Abstract
Comorbidities adversely affect the quality of life and survival of patients with chronic obstructive pulmonary disease (COPD), and timely identification and management of comorbidities are important in caring for COPD patients. This study aimed to investigate the impact of COPD on long-term developmental trajectories of its comorbidities. From 2010 to 2013, all spirometry-confirmed COPD patients with a 5-year follow-up period were identified as the cases. The prevalence of comorbidities and their trajectories in COPD cases were obtained and compared with those in non-COPD controls matched for age, sex, smoking status and Charlson comorbidity index (CCI). Over the study period, a total of 682 patients, 341 each in COPD and control groups were included, with a mean age of 69.1 years and 89% male. The baseline mean CCI was 1.9 for both groups of patients and significantly increased to 3.4 and 2.7 in COPD and control groups after 5 years, respectively (both P < 0.001). Through the 5-year follow-up, a significant increase in the prevalence of all comorbidities of interest was observed in the COPD cohort and the incidence was remarkably higher for hypertension [incidence rate ratio (IRR) 1.495; 95% confidence interval (CI) 1.017–2.198], malignancy (IRR 2.397; 95% CI 1.408–4.081), diabetes mellitus (IRR 2.927; 95% CI 1.612–5.318), heart failure (IRR 2.531; 95% CI 1.502–4.265) and peptic ulcer disease (IRR 2.073; 95% CI 1.176–3.654) as compared to the non-COPD matched controls. In conclusion, our findings suggest that the presence of COPD may be considered a pathogenic factor involved in the development of certain comorbidities.
Similar content being viewed by others
Introduction
Chronic obstructive pulmonary disease (COPD), featured by persistent airflow limitation and respiratory symptoms, is one of the most important global health threats and the third leading cause of death worldwide1. Compared to non-COPD individuals, patients with COPD are more likely to suffer from comorbidities, such as cardiovascular disease and metabolic syndrome2,3,4,5. The increased prevalence may be attributable to shared risk factors (e.g., smoking) or pathophysiological mechanisms (e.g., chronic inflammation)1,3. Comorbidities are of paramount importance since it is well known that some of them, such as heart failure, coronary artery disease and diabetes mellitus, have a significant impact on health status and health care utilization and increase the risks of hospitalization and mortality in COPD patients2,6,7,8,9.
It has been suggested that comorbidities should be routinely looked for and appropriately treated in any patient with COPD as per clinical practice guideline1, although the evidence to support this contention and practical recommendations are still lacking. Over the past 20 years, a barrage of publications have delineated a clearer picture of the association between COPD and its comorbidities2,3,4,6,7,8; however, little if any is known about the trajectories in the comorbidity burden along the clinical course of patients with COPD. In this regard, it would be valuable to better understand this issue because the messages can inform health decision-making and guideline development.
In this study, we therefore investigated the prevalence of comorbidities in spirometry-confirmed COPD patients and observed their evolution through the 5-year follow-up period. Meanwhile, a matched non-COPD cohort was assembled to determine the impact of COPD on the developmental trajectories of those comorbidities.
Methods
Study design and settings
This long-term retrospective cohort study was conducted at the National Taiwan University Hospital, a tertiary-care referral center in Northern Taiwan. In our institution, patients with COPD were in principle assessed and treated based on the contemporary Global Initiative for Chronic Obstructive Lung Disease (GOLD) guidelines10,11. The Research Ethics Committee of the National Taiwan University Hospital has approved the study protocol and waived the informed consent because of descriptive and retrospective nature of the study.
Study population
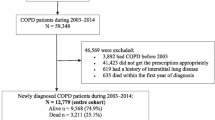
To assemble the COPD cohort with a 5-year follow-up period for evaluating the evolution of the comorbidities, all patients having a spirometry test from June 2010 to December 2013 were screened for eligibility in this study (Fig. 1). Inclusion criteria included: (a) persistent airflow limitation, defined as a proportion of the forced vital capacity exhaled in the first second (FEV1/FVC) < 0.7 after administration of a bronchodilator; (b) a clinical diagnosis of COPD plus a prescription of any COPD medications, including β2-agonists, anticholinergics and methylxanthines; and (c) having follow-up for COPD prescriptions for at least 5 consecutive years. The date of COPD diagnosis was defined as the index date. Patients were excluded if they had any of the following: (a) prior history of COPD before June 2010; (b) age < 40 years; (c) non-smokers; (d) loss of follow-up or death within 5 years of the index date.
