotoxicity of nine selected representative E. coli isolates (S1-S9) to HeLa cells. To detect colibactin activity, cells must be exposed to live bacteria to allow for precolibactin transport into the bacterial periplasmic space, followed by maturation31. Conversely, CDT and CNF cytotoxicity are only detectable using sonicate or supernatant preparations32. Live pks+ E. coli isolates S1-S6 induced megalocytic cytotoxicity in HeLa cells in a manner corresponding to multiplicity of infection, indicating contact-dependent colibactin activity (Supplementary Figure S4). HeLa cells treated with sonicate from cnf+ E. coli isolates S1-S3 displayed multinucleation and megalocytic cytotoxicity in a dose-dependent manner (Supplementary Figure S4). HeLa cells treated with sonicate from the cdt+ E. coli isolate S6 displayed megalocytosis and multinucleation in a dose-dependent manner (Supplementary Figure S4). These phenotypes were not observed in HeLa cells treated with live pks- E. coli isolates S7, S8, and S9, or with the sonicates of cnf− or cdt− isolates S4, S5, S7, S8, and S9.
Serotyping
Nine representative E. coli isolates (S1-S9) from all three colonies were sent to the E. coli Reference Center at Pennsylvania State University for serotyping and further virulence factor profiling (Table 1). Three of the isolates were O6:H+, all of which were in phylogenetic group B2 and pks+, but otherwise differed by genotype and source colony. Two of the isolates were O2:H14, both of which were in phylogenetic group B2 and encoded pks, cnf, and the mutant hemolysin gene, but each isolate originated from a different colony. The remaining pks+ isolate was cdt+, serotype O7:H7, and was in phylogenetic group B2. The three remaining isolates represented all three marmoset colonies and were negative for all tested virulence factors. The isolate from marmoset colony A was serotype O128:H2 and phylogroup B1. The isolate from marmoset colony B was serotype O26:H32 and phylogenetic group B2, which was unusual for a pks- E. coli isolate. The isolate from marmoset colony C was serotype O139:H19 and phylogroup B1. This isolate tested positive for cnf1 by PCR at the E. coli Reference Center at Pennsylvania State University, which conflicted with our negative PCR result as well as with later genome sequencing results. None of the nine E. coli isolates serotyped were positive for elt, estA, estB, stx1, stx2, eae, or cnf2.
Draft genome sequencing and comparative analysis
Whole genome sequences of five representative E. coli isolates (S1, S3, S4, S5, and S8) were evaluated for the presence of known virulence factor genes and for comparative analysis with other cyclomodulin-encoding E. coli from humans, rhesus macaques, and laboratory rodents. The genome sizes, GC contents, protein-coding sequences and RNA genes of the representative marmoset E. coli isolates were comparable to those of pks+ E. coli strains IHE3034 and NC101, as well as cnf+ E. coli strain UTI89 (Table 2). Gene sequences homologous to the pks island found in IHE3034 and NC101 were present in marmoset E. coli isolates S1, S3, S4, and S5, as predicted by the results of PCR and in vitro cytotoxicity assays. Syntenic alignments were confirmed for the pks island in all four predicted marmoset E. coli isolates with E. coli strains IHE3034 and NC101. (Supplementary Figure S5) Isolate S4 was found to have a putative hybrid gene for clbJ-K, similar to a gene sequence found previously in E. coli isolates from laboratory and pet rats28,33.
Gene sequences homologous to the hlyCABD-cnf1 operon present in UTI89 were detected in marmoset E. coli isolates S1 and S3, as predicted by the results of PCR and in vitro cytotoxicity assays. Syntenic alignments were confirmed for the hlyCABD-cnf1 operon in all predicted marmoset hlyA+ E. coli isolates with E. coli strain UTI89 (Supplementary Figure S5). Isolate S1 contained a 1081 bp insertion in the hlyA gene sequence as predicted by PCR and amplicon sequencing. Isolate S4 encoded all four hemolysin genes, but not the cnf1 gene sequence, consistent with PCR results, in vitro cytotoxicity in HeLa cells, and hemolytic phenotype.
Additional virulence factor genes were detected in the marmoset E. coli isolates (Table 2), including serine protease autotransporters of Enterobacteriaceae (SPATEs) (pic, vat), survival and immune evasion factors (gad, iss), iron acquisition (iroN), adherence (lpfA, sfaS), and synthesis and secretion genes for bacteriocins (cba, cma, mcmA, mchB, mchC, mchF). All five marmoset E. coli isolates encoded the mdfA gene, a broad-spectrum multidrug efflux pump that contributes to antibiotic resistance. Sequences for other cyclomodulins cnf2, cdtABC and cif, were not detected. Genes for virulence factors elt, estA, estB, stx1, stx2, and eae were not detected, consistent with previous PCR results. Several isolates (S1, S3, S4, and S5) had similar virulence factor profiles to necrotoxigenic (NTEC) and uropathogenic (UPEC) strains of E. coli, including CNF1, α-Hemolysin (αHly), fimbrial adhesins (SfaS), increased serum survival (ISS), and siderophore receptors (IroN). Similar Genome Finder showed isolates S3, S4 and S5 closely matched UPEC strains that caused urinary tract infections and bacteremia in humans and animals worldwide. Isolate S1 was similar to antibiotic resistant strains from human feces, and S8 showed similarity to potential foodborne contaminants. Similar genome descriptions and references are listed in Supplementary Table S3.
