Abstract
A significantly negative reversion in the QuantiFERON-TB Gold In-tube (QFT-GIT) test is reported in patients on dialysis, which makes the results unreliable. The CD4 and CD8 responses of the QFT-Gold plus (QFT-Plus) may have better positive consistency, but this needs to be investigated. We enrolled dialysis patients with baseline positive QFT-GIT0 results and conducted two rounds of follow-up paired QFT-GIT1&2 and QFT-Plus1&2 tests at an interval of 6 months. The positive consistency, concordance, and discordance of the QFT results were analyzed. A total of 236 patients on dialysis were screened, and 73 participants with positive QFT-GIT0 results were enrolled. The baseline QFT-GIT0 response was higher in the 1st QFT-Plus1(+) group than in the QFT-Plus1(−) group, but insignificantly different between the 1st QFT-GIT1(+) and QFT-GIT1(−) groups. The two assays had good correlation when concurrently tested. Fifty-three subjects completed a second round of the QFT-GIT2 and QFT-Plus2. Persistent positivity was higher with the QFT-Plus2 (81.8%) than with the QFT-GIT2 (58.8%, p = 0.040). The QFT-GIT1 and QFT-Plus1 CD4 responses were higher in patients with persistent positivity than in those with negative reversion, whereas the difference of the QFT-Plus TB1 and TB2 data, representative of the CD8 response, were similar between positive persistence and negative reversion. In conclusion, the QFT-Plus provides more reliable positive consistency than does the QFT-GIT. The CD4 interferon-γ response might play a role in maintaining positivity of LTBI.
Similar content being viewed by others
Introduction
Tuberculosis (TB) remains one of the most common infectious diseases in the world. According to the World Health Organization, around 10.0 million TB cases and an estimated 1.2 million TB deaths occurred in HIV-negative people in 20181. In fact, an estimated one quarter of the world’s people carry Mycobacterium tuberculosis (M.tb)2. When elderly and immunocompromised patients develop active TB, mortality and morbidity are still high3,4. In the future of the management of active TB, latent TB infection (LTBI) intervention is one strategy for preventing reactivation and reducing transmission5,6.
Among the high-risk populations for TB reactivation, patients on dialysis have a higher incidence of TB (10–25 times higher) than the general population7,8. Notably, dialysis patients have high mortality once active TB develops due to the atypical manifestation and delayed diagnosis9,10. Therefore, in addition to those with close contact with TB, patients receiving dialysis are also highly prioritized for LTBI intervention in Taiwan5.
However, LTBI is diagnosed indirectly by immune assays such as the tuberculin skin test and interferon-gamma release assays (IGRAs), which, due to immune depression, sometimes yield false negative results in patients receiving dialysis11,12. Although our prior study using QuantiFERON-TB Gold In-tube (QFT-GIT) demonstrated an acceptable indeterminate rate of < 5% in dialysis patients12, the negative reversion rate at 6-month follow-up was 44–48%13,14, which was higher than the 33–35% in contacts or healthcare workers15,16. Notably, the greater consistency of positive QFT-GIT tests, a kind of IGRA, is reportedly correlated with the higher incidence of TB in patients receiving dialysis14. The hazard ratio for TB development increases by 10.4- to 14.4-fold if QFT-GIT results remain positive after 6 months14.
A test with better consistency of positive results is needed because serial follow-up using IGRA is time-consuming and wasteful of resources. Currently, a new test, the QuantiFERON-TB Gold Plus (QFT-Plus), has modified epitopes for CD4 and CD8 lymphocyte-specific reactions. Better LTBI diagnosis and lower non-specific reactions have been reported17,18,19,20. But all the reports to date have been based on cross-sectional designs, either for active TB or for LTBI, and they have lacked discussion of immunocompromised patients. Although the QFT-Plus might have a more specific M.tb response and have positive consistency, it still needs to be validated. In the present study, we aimed to examine the consistency of positive LTBI status after 6 months by directly comparing QFT-Plus and QFT-GIT assays in dialysis patients.