To compare the change in the comorbidity burden between COPD and non-COPD patients, all subjects with a spirometry test showing post-bronchodilator FEV1/FVC ≥ 0.7 and a current or former smoking status were randomly selected to assemble the non-COPD cohort after matching for age, sex, Charlson comorbidity index (CCI) and year for spirometry. A minimal 5-year follow-up period after the date of spirometry was also a prerequisite for the controls.
Data collection
Data retrieved at baseline were age, sex, body mass index, smoking status, comorbidities needed to calculate the CCI and FEV1% of the predicted value. During the follow-up period, messages regarding the development of comorbidities of interest was pursued. Through literature review, we included hypertension, coronary artery disease, malignancy, diabetes mellitus, hyperlipidemia, heart failure, peptic ulcer disease, peripheral arterial disease, atrial fibrillation and liver cirrhosis as the comorbidities of interest since those comorbidities have been shown to be prognostically detrimental among the COPD patients1,8,12. Meanwhile, similar information was obtained from the control subjects at baseline and during follow-up. To obtain the aforementioned information, patient medical records were reviewed in detail. The presence and development of all the comorbidities of interest needed to be affirmed by both the clinician's diagnosis and objective criteria for the diseases13,14,15,16,17,18,19,20.
Endpoints
Outcomes of interest in this study were the baseline prevalence of medical record notes of the above-specified comorbidities and their incidence along the 5-year follow-up in the COPD patients. We also sought to realize the impact of COPD on comorbidity trajectories by comparing to non-COPD controls.
Statistical analysis
Categorical variables were presented as number (%) and continuous variables were displayed as mean ± standard deviation. Comparisons of variables between COPD patients and controls were performed using the chi-square or Student’s t-test, as appropriate. The P for trend was used to evaluate the trend of comorbidity prevalence along the follow-up time. Repeated measures analysis of variance was conducted to compare the trend of change in the prevalence of comorbidities between the COPD and control cohorts. The 5-year incidence rate ratios (IRRs) with their 95% confidence intervals (CIs) for each comorbidity of interest were also calculated. The multivariate logistic regression model was constructed to identify independent risk factors associated with the development of two or more comorbidities of interest over the 5-year follow-up and odds ratios (ORs) with 95% CIs were reported. Data analysis was performed using the SPSS software (Version 20.0; IBM Corp., Armonk, NY, US). A two-tailed P value of < 0.05 was deemed statistically significant.
Ethics declarations
The study protocol was approved by the Research Ethics Committee of the National Taiwan University Hospital and informed consent was waived because of the retrospective nature of the study.
Results
Baseline characteristics of patients
A total of 756 patients newly diagnosed with spirometry-confirmed COPD were identified between June 2010 and December 2013 (Fig. 1). Of those, 369 subjects had at least 5-year follow-up period from the index date of COPD diagnosis to evaluate the long-term evolution of their comorbidities. After the matching process, the study sample included 341 COPD patients and an equal number of matched non-COPD individuals. Table 1 shows the characteristics of the study cohort. The mean age of COPD patients was 69.4 years and 89% were males. The average FEV1% of the predicted value for patients with COPD was 0.70 ± 0.23 and the majority (N = 277, 81%) of those were categorized as GOLD stage 1 or 2. Compared to the COPD cohort, non-COPD controls were more like to be overweight (55% vs. 42%).
Burden of comorbidities
In terms of comorbidity burden, the baseline CCI was the same for both groups of patients; however, a significant difference in the prevalence of certain comorbidities of interest was observed (Table 2). At baseline, a higher proportion of non-COPD controls had comorbid hypertension (43% vs. 29%), malignancy (24% vs. 16%), diabetes mellitus (26% vs. 16%), hyperlipidemia (20% vs. 11%) and peptic ulcer disease (14% vs. 7%) than did COPD patients.