Discussion
Escherichia coli is normally a commensal organism colonizing the lower GI tract of common marmosets. However, certain strains of E. coli encode virulence factors which can cause disease. Enteropathogenic E. coli (EPEC) has previously been implicated in diarrhea, hemorrhagic typhlocolitis, and ileitis in laboratory marmosets22,23,24. Marmoset EPEC strains demonstrated similarities in virulence factors, pathogenic properties, and genomic features to human strains, indicating they are likely equivalent pathotypes and possibly capable of zoonotic transmission22,34. Additionally, marmosets have been experimentally infected with uropathogenic E. coli (UPEC) which caused lower urinary tract infection and pyelonephritis35. However, the prevalence and pathogenicity of cyclomodulin-encoding E. coli in laboratory common marmosets is not currently known.
Because of the diversity and ubiquity of E. coli, it can be challenging to determine whether an isolated strain is pathogenic. This determination is made based on the source, biotype, serotype, phylogenetic background, virulence factor profile, and demonstration of virulence properties using in vitro and in vivo models. Biotyping is commonly used to phenotypically differentiate unique E. coli isolates by characterization of the biochemical metabolism profile. Many marmosets were shown to be colonized by multiple unique E. coli isolates by this method. Phylogenetic typing is a simple method using multiplex PCR to assign an isolate into one of five phylogroups, which significantly correspond with the isolate’s pathogenicity36. This makes it feasible for use on a large scale to screen for potentially pathogenic E. coli in a given population. Pathogenic E. coli strains typically belong to phylogroups B2 or D, whereas those in phylogroups A and B1 are usually considered commensals37. Ninety-eight percent of the cyclomodulin-encoding E. coli isolated from laboratory marmosets belong to phylogenetic group B2, which is consistent with strains isolated from humans and other species11,26,27,28,33,38. The distribution of phylogroups was significantly different in Colony B, which had 61% prevalence of the non-pathogenic phylogroup A, whereas the prevalence of phylogroup A in Colony A was only 3%, and it was not isolated in Colony C. Potentially pathogenic E. coli classified in phylogroup B2 had a 44% prevalence overall, and was associated with the presence of virulence factor genes pks, cnf and hlyA, especially in Colonies A and C. Marmoset colony B had a significantly lower prevalence of pks+ (6%) and cnf+ (0%) E. coli isolates than the other two colonies.
Overall, 40% of the laboratory marmoset E. coli isolates were pks+. This is similar to the prevalence of pks+ E. coli in laboratory macaques (30.1%)26. Prevalence of pks+ E. coli in healthy humans ranged from 4.3 to 22%7,39. The majority (84%) of pks+ E. coli isolates from marmosets were also cnf+, and all cnf+ E. coli isolates were also pks+. Double-positive isolates (pks+/cnf+) have been characterized from both healthy humans and patients with urosepsis38, in contrast to surveys in humans and macaques where cnf is occasionally present in pks− isolates26,40. Nearly 34% of the marmoset E. coli isolates were cnf+, similar to what has been found previously in other laboratory nonhuman primates (20.9%)26. In contrast, a similar survey of cnf+ E. coli in healthy humans showed a prevalence of only 2%38.
Only one E. coli isolate was cdt+ (0.7% prevalence). Other surveys have not found cdt+ E. coli in human patients with diverticulosis, nor in macaques26,32.
Previous studies have shown an association between cnf and hemolysis29,38,40, which is consistent with the proximity of the cnf gene to the hemolysin gene. Interestingly, 96% of the cnf + isolates from marmosets did not demonstrate hemolysis due to an insertion event in the hlyA gene. Strains of E. coli with a similar deactivating insertion in the hlyA gene were previously isolated from laboratory rats from a specific vendor28.
HeLa cells infected with live bacteria of select pks+ E. coli isolates demonstrated megalocytosis, cytopathic morphology consistent with colibactin, and depending on the multiplicity of infection (MOI). In this assay, the cytopathic effect requires direct contact with live cells, presumably due to the instability of colibactin, which is matured upon secretion. Consistent with this known mechanism, HeLa cells treated with sonicates of pks+/cnf−/cdt− E. coli were indistinguishable from those treated with nonpathogenic K12 E. coli. HeLa cells treated with sonicates of select cnf+ E. coli isolates demonstrated multinucleation and megalocytosis, morphology consistent with cytotoxicity from CNF, in a manner dependent on the protein concentration of cnf+ E. coli sonicates. HeLa cells treated with the sonicate from S6, the single cdt+ E. coli isolate demonstrated cellular distension and multinucleation consistent with cytotoxicity from CDT and dependent on the protein concentration of the sonicate. Cells treated with cyclomodulin-negative E. coli strains were indistinguishable from those treated with nonpathogenic K12 strain. These results were consistent with findings from previous studies showing cytotoxic effects of pks+, cnf+ and cdt+ E. coli in vitro11,26,28,29,33.