Results
Participant enrollment
A total of 236 patients on maintenance dialysis at hospital 1 and 234 patients of hospital 2 were screened for LTBI (Fig. 1). Among them, 108 patients (44 [18.6%] from hospital 1 and 64 [27.4%] from hospital 2) had positive QFT-GIT results. Thirty-five of these patients rejected the invitation to participate in the study. Thereafter, 73 subjects were enrolled in the first round of testing of the QFT-GIT and QFT-Plus together. Around 6 months later, at the follow-up testing of the QFT-GIT and QFT-Plus, twenty participants withdrew from the study, leaving fifty-three participants in the second round.
Baseline test of QFT-GIT0 and first follow-ups of QFT-GIT1 and QFT-Plus1
The 73 participants were 56.7 ± 11.2 years old (mean ± standard deviation). The majority were men (71%) and patients receiving hemodialysis (80.8%) (Table 1). After the 1st round of paired tests, fifty-seven participants received LTBI treatment during follow-up, with similar distributions of QFT-GIT1(+) and QFT-Plus1(+) results. Weekly high-dose rifapentine plus isoniazid for 3 months was prescribed to 74%, and 9 months of isoniazid daily were prescribed to the remaining 26%. The mean IFN-γ response of the baseline QFT-GIT (QFT-GIT0) was 2.31 ± 2.40 IU/ml for all. The first round of follow-up QFT-GIT and QFT-Plus results were QFT-GIT(+) for 49 subjects (67.1% of 73 patients) and QFT-Plus(+) for 47 subjects (64.4%). The demographic data, including BCG scar and QFT-GIT0, were not significantly different between the first QFT-GIT(+) and QFT-Plus (+) groups. The QFT-GIT0 response was higher in the QFT-Plus1(+) group than in the QFT-Plus1(−) group (2.93 vs. 1.18 IU/ml, p = 0.002). By contrast, the QFT-GIT0 response was not significantly different between the QFT-GIT1(+) and QFT-GIT1(−) cases (2.65 vs. 1.60 IU/ml, p = 0.080) (Table S1, supplement file).
As shown in Table 2, for the IFN-γ response of the first follow-up test of the QFT-GIT (QFT-GIT1), the TB1 tube in the first test of the QFT-Plus (T1 of QFT-Plus1) and the TB2 tube in the first test of the QFT-Plus1 (T2 of QFT-Plus1) had good correlation with the IFN-γ response of the QFT-GIT0 (Spearman correlation r: 0.520, 0.611, and 0.611, respectively [all p < 0.001]). However, the QFT-GIT0 had no significant correlation with the IFN-γ level of the TB2 tube minus the TB1 tube of the QFT-Plus (T2–T1 of the QFT-Plus1) (Spearman correlation coefficient r: 0.085, p = 0.477).
Discordance and concordance of first follow-up QFT-Plus and QFT-GIT tests
Regarding the QFT1 response, the QFT-GIT1 had good correlation with T1 of the QFT-Plus1 and T2 of the QFT-Plus1 (r = 0.879 and 0.895, respectively, both p < 0.001) (Table 2). But T2–T1 of the QFT-Plus1 had non-significant correlation with the QFT-GIT1 (r = 0.150, p = 0.206). For binary results (positive or negative), the concordance rate of the QFT-GIT1 and the QFT-Plus1 was 83.6%, whereas the discordance rate was 16.4% ([Table S2 in supplement file]). The discordant QFT-Plus (+)/QFT-GIT(−) cases were younger (45.9 vs. 58.8 years, p = 0.017) but had similar T1/T2 responses (p = 0.250 and 0.221, respectively) to those of the concordant QFT-Plus (+)/QFT-GIT(+) group (Table S3 in supplement file). In the discordant QFT-Plus (−)/QFT-GIT(+) subgroup, the QFT response was 0.58 IU/ml, lower than that of the positive concordant subgroup (p = 0.027).
Concordance of first and second follow-ups of QFT-GIT and QFT-Plus
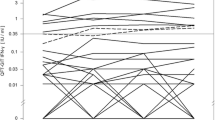
A total of 53 subjects (34 QFT-GIT1 positive and 33 QFT-Plus1 positive) completed the second follow-up round of paired QFT-GIT and QFT-Plus tests (Table 3 and Fig. 2). Persistent positivity was found in 20 subjects by the QFT-GIT (58.8%) and in 27 by the QFT-Plus (81.8%, p = 0.040). The Cohen’s kappa values of the QFT-GIT and QFT-Plus were 0.34 and 0.61, respectively. Negative concordances of the QFT-GIT and QFT-Plus were similar (78.9% vs. 80%). Even after the exclusion of 1 patient who had old TB and completed the two rounds of tests, the QFT-Plus test had better positive consistency than the QFT-GIT test (81.3% vs. 58.8%, p = 0.047).
Demographic differences between persistent positivity and negative reversion patients
For the patients with persistent positive results on two subsequent QFT2 tests, no significant differences from their corresponding negative reversion groups existed in age, dialysis type, diabetes mellitus, BCG vaccination scar or LTBI treatment rate (Table 4). However, more men had persistent positive QFT-Plus results than negative reversion (77.8% vs. 33.3%, p = 0.053). The baseline test of the QFT-GIT0 (2.47 ± 0.55 vs. 1.20 ± 0.81, p < 0.001) (Table 4) and that of the QFT-GIT1 (3.68 ± 2.47 vs. 0.98 ± 0.81, p < 0.001) were higher in patients with persistent positivity than in those with negative reversion of QFT-GIT2. The T1 and T2 responses of the QFT-Plus1 were also higher in those with persistent positivity than in the negative reversion group. But the T2–T1 levels of subsequent QFT-Plus1 tests were not significantly different between the persistent positivity and negative reversion groups.
For patients with persistent positive results of the QFT-Plus2, their QFT-GIT1, T1 and T2 of the QFT-Plus1 were significantly higher than those with reversion (2.39 ± 2.22 vs. 0.63 ± 0.21, p < 0.001; 2.13 ± 1.96 vs. 0.55 ± 0.30, p < 0.001; and 2.27 ± 2.01 vs. 0.62 ± 0.28, p < 0.001, respectively). But the values of the baseline QFT-GIT0 tests were not significantly different between the persistent positivity and negative reversion groups (2.25 ± 0.43 vs. 2.86 ± 3.56, p = 0.924). As for the QFT-GIT, there were no significant differences between T2–T1 of the QFT-Plus1 and the QFT-Plus2 persistent positivity and negative reversion results.
Discussions
To examine the positive consistency of the new QFT-Plus test, we screened 236 patients on dialysis. Among them, 73 were enrolled and 53 completed the second round of follow-up paired QFT-GIT and QFT-Plus tests in the present study. The correlation between the two tests when they were administered concurrently was excellent (Pearson’s correlation > 0.9). The negative reversion rate of the QFT-GIT was as high as 41.2% at the 6-month follow-up, higher than that of the QFT-Plus (12.9%, p = 0.040). Higher first-round IFN-γ levels of both the QFT-GIT and the QFT-Plus were significantly correlated with persistent positivity at the follow-up.
A diagnosis of LTBI is defined as a positive result from an immune assay for M.tb after active TB exclusion and based on the life-long presence of M.tb infection without adequate anti-TB treatment21,22. Negative reversion of the immune assay for M.tb antigens may indicate regression of the immune response, especially in immunocompromised patients with underlying comorbidities13,15. In our previous study, QFT-GIT negative reversion was found to be as high as 45.9% in patients on dialysis13, which is similar to that in the present study (41.2% by QFT-GIT). This inconsistent finding is concerning for LTBI screening among high-risk populations who have no clear history of TB exposure that could serve as a timepoint for commencement of LTBI survey. Furthermore, random LTBI testing to screen the dialysis population with QFT-GIT may produce false negative results if the immune response has fallen below the cut-off value of the immune test. Hence, optimal LTBI screening tests with high consistency would provide more reliable information for LTBI management in high-risk groups.