Serotyping uses antibodies to characterize the O-polysaccharide antigens, flagellar H-antigens, and capsular K-antigens found on the surface of the bacteria. Certain serotypes are more frequently associated with specific pathotypes, such as EHEC O157:H741. Serotypes of select E. coli isolates from marmosets suggested the potential for pathogenicity. The most common serotypes were O2 (S1–S2) and O6 (S3–S5) (Table 1), both of which are frequently associated with urinary tract infection (UTI) in humans and animals42,43. Genome sequence analysis supports the potential of these isolates to cause UTI. Virulence factors associated with UPEC pathotype were found in isolates S1–S5, including S-fimbriae (sfaS), hemolysin (hlyA), cnf, iron uptake genes (iroN), and other colonization and survival factors (pic, vat)2. Whether these E. coli serotypes are associated with UTI in marmosets requires further study. E. coli in serogroups O26 and O128:H2 have been associated with enterohemorrhagic E. coli (EHEC) infections in humans and animals44. However, these isolates (S7 and S9) tested negative by PCR for the EHEC virulence factors stx1, stx2, and eae, in addition to being negative for hlyA, pks, and cnf, so they are unlikely to be pathogenic. Nonpathogenic O7:H7 E. coli negative for both eae and stx were isolated from laboratory rabbit fecal samples45. Finally, O139 is commonly involved in edema disease in post-weaning pigs46.
Whole genome sequence analysis of select E. coli isolates raised an interesting question about genetic variants of pks. Isolate S4 had a putative hybrid gene for clbJ-K. This hybrid gene contains approximately 90% of the clbJ sequence, and 45% of the clbK sequence, when compared to the IHE3034 genome. The putative clbJ-K hybrid gene is predicted to translate into a 2440-amino acid hybridized protein that contains two NPRS modules as well as an oxidase domain. Isolate S4 exhibited cytotoxicity to HeLa cells similar to other pks+ E. coli isolates tested (Supplementary Figure S4), suggesting the hybrid gene produces functional colibactin. Similar clbJ-K hybrid genes were found in E. coli isolates from laboratory and pet rats; and these isolates caused cytotoxicity and DNA damage in HeLa cells following in vitro infection28,33.
The fact that differences in prevalence were detected years after animals were transferred from each colony of origin to MIT suggests that these E. coli strains can stably colonize marmosets over multiple years. This is supported by the fact that similar E. coli isolates were cultured from 60% of marmosets 12 and 24 months previously. Furthermore, the presence of similar E. coli isolates in animals that were born at the MIT facility suggests these strains are passed to infants from family members. This observation is further validated by the unique cyclomodulin gene distribution found in Families 1 and 2 from Colony C, where 24% of individuals carried pks+/cnf− isolates of E. coli compared to 0% of the remainder of marmosets in Colony C.
Interestingly, 42/56 pks+ E. coli isolates harbored a hemolysin A gene with an inactivating insertional mutation (designated hlyA::ISKpn28). Thirty-four of the 42 hlyA::ISKpn28 strains had identical biochemical profiles (API code 5144552), and two representative strains were serotype O2:H14. The remaining 8 hlyA::ISKpn28 strains had identical biochemical profiles to each other (API code 7144552), and possessed arginine dihydrolase activity unlike the 34 hlyA::ISKpn28 isolates. All 42 of these isolates were only cultured from Colony A and C animals, suggesting there could be clonal transmission within and between the marmoset colonies. Future studies using whole genome sequencing will be required to confirm clonality as well as the need to evaluate the dynamics of E. coli transmission in marmoset colonies and family units.
Common marmosets in captivity are frequently afflicted with IBD-like disease in which the etiology and pathogenesis are presently unknown. In humans, cyclomodulin-encoding E. coli have been demonstrated in higher frequency in patients with colorectal cancer, familial adenomatous polyposis, and IBD than in healthy controls or in patients with other GI diseases7,9,32,39. Although this study did not show an association between cyclomodulin-encoding E. coli and clinical GI disease in marmosets, it does not exclude their potential role in subclinical intestinal inflammation in marmosets. One limitation of this study is that the evolving nature of the clinical diagnosis of CLE in marmosets means that some of the animals in this study may have been in early subclinical stages of the disease. It is possible that some strains of E. coli present in the GI tract may not have been isolated from a rectal swab culture, although microbiome comparison of rectal swabs to feces in marmosets showed good agreement47. Finally, the fact that pks is present in other Enterobacteriaceae species raises the possibility that colibactin-producing bacteria are more prevalent in this population than we detected by culture of fecal material for E. coli only. These issues can be addressed in future studies with improved antemortem diagnostics including markers of intestinal inflammation, as well as clinical correlations with metagenomic data.
This report is the first to characterize the presence of cyclomodulin-encoding E. coli in marmosets. We have demonstrated that laboratory common marmosets can be colonized by cyclomodulin-encoding E. coli and have further shown that the prevalence of these cyclomodulin genes varies significantly depending on the source of the animal. Although we hypothesized that pks+, cnf+ and cdt+ E. coli is present in animals clinically afflicted with gastrointestinal disease at greater frequency than in clinically normal animals, no such association was detected in this cohort of marmosets. However, E. coli encoding colibactin, CNF, and to a lesser extent CDT, colonizing laboratory common marmosets may influence clinical and subclinical disease or contribute to physiological variation between animals from different sources, thus potentially affecting reproducibility in this model.