The positive consistency of the QFT-Plus at follow-up was higher (81.8%) than that of the QFT-GIT (58.8%), which might be explained by the greater sensitivity of the QFT-Plus in detecting LTBI consistently. For example, in the first round of the QFT-GIT1(−) tests, the QFT-Plus1 detected specific cases with a high response (T1: 0.98 IU/ml [Table S3]) beyond the detection grey zone (< 0.8 IU/ml) proposed by Metcalfe et al23. In addition, the difference in QFT-GIT0 between the QFT-Plus1(+) and (−) results was larger than that between the QFT-GIT0(+) and (−) results, indicating that the QFT-Plus might have a better discriminating ability. In addition, CD8 antigens were added to the QFT-Plus to augment CD8 effector or memory responses in individuals with defective or low CD4 responses. However, the CD8 response was not significantly different between the persistent positivity and negative reversion groups. By contrast, the CD4 (TB1 tube) or CD4 plus CD8 (TB2 tube) responses were both well correlated with persistent positivity (Table 4). Therefore, the present study indicates that remote or chronic M.tb infection might be more highly correlated with the CD4 response than with the CD8 response24. In this cohort of patients on dialysis, the CD4 antigens of the QFT-Plus, including ESAT 6 and CFP10 peptides but no TB7.7, as compared with the QFT-GIT, might be the dominant and consistent inducers of the memory CD4 T cell response. Our study demonstrates a more reliable response of M.tb-specific CD4 T cell response over time for the QFT-Plus than for the QFT-GIT. Even though both tests measure the CD4 response, it was reported that the QFT-Plus might be more useful in detecting LTBI in elderly18 and immunocompromised patients25. However, determining the exact mechanism of why the QFT-Plus can maintain the positive consistency well will require further study and large-scale validation.
It should be noted that the response decreased from the first to the second round, regardless of which QFT assay was used, indicating a decaying immune response to M.tb antigens. This finding is consistent with previous reports that high initial values might predict positive consistency13, but so far, the results have been too inconclusive to titrate a cut-off value. Because follow-up is time-consuming, our previous report also showed that additional inflammatory markers at the baseline could be used to discriminate persistent positivity from negative reversion, at a possible cost of more than one test26. The present study indicates that an initial positive QFT-Plus result has a likelihood of being consistent at 6-month follow-up of higher than an 80%, so this test could be considered as a one-time test.
Regarding the results of the QFT-GIT0, QFT-GIT1 and QFT-Plus1, respectively, the positive consistency rates were both around two-thirds, with no inter-assay difference (67% vs. 64%). Because of a lack of baseline QFT-Plus data, the QFT-GIT0 might be specific only for QFT-GIT1 follow-up. This might indicate that initial screening is a critical point in the positive judgement and has the same importance as its detection consistency. Another issue is that we recruited several patients with old TB cases, who might not have been candidates for LTBI screening. We only aimed to test the positive consistency by IGRA assay. When the results were re-analyzed for the patients without a history of old TB, the positive consistency of the QFT-Plus was still higher than that of the QFT-GIT.
This study had several limitations. First, the sample size in the present study was not large. In addition, the study design was not a randomized comparison. However, the two assays were compared using the same blood samples so as to avoid inter-subject variation. Third, the subjects were recruited from a set of patients with positive IGRA tests, so the results could not be used to interpret positive conversion from negative results. Fourth, we did not enroll patients with tuberculosis or household contacts. Further application of the CD8 response in the QFT-Plus might have a potential for disease status discrimination27. No participants had a history of recent TB contact, which could have affected the performance of the QFT-Plus28, especially the CD8 response29,30.
In conclusion, the present study demonstrates that the QFT-Plus had a higher positive result consistency (81.8%) at a 6-month follow-up as compared with the QFT-GIT. The inter-assay correlation was remarkably high. Another finding was that the CD4 response of the QFT-Plus might be important for positive consistency of LTBI. Although larger studies are needed to further validate the findings, we conclude that the new QFT-Plus has more reliable positive results than those of the QFT-GIT and is therefore more suitable for TB screening of dialysis patients.
Methods
Participants
Subjects were recruited from two tertiary-care medical centers in northern Taiwan in 2016–2019 under IRB approval (Nos. 201603066DIPB and 108067-F). Written informed consent was obtained. Patients on maintenance dialysis underwent LTBI screening according to the routine health policy of one hospital and by invitation in a research survey at the other. The LTBI screening included chest plain film and the QFT-GIT. If radiography indicated possible TB, three sets of sputum cultures for M.tb were obtained. Patients on dialysis with positive QFT-GIT results but no evidence of active TB were included. Patients with diagnoses of human immunodeficiency virus infection or active TB were excluded. Identifiers of participants were removed in accordance with the Health Insurance Portability and Accountability Act (HIPAA) Privacy Rule. We confirmed that all experiments were performed in accordance with relevant guidelines and regulations.
Study design
After a participant had been enrolled, the QFT-GIT and QFT-Plus (QIAGEN, Germany) were performed concurrently on the same blood sample according to the manufacturer’s instructions17,31. The interferon-gamma (IFN-γ) level of the post-reaction supernatant was then measured with an enzyme-linked immuno-sorbent assay. The IFN-γ response of the QFT kits was the IFN-γlevel in the TB antigen tube minus that in the negative-control tube in the QFT-GIT and the TB1 or TB2 tube minus the negative-control tube in the QFT-Plus. Results were interpreted as positive, negative, or indeterminate32,33. Around 6 months later, the QFT-GIT and QFT-Plus were followed up together on the same participants and on the same samples of whole blood. Regarding the primary end-point, we compared the proportions of persistent positive results (consistency) of the different QFT kits.
Data collection
We recorded participant’s demographics, including age, gender, and comorbidities, as well as dialysis type (hemodialysis or peritoneal dialysis). Standardized case report forms were used and completed with default options. The Bacillus Calmette Guérin (BCG) vaccination scar was checked. The IFN-γ levels of the QFT-GIT and QFT-Plus were recorded.
Statistical analyses
Pearson’s chi-squared test or Fisher’s exact test was used to compare categorical variables, and continuous variables were compared using the Student’s t test or the Mann–Whitney U test, where appropriate. Pearson’s correlation analysis was used to assess relationships between the first and second pairs of the QFT-GIT and QFT-Plus assays. All analyses were performed in SPSS software (version 19.0 for Windows, SPSS Inc.).
Ethics approval and consent to participate
The Research Ethics Committee of National Taiwan University Hospital (IRB No.: No. 201603066DIPB) and the Research Ethics Committee of Far-Eastern Memorial Hospital (No. 108067-F) approved this study.
Consent for publication
Written informed consent was obtained from the participants at the time of enrollment.
References
World Health Organization, Geneva, Switzerland, 2019.
Cohen, A., Mathiasen, V. D., Schon, T. & Wejse, C. The global prevalence of latent tuberculosis: a systematic review and meta-analysis. Eur. Respir. J. https://doi.org/10.1183/13993003.00655-2019 (2019).
Liu, J., Yan, J., Wan, Q., Ye, Q. & Huang, Y. The risk factors for tuberculosis in liver or kidney transplant recipients. BMC Infect. Dis. 14, 387. https://doi.org/10.1186/1471-2334-14-387 (2014).
Chou, K. J. et al. Tuberculosis in maintenance dialysis patients. Nephron 88, 138–143. https://doi.org/10.1159/000045974 (2001).
Getahun, H. et al. Management of latent Mycobacterium tuberculosis infection: WHO guidelines for low tuberculosis burden countries. Eur. Respir. J. 46, 1563–1576. https://doi.org/10.1183/13993003.01245-2015 (2015).