Materials and methods
Animals
Common marmosets (Callithrix jacchus) were housed in an AAALAC-accredited institution. Studies were conducted on an animal use protocol approved by the Massachusetts Institute of Technology Committee on Animal Care, and all experiments were performed in accordance with relevant guidelines and regulations. Marmosets were socially housed in breeding pairs or small family groups unless a suitable mate was unavailable. Enclosures were enriched stainless steel and polycarbonate cages (inner dimensions 56 in. × 28 in. × 28 in.) in a housing room maintained at 23.3 ± 1.0 °C, with a relative humidity of 30% to 70% and a 12:12-h light:dark cycle. Diet consisted of extruded biscuits (Teklad New World Primate Diet 8794, Envigo, Madison, WI) soaked lightly in water, supplemented with canned diet (ZuPreem, Premium Nutritional Products, Shawnee, KS), washed fruits and vegetables, and various protein sources. Chlorinated reverse-osmosis-purified water was provided ad libitum. Marmosets were observed at least twice daily by veterinary staff, and individual health records were maintained.
Marmosets originating from three different institutions were maintained in separate breeding colonies with strict biosecurity to minimize microbial contamination. Colony A came from a contract research organization in 2017. Colony B came from a commercial vendor in 2016. Colony C came from the New England Primate Center in 2014. Animals were seronegative for squirrel monkey cytomegalovirus, Saimiriine herpesvirus 1, Saimiriine herpesvirus 2, and measles virus (VRL Laboratories, San Antonio, TX) on arrival to MIT. Semiannual health monitoring included sedated physical examinations, hematology, and surveillance for fecal pathogens.
Culture and isolation
Rectal swabs were collected during semiannual examinations from all marmosets over 6 months of age in 2018, and from a subset of animals in 2016 and 2017. Rectal swabs were enriched in Gram-negative broth (Becton Dickenson, Franklin Lakes, NJ), then streaked onto MacConkey lactose agar plates (Remel, Lenexa, KS). Lactose-fermenting colonies were selected, then plated onto sheep blood agar plates (Remel) to determine hemolysis. All bacterial cultures were incubated overnight at 37 °C. Isolates phenotypically consistent with E. coli were biochemically characterized using API 20 E (Biomérieux, Marcy l’Etoile, France).
DNA extraction and PCR amplification
Escherichia coli colonies were suspended in sterile phosphate buffered saline (PBS), boiled for 10 min, and centrifuged at 12,000g for 10 min. Supernatant was used for PCR analysis. Primers for clbQ were used to identify pks genes26. Primers for cnf were used that amplify both cnf1 and cnf2. Primers for all known variants of cdtB were used to detect cdt. Primers for hlyA were used to identify hemolysin genes. To determine the phylogenetic groups of isolates, multiplex PCR analysis was conducted using primers for svg, chuA, yjaA, uidA, and TspE4.C2. Amplicons produced distinct banding patterns with gel electrophoresis, allowing for differentiation of phylogroups A, B1, B2, and D36,37,48. The primers and annealing temperatures used are shown in Supplementary Table S4. Gels were stained with ethidium bromide and imaged using Syngene GeneSnap software. Gel images were edited for clarity. Unedited images of gels are shown in Supplementary Figure S6. Sanger sequencing (Quintara Biosciences, Cambridge, MA) of amplicons from select isolates was used to confirm sequence identity and to compare wildtype and mutant hlyA sequences.
Clinical associations
Medical records were evaluated for temporal correlations from animals which had gastrointestinal clinical signs with culture of cyclomodulin-encoding E. coli isolates. Animals with more than three consecutive days of diarrhea were treated with oral enrofloxacin (5 mg/kg once daily for 5 days) and fecal samples were tested for GI pathogens. A presumptive diagnosis of IBD-like disease was made when an adult animal had bodyweight below 325 g and hypoalbuminemia below 3.5 g/dL with no identified infectious cause49. These animals were maintained on oral budesonide (0.5–0.75 mg once daily) to manage clinical signs. In animals with repeated bouts of vomiting and inappetence coupled with weight loss, a presumptive diagnosis of duodenal ulceration was made by palpation of a cranial abdominal mass, and often confirmed with abdominal ultrasound50. Many elements of this syndrome are similar to the duodenal dilation syndrome recently described51. These animals were maintained on oral sucralfate (100–200 mg/kg once daily) to manage clinical signs.
Animals with E. coli isolates collected in 2016 and 2017 were evaluated for stability of colonization by cyclomodulin-encoding E. coli over time. Animal records were also evaluated for associations of familial relationships with colonization by similar E. coli isolates based on biochemical analyses, phylogenetic groups, and virulence factor genotypes.