Lonnroth, K. et al. Towards tuberculosis elimination: an action framework for low-incidence countries. Eur. Respir. J. 45, 928–952. https://doi.org/10.1183/09031936.00214014 (2015).
Harries, A. In Toman’s tuberculosis Case detection, treatment and monitoring (ed. Frieden, T. R.) 46–50 (World Health Organization, Geneva, 2004).
Smirnoff, M., Patt, C., Seckler, B. & Adler, J. J. Tuberculin and anergy skin testing of patients receiving long-term hemodialysis. Chest 113, 25–27 (1998).
Venkata, R. K., Kumar, S., Krishna, R. P., Kumar, S. B. & Padmanabhan, S. Tuberculosis in chronic kidney disease. Clin. Nephrol. 67, 217–220 (2007).
Fang, H. C. et al. Tuberculosis in patients with end-stage renal disease. Int. J. Tuberc. Lung. Dis. 8, 92–97 (2004).
Sester, M. et al. Risk assessment of tuberculosis in immunocompromised patients. A TBNET study. Am. J. Respir. Crit. Care Med. 190, 1168–1176. https://doi.org/10.1164/rccm.201405-0967OC (2014).
Shu, C. C. et al. Predictors and prevalence of latent tuberculosis infection in patients receiving long-term hemodialysis and peritoneal dialysis. PLoS ONE 7, e42592. https://doi.org/10.1371/journal.pone.0042592 (2012).
Shu, C. C. et al. Dynamic changes in positive interferon-gamma release assay in a dialysis population: an observational cohort study. J. Infect. 67, 529–535. https://doi.org/10.1016/j.jinf.2013.07.029 (2013).
Shu, C. C. et al. Risk of tuberculosis among patients on dialysis: the predictive value of serial interferon-gamma release assay. Medicine (Baltimore) 95, e3813. https://doi.org/10.1097/md.0000000000003813 (2016).
Adetifa, I. M. et al. Interferon-gamma ELISPOT as a biomarker of treatment efficacy in latent tuberculosis infection: a clinical trial. Am. J. Respir. Crit. Care Med. 187, 439–445. https://doi.org/10.1164/rccm.201208-1352OC (2013).
Ringshausen, F. C. et al. Predictors of persistently positive Mycobacterium-tuberculosis-specific interferon-gamma responses in the serial testing of health care workers. BMC Infect. Dis. 10, 220. https://doi.org/10.1186/1471-2334-10-220 (2010).
Barcellini, L. et al. First evaluation of QuantiFERON-TB Gold Plus performance in contact screening. Eur. Respir. J. 48, 1411–1419. https://doi.org/10.1183/13993003.00510-2016 (2016).
Chien, J. Y. et al. QuantiFERON-TB Gold Plus is a more sensitive screening tool than QuantiFERON-TB gold in-tube for latent tuberculosis infection among older adults in long-term care facilities. J. Clin. Microbiol. https://doi.org/10.1128/JCM.00427-18 (2018).
Lee, M. R. et al. CD8 response measured by QuantiFERON-TB Gold Plus and tuberculosis disease status. J. Infect. 78, 299–304. https://doi.org/10.1016/j.jinf.2019.01.007 (2019).
Yi, L. et al. Evaluation of QuantiFERON-TB Gold Plus for detection of Mycobacterium tuberculosis infection in Japan. Sci. Rep. 6, 30617. https://doi.org/10.1038/srep30617 (2016).
Horsburgh, C. R. Jr. Priorities for the treatment of latent tuberculosis infection in the United States. N. Engl. J. Med. 350, 2060–2067. https://doi.org/10.1056/NEJMsa031667 (2004).
Vynnycky, E. & Fine, P. E. Lifetime risks, incubation period, and serial interval of tuberculosis. Am. J. Epidemiol. 152, 247–263. https://doi.org/10.1093/aje/152.3.247 (2000).
Metcalfe, J. Z. et al. Test variability of the QuantiFERON-TB gold in-tube assay in clinical practice. Am. J. Respir. Crit. Care Med. 187, 206–211. https://doi.org/10.1164/rccm.201203-0430OC (2013).