Cytotoxicity assays
Escherichia coli strains used as controls in the cytotoxicity assays included K12 (pks-/cnf-/cdt-), V27 (a pks+/cnf−/cdt+ control from the E. coli Reference Center at Penn State), NC101 (a pks+/cnf−/cdt− mouse isolate generously gifted from Dr. Christian Jobin), and 1701240014 (a pks−/cnf+/cdt− rat isolate). Nine E. coli isolates from clinically normal marmosets were selected for more detailed analysis, and designated S1 through S9. These isolates represented all three marmoset colonies and all cyclomodulin genotype combinations present, including pks+/cnf+/cdt−, pks+/cnf−/cdt+, pks+/cnf−/cdt−, and pks−/cnf−/cdt−. These isolates were submitted for serotyping, and five of these isolates were selected for whole genome sequencing. HeLa S3 cells (CCL2.2, ATCC, Manassas, VA) were cultured in Eagle minimal essential medium (EMEM, ATCC) containing 10% FCS (Sigma, St Louis, MO) and 1% antibiotic–antimycotic (Gibco, Gaithersburg, MD) at 37 °C with 5% CO2. For both assays, 5,000 cells per well were seeded onto 96-well cell culture plates and incubated for 24 h (doubling time). Cells were then treated as described in the following sections. At the end of experiments, plates were stained with Diff-quick (Thermo Fisher Scientific, Waltham, MA). Cells were inspected for confluence and morphologic changes. Images were captured at 20× magnification with a Zeiss Axiovert-10 microscope (Jena, Germany) using Image Pro-Plus software version 7.0 (Media Cybernetics, Rockville, MD).
Cell culture assay for colibactin cytotoxicity
The assay was performed as described previously with modifications11,27. Isolates of E. coli cultured overnight were incubated in LB broth for 2 h at 37 °C to reach logarithmic growth phase, then adjusted using OD600nm in 1% FCS EMEM to concentrations corresponding to multiplicities of infection (MOI) of 100, 25 and 5 bacteria per cell. After inoculation into 24-h non-confluent HeLa cultures, the 96-well plates were centrifuged at 200 g for 10 min to facilitate bacterial interaction, then incubated at 37 °C with 5% CO2 for 4 h. Cells were washed with EMEM and then incubated in EMEM containing 10% FCS and 200 µg/mL gentamicin (Gibco) at 37 °C with 5% CO2 for 72 h.
Cell culture assay for sonicate cytotoxicity
Overnight cultures of E. coli isolates were suspended in PBS and pelleted by centrifugation at 12,000 rpm for 10 min at 4 °C. The pellets were resuspended in 1.5 mL of PBS and sonicated on ice (amplitude: 35, power: 7 W) for a total of 5 min divided into 30-s intervals, with 60-s breaks between intervals to prevent overheating. Sonicated samples were centrifuged at 12,000 rpm for 10 min at 4 °C. Supernatants were collected and filter-sterilized through 0.2 µm filters. Total proteins were quantified using the BCA assay (Thermo Fisher Scientific), then 24-h non-confluent HeLa cultures were treated with crude bacterial sonicate (80, 140, or 220 µg/mL total protein) or PBS (40 µL) and incubated at 37 °C with 5% CO2 for 72 h.
Serotyping
The nine representative E. coli isolates, S1–S9, were submitted to the E. coli Reference Center at The Pennsylvania State University for serotype testing, which included O and H typing as well as PCR analyses for heat-labile enterotoxin (elt), heat stable enterotoxins A and B (estA and estB), Shiga toxins 1 and 2 (stx1 and stx2), cytotoxic necrotizing factor 1 and 2 (cnf1 and cnf2), and intimin gamma (eae).
Draft genome sequencing and comparative analysis
Genomic DNA was isolated using the High Pure PCR Template Preparation Kit (Roche Molecular Biochemicals, Indianapolis, IN) following the manufacturer’s protocol for bacterial cell samples. DNA libraries were prepared using the QIAseq FX DNA Library Kit (Qiagen, Valencia, CA) following the manufacturer’s protocol for 500 bp fragments. DNA libraries were sequenced using 2 × 300 bp paired-end reads by Illumina MiSeq by the MIT BioMicro Center. Raw sequenced reads were decontaminated of adapter sequences and quality trimmed to a Phred quality score (Q) ≥ 10 using BBDuk from the BBMap package version 38.34 (http://sourceforge.net/projects/bbmap/). Decontaminated reads were then assembled into contigs with SPAdes followed by genome annotation with RAST, both services hosted by PATRIC52 .Sequences encoding putative virulence factor and antibiotic-resistance genes were identified using PathogenFinder 1.153, VirulenceFinder 2.054, and ResFinder 3.255 using the 90% identity and 60% minimum length threshold parameters, as described previously26. Syntenic relationships of pks genes and the hlyCABD-cnf1 operon between genomes were determined with SimpleSynteny v1.456, as described previously26. The “Similar Genome Finder” tool, hosted by PATRIC52, was used to identify E. coli genomes that were related to the marmoset E. coli isolate genomes. Sequences have been deposited in GenBank under the following accession numbers JAADCB000000000, JAADBZ000000000, JAADCA000000000, JAADCC000000000, and JAADBY000000000 for isolates S1, S3, S4, S5, and S8, respectively (Table 2).
Statistical analysis
A webtool was used to calculate expected contingency tables and to perform 2-tailed Fisher exact tests to evaluate categorical data with small group sizes. Differences were determined between categories of virulence factor, phylogroup, and API code, among the three marmoset colonies and taking into account marmoset age, sex and health status57. Statistical significance was set at a P value of less than 0.05.
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Mainil, J. Escherichia coli virulence factors. Vet. Immunol. Immunopathol. 152, 2–12. https://doi.org/10.1016/j.vetimm.2012.09.032 (2013).
Kaper, J. B., Nataro, J. P. & Mobley, H. L. Pathogenic Escherichia coli. Nat. Rev. Microbiol. 2, 123–140. https://doi.org/10.1038/nrmicro818 (2004).
El-Aouar Filho, R. A. et al. Heterogeneous family of cyclomodulins: smart weapons that allow bacteria to hijack the eukaryotic cell cycle and promote infections. Front. Cell. Infect. Microbiol. 7, 208. https://doi.org/10.3389/fcimb.2017.00208 (2017).
Bossuet-Greif, N., et al. The colibactin genotoxin generates DNA interstrand cross-links in infected cells. mBio 9, e02393–17. https://doi.org/10.1128/mbio.02393-17 (2018).
Wilson, M.R., et al. The human gut bacterial genotoxin colibactin alkylates DNA. Science 363, eaar7785. https://doi.org/10.1126/science.aar7785 (2019).
Xue, M., et al. Structure elucidation of colibactin and its DNA cross-links. Science 365, eaax2685. https://doi.org/10.1126/science.aax2685 (2019).
Iyadorai, T. et al. Prevalence and association of pks+ Escherichia coli with colorectal cancer in patients at the University Malaya Medical Centre, Malaysia. PLoS ONE 28, e0228217. https://doi.org/10.1371/journal.pone.0228217 (2020).
Pleguezuelos-Manzano, C., et al. Mutational signature in colorectal cancer caused by genotoxic pks+ E. coli. Nature 580, 269–273. https://doi.org/10.1038/s41586-020-2080-8 (2020).
Prorok-Hamon, M. et al. Colonic mucosa-associated diffusely adherent afaC+ Escherichia coli expressing lpfA and pks are increased in inflammatory bowel disease and colon cancer. Gut 63, 761–770. https://doi.org/10.1136/gutjnl-2013-304739 (2014).
Faïs, T., Delmas, J., Barnich, N., Bonnet, R. & Dalmasso, G. Colibactin: more than a new bacterial toxin. Toxins (Basel). 10, E151. https://doi.org/10.3390/toxins10040151 (2018).
Nougayrède, J. P. et al. Escherichia coli induces DNA double-strand breaks in eukaryotic cells. Science 313, 848–851. https://doi.org/10.1126/science.1127059 (2006).
Fabbri, A. et al. The Escherichia coli protein toxin cytotoxic necrotizing factor 1 induces epithelial mesenchymal transition. Cell. Microbiol. 22, e13138. https://doi.org/10.1111/cmi.13138 (2020).
Knust, Z. & Schmidt, G. Cytotoxic necrotizing factors (CNFs)-a growing toxin family. Toxins (Basel) 2, 116–127. https://doi.org/10.3390/toxins2010116 (2010).
Orden, J. A. et al. Necrotoxigenic Escherichia coli from sheep and goats produce a new type of cytotoxic necrotizing factor (CNF3) associated with the eae and ehxA genes. Int. Microbiol. 10, 47–55 (2007).
Welch, R.A. Uropathogenic Escherichia coli-associated exotoxins. Microbiol. Spectr. 4. https://doi.org/10.1128/microbiolspec.UTI-0011-2012 (2016).
Bielaszewska, M., Aldick, T., Bauwens, A. & Karch, H. Hemolysin of enterohemorrhagic Escherichia coli: structure, transport, biological activity and putative role in virulence. Int. J. Med. Microbiol. 304, 521–529. https://doi.org/10.1016/j.ijmm.2014.05.005 (2014).
Smith, Y. C., Rasmussen, S. B., Grande, K. K., Conran, R. M. & O’Brien, A. D. Hemolysin of uropathogenic Escherichia coli evokes extensive shedding of the uroepithelium and hemorrhage in bladder tissue within the first 24 hours after intraurethral inoculation of mice. Infect. Immun. 76, 2978–2990. https://doi.org/10.1128/IAI.00075-08 (2008).
Strack, K. et al. Induction of erythrocyte microvesicles by Escherichia Coli alpha hemolysin. Biochem. J. 476, 3455–3473. https://doi.org/10.1042/BCJ20190546 (2019).
Ge, Z., Schauer, D. B. & Fox, J. G. In vivo virulence properties of bacterial cytolethal-distending toxin. Cell. Microbiol. 10, 1599–1607. https://doi.org/10.1111/j.1462-5822.2008.01173.x (2008).
Marini, R.P., Fox, J.G., Wachtman, L.M., Mansfield, K. & Tardif, S.D. (eds.) The Common Marmoset in Captivity and Biomedical Research, (Elsevier Science Publishing Co Inc, 2019).
Parambeth J.C., et al. Serum cobalamin and folate concentrations in common marmosets (Callithrix jacchus) with chronic lymphocytic enteritis. Comp. Med. 69, 135–143. https://doi.org/10.30802/AALAS-CM-18-000045 (2019).
Carvalho, V. M. et al. Characterization of monkey enteropathogenic Escherichia coli (EPEC) and human typical and atypical EPEC serotype isolates from neotropical nonhuman primates. J. Clin. Microbiol. 41, 1225–1234. https://doi.org/10.1128/jcm.41.3.1225-1234.2003 (2003).