Prezzemolo, T. et al. Functional signatures of human CD4 and CD8 T cell responses to Mycobacterium tuberculosis. Front. Immunol. 5, 180. https://doi.org/10.3389/fimmu.2014.00180 (2014).
Kim, S. H., Jo, K. W. & Shim, T. S. QuantiFERON-TB Gold PLUS versus QuantiFERON- TB Gold In-Tube test for diagnosing tuberculosis infection. Korean J. Intern. Med. 35, 383–391. https://doi.org/10.3904/kjim.2019.002 (2020).
Shu, C. C. et al. Inflammatory markers and clinical characteristics for predicting persistent positivity of interferon gamma release assay in dialysis population. Sci. Rep. 6, 34577. https://doi.org/10.1038/srep34577 (2016).
Rozot, V. et al. Mycobacterium tuberculosis-specific CD8+T cells are functionally and phenotypically different between latent infection and active disease. Eur. J. Immunol. 43, 1568–1577. https://doi.org/10.1002/eji.201243262 (2013).
Day, C. L. et al. Functional capacity of Mycobacterium tuberculosis-specific T cell responses in humans is associated with mycobacterial load. J. Immunol. 187, 2222–2232. https://doi.org/10.4049/jimmunol.1101122 (2011).
Barcellini, L. et al. First evaluation of QuantiFERON-TB Gold Plus performance in contact screening. Eur. Respir. J. 47, 1587–1590 (2016).
Nikolova, M. et al. Antigen-specific CD4- and CD8-positive signatures in different phases of Mycobacterium tuberculosis infection. Diagn. Microbiol. Infect. Dis. 75, 277–281. https://doi.org/10.1016/j.diagmicrobio.2012.11.023 (2013).
Lalvani, A. et al. Rapid detection of Mycobacterium tuberculosis infection by enumeration of antigen-specific T cells. Am. J. Respir. Crit. Care Med. 163, 824–828. https://doi.org/10.1164/ajrccm.163.4.2009100 (2001).
Dyrhol-Riise, A. M. et al. Diagnosis and follow-up of treatment of latent tuberculosis; the utility of the QuantiFERON-TB Gold In-tube assay in outpatients from a tuberculosis low-endemic country. BMC Infect. Dis. 10, 57. https://doi.org/10.1186/1471-2334-10-57 (2010).
Banach, D. B. & Harris, T. G. Indeterminate QuantiFERON(R)-TB Gold results in a public health clinic setting. Int. J. Tuberc. Lung Dis. 15, 1623–1630. https://doi.org/10.5588/ijtld.11.0017 (2011).
Acknowledgements
The authors thank the staff of the Eighth Core Lab of the Department of Medical Research of National Taiwan University Hospital for their technical support. In addition, we thank Prof. Hsien-Ho Lin, who is affiliated with the College of Public Health, National Taiwan University, Taipei, Taiwan, for his help in study design.
Funding
This study was supported by the Ministry of Health and Welfare (Nos. MOHW107-TDU-B-211-123002 and 108-TDU-B-211-133002). The QIAGEN company provided tubes of QuantiFERON-G plus for 200 tests. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
Author information
Authors and Affiliations
Contributions
Dr. C.C.S. conceived and coordinated the study with Profs. J.Y.W. and C.J.Y. Drs. C.C.S., P.H.W., and S.Y.L. recruited patients and conducted the study along with Drs. S.S.J.L., C.Y.L., S.W.L. and Y.F.W. All the authors were involved in data interpretation and analysis. Drs. C.C.S. and P.H.W. were responsible for the manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, PH., Lin, SY., Lee, S.SJ. et al. CD4 response of QuantiFERON-TB Gold Plus for positive consistency of latent tuberculosis infection in patients on dialysis. Sci Rep 10, 21367 (2020). https://doi.org/10.1038/s41598-020-78374-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-78374-3
- Springer Nature Limited