Hayashimoto, N. et al. Survey and experimental infection of enteropathogenic Escherichia coli in common marmosets (Callithrix jacchus). PLoS ONE 11, e0160116. https://doi.org/10.1371/journal.pone.0160116 (2016).
Thomson, J. A. & Scheffler, J. J. Hemorrhagic typhlocolitis associated with attaching and effacing Escherichia coli in common marmosets. Lab. Anim. Sci. 46, 275–279 (1996).
Bakthavatchalu, V. et al. Cytotoxic Escherichia coli strains encoding colibactin isolated from immunocompromised mice with urosepsis and meningitis. PLoS ONE 13, e0194443. https://doi.org/10.1371/journal.pone.0194443 (2018).
Feng, Y. et al. Cytotoxic Escherichia coli strains encoding colibactin and cytotoxic necrotizing factor (CNF) colonize laboratory macaques. Gut Pathog. 9, 1–15. https://doi.org/10.1186/s13099-017-0220-y (2017).
García, A. et al. Cytotoxic Escherichia coli strains encoding colibactin colonize laboratory mice. Microbes Infect. 18, 777–786. https://doi.org/10.1016/j.micinf.2016.07.005 (2016).
Kurnick, S.A., et al. Genotoxic Escherichia coli strains encoding colibactin, cytolethal distending toxin, and cytotoxic necrotizing factor in laboratory rats. Comp. Med. 69, 103–113. https://doi.org/10.30802/AALAS-CM-18-000099 (2019).
Marini, R. P. et al. Characterization of hemolytic Escherichia coli strains in ferrets: recognition of candidate virulence factor CNF1. J. Clin. Microbiol. 42, 5904–5908. https://doi.org/10.1128/jcm.42.12.5904-5908.2004 (2004).
Siguier, P., Perochon, J., Lestrade, L., Mahillon, J. & Chandler, M. ISfinder: the reference centre for bacterial insertion sequences. Nucleic Acids Res. 34 (Database issue), D32-D36. http://www-is.biotoul.fr (2006).
Volpe, M. R. et al. In vitro characterization of the colibactin-activating peptidase ClbP enables development of a fluorogenic activity probe. ACS Chem. Biol. 14, 1097–1101. https://doi.org/10.1021/acschembio.9b00069 (2019).
Buc, E., et al. High prevalence of mucosa-associated E. coli producing cyclomodulin and genotoxin in colon cancer. PLOS ONE 8, e56964. https://doi.org/10.1371/journal.pone.0056964 (2013).
Fabian, N. J., Mannion, A. J., Feng, Y., Madden, C. M. & Fox, J. G. Intestinal colonization of genotoxic Escherichia coli strains encoding colibactin and cytotoxic necrotizing factor in small mammal pets. Vet. Microbiol. 240, 108506. https://doi.org/10.1016/j.vetmic.2019.108506 (2020).
Carvalho, V.M., Irino, K., Onuma, D. & Pestana de Castro, A.F. Random amplification of polymorphic DNA reveals clonal relationships among enteropathogenic Escherichia coli isolated from non-human primates and humans. Braz. J. Med. Biol. Res. 40, 237–241. https://doi.org/10.1590/s0100-879x2007000200010 (2007).
Rocha, H., Da Silva Teles, E. & Brito, E. Studies on experimental bacteremia and pyelonephritis in the marmoset (Callithrix jacchus). Proc. Soc. Exp. Biol. Med. 129, 506–509. https://doi.org/10.3181/00379727-129-33356 (1968).
Bidet, P. et al. Detection and identification by PCR of a highly virulent phylogenetic subgroup among extraintestinal pathogenic Escherichia coli B2 strains. Appl. Environ. Microbiol. 73, 2373–2377. https://doi.org/10.1128/AEM.02341-06 (2007).
Clermont, O., Christenson, J. K., Denamur, E. & Gordon, D. M. The Clermont Escherichia coli phylo-typing method revisited: improvement of specificity and detection of new phylo-groups. Environ. Microbiol. Rep. 5, 58–65. https://doi.org/10.1111/1758-2229.12019 (2013).
Dubois, D. et al. Cyclomodulins in urosepsis strains of Escherichia coli. J. Clin. Microbiol. 48, 2122–2129. https://doi.org/10.1128/jcm.02365-09 (2010).
Dejea, C. M. et al. Patients with familial adenomatous polyposis harbor colonic biofilms containing tumorigenic bacteria. Science 359, 592–597. https://doi.org/10.1126/science.aah3648 (2018).
Raisch, J. et al. Colon cancer-associated B2 Escherichia coli colonize gut mucosa and promote cell proliferation. World J. Gastroenterol. 20, 6560–6572. https://doi.org/10.3748/wjg.v20.i21.6560 (2014).
Fratamico, P. M. et al. Advances in molecular serotyping and subtyping of Escherichia coli. Front. Microbiol. 7, 644. https://doi.org/10.3389/fmicb.2016.00644 (2016).
Cunha, M. P. V. et al. Pandemic extra-intestinal pathogenic Escherichia coli (ExPEC) clonal group O6–B2-ST73 as a cause of avian colibacillosis in Brazil. PLoS ONE 12, e0178970. https://doi.org/10.1371/journal.pone.0178970 (2017).
Delannoy, S. et al. The Escherichia coli serogroup O1 and O2 lipopolysaccharides are encoded by multiple O-antigen gene clusters. Front. Cell. Infect. Microbiol. 7, 30. https://doi.org/10.3389/fcimb.2017.00030 (2017).
Eichhorn, I. et al. Highly virulent non-O157 enterohemorrhagic Escherichia coli (EHEC) serotypes reflect similar phylogenetic lineages, providing new insights into the evolution of EHEC. Appl. Environ. Microbiol. 81, 7041–7047. https://doi.org/10.1128/AEM.01921-15 (2015).
García, A. & Fox, J. G. The rabbit as a new reservoir host of enterohemorrhagic Escherichia coli. Emerg. Infect. Dis. 9, 1592–1597. https://doi.org/10.3201/eid0912.030223 (2003).
Do, K. H., Byun, J. W. & Lee, W. K. Prevalence of O-serogroups, virulence genes, and F18 antigenic variants in Escherichia coli isolated from weaned piglets with diarrhea in Korea during 2008–2016. J. Vet. Sci. 20, 43–50. https://doi.org/10.4142/jvs.2019.20.1.43 (2019).
Artim, S. C., Sheh, A., Burns, M. A. & Fox, J. G. Evaluating rectal swab collection method for gut microbiome analysis in the common marmoset (Callithrix jacchus). PLoS ONE 14, e0224950. https://doi.org/10.1371/journal.pone.0224950 (2019).
Clermont, O., Bonacorsi, S. & Bingen, E. Rapid and simple determination of the Escherichia coli phylogenetic group. Appl. Environ. Microbiol. 66, 4555–4558. https://doi.org/10.1128/aem.66.10.4555-4558.2000 (2000).
Baxter, V. K. et al. Serum albumin and body weight as biomarkers for the antemortem identification of bone and gastrointestinal disease in the common marmoset. PLoS ONE 8, e82747. https://doi.org/10.1371/journal.pone.0082747 (2013).
Artim, S.C., Burns, M.A., Sheh, A., Fox, J.G. & Muthupalani, S. P139 A syndrome of duodenal ulceration with strictures in a colony of common marmosets (Callithrix jacchus) [abstract]. In: Abstracts of Scientific Presentations 2019 AALAS National Meeting. J. Am. Assoc. Lab. Anim. Sci. 58, 607–726, (2019).
Mineshige, T. et al. Novel gastrointestinal disease in common marmosets characterised by duodenal dilation: a clinical and pathological study. Sci. Rep. 10, 3793. https://doi.org/10.1038/s41598-020-60398-4 (2020).
Wattam, A. R. et al. Improvements to PATRIC, the all-bacterial Bioinformatics Database and Analysis Resource Center. Nucleic Acids Res. 45, D535–D542. https://doi.org/10.1093/nar/gkw1017 (2017).
Cosentino, S., Larsen, M. V., Aarestrup, F. M. & Lund, O. PathogenFinder–distinguishing friend from foe using bacterial whole genome sequence data. PLoS ONE 8, e77302. https://doi.org/10.1371/journal.pone.0077302 (2013).
Joensen, K. G. et al. Real-time whole-genome sequencing for routine typing, surveillance, and outbreak detection of verotoxigenic Escherichia coli. J. Clin. Micobiol. 52, 1501–1510. https://doi.org/10.1128/jcm.03617-13 (2014).
Zankari, E. et al. Identification of acquired antimicrobial resistance genes. J. Antimicrob. Chemother. 67, 2640–2644. https://doi.org/10.1093/jac/dks261 (2012).
Veltri, D., Malapi-Wight, M. & Crouch, J. A. SimpleSynteny: a web-based tool for visualization of microsynteny across multiple species. Nucleic Acids Res. 44, W41–W45. https://doi.org/10.1093/nar/gkw330 (2016).
Kirkman, T.W. Statistics to Use. http://www.physics.csbsju.edu/stats/ (1996) (Accessed 26 Mar 2019).
Acknowledgements
Research reported in this publication was supported by principal investigator James G. Fox’s grants from NIH T32 (RR07036) and P30 (P30ES02109), as well as a grant from the McGovern Institute at Massachusetts Institute of Technology. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH or the McGovern Institute.
Author information
Authors and Affiliations
Contributions
Supervised the work: J.G.F. Conceived and designed the study: J.G.F., A.J.M., Y.F., and A.S. Performed the study: C.S.M., A.J.M., Y.F., C.M.M., S.C.A., J.L.H., M.A.B., G.G.A., and M.D. Analyzed the data: C.S.M. and A.J.M. Wrote and edited manuscript: C.S.M., A.J.M. and J.G.F. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
McCoy, C.S., Mannion, A.J., Feng, Y. et al. Cytotoxic Escherichia coli strains encoding colibactin, cytotoxic necrotizing factor, and cytolethal distending toxin colonize laboratory common marmosets (Callithrix jacchus). Sci Rep 11, 2309 (2021). https://doi.org/10.1038/s41598-020-80000-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-80000-1
- Springer Nature Limited