Comorbidity evolution
Figure 2 displays the developmental trajectories of the CCI and comorbidities of interest in COPD and matched control patients. Over the 5-year follow-up period, a significant increase in the CCI was observed in both COPD patients and controls, but the trend was more prominent in patients with COPD (between-group P = 0.001). Hypertension, coronary artery disease, malignancy, diabetes mellitus and hyperlipidemia were the leading comorbidities of interest for both groups of patients on enrollment. Through the subsequent 5 years, there was a significantly increasing trend in the prevalence of all those comorbidities among the COPD patients; however, the increasing trend was only remarkable for hypertension, coronary artery disease and hyperlipidemia in the control patients. With regards to less prevalent comorbidities, namely, heart failure and peptic ulcer disease, a dissimilarity in the trend of increasing prevalence was also found between two patient groups, with more prominent change in the COPD patients.
For the annual incidence, hypertension (3.5%), heart failure (2.7%), hyperlipidemia (2.6%), malignancy (2.6%) and diabetes mellitus (2.5%) were the top five comorbidities in the COPD cohort during the 5-year follow-up (Fig. 2). Table 3 shows the 5-year IRRs of comorbidities between COPD and non-COPD matched control patients. Significant differences between groups were seen in diabetes mellitus (IRR 2.927; 95% CI 1.612–5.318), heart failure (IRR 2.531; 95% CI 1.502–4.265), malignancy (IRR 2.397; 95% CI 1.408–4.081), peptic ulcer disease (IRR 2.073; 95% CI 1.176–3.654) and hypertension (IRR 1.495; 95% CI 1.017–2.198).
Risk factors with comorbidity development
Through the 5-year follow-up, approximately one-fourths (N = 157, 23%) of the total patient cohort developed two or more comorbidities of interest. Using multivariate logistic regression analysis (Table 4), taking into account patients’ baseline characteristics, i.e., a diagnosis of COPD, age, sex, body mass index and smoking status, we identified the presence of COPD (OR 1.527; 95% CI 1.054–2.212), age ≥ 65 years (OR 1.651; 95% CI 1.101–2.475) and current smoker (OR 1.675; 95% CI 1.150–2.439) as the independent risk factors associated with the development of two or more comorbidities.
Discussion
To the best of our knowledge, this is the first study to elaborate the developmental trajectories of the comorbidity burden through a long 5-year follow-up in patients with COPD. We found a significant increasing trend in the prevalence of all those comorbidities of interest in COPD patients. Compared to non-COPD matched controls, a higher incidence of hypertension, malignancy, diabetes mellitus, heart failure and peptic ulcer disease was observed among patients with COPD. In addition, the presence of COPD was associated with an increased odds of developing two or more comorbidities during the 5-year follow-up period. Taken together, our findings provide a broader view of the association between COPD and comorbidities and suggest that COPD per se exaggerates the risk of developing comorbidities. The information is practically useful in the evaluation and management of comorbidities in the COPD patients.
The novel information provided herein is to delineate the time course of pertinent comorbidities following a diagnosis of COPD. Since those comorbidities are prognostically associated with COPD, the messages, from a broad viewpoint, may inform guideline developers and health policy makers to propose an ideal strategy for comorbidity screening among patients with COPD. Moreover, from the individual viewpoint, the health care providers may be able to deliver better care of patients via improved understanding of comorbidity trajectories in COPD and timely identification and management of these comorbidities. Despite that it remains unsolved whether routine screening for comorbid conditions in COPD contributes to a better clinical outcome5,21, our study detailing the year-to-year change in the prevalence of comorbidities would certainly broaden our horizons for dealing with COPD from a longitudinal and whole-patient perspective.
As COPD becomes more and more understood to be a systemic inflammatory disease22, the view of COPD itself as an important etiologic factor for comorbidities has been an area of intense investigations. Several pieces of evidence demonstrate that the presence of COPD is associated with an increased risk of developing a number of comorbidities3,4,5. However, from another perspective, the concept of multimorbidity, i.e., the co-occurrence of multiple chronic diseases and medical conditions within one person, in the management of COPD has been insightfully studied in several works, indicating that COPD may just be one of a cluster of multiple conditions resulting from common risk factors8,23,24. Compared to other studies25,26, the present work is unique in terms of the matching strategy. Besides age, sex and smoking status, the non-COPD controls were matched to the COPD subjects for their comorbidities using the CCI. In this case, the COPD and non-COPD patients had similar baseline comorbidity burden and it would be more reasonable and appropriate to compare the change in comorbidities between the two groups of patients. Along this line, the prevalence of most comorbidities of interest at baseline was lower in our COPD cohort than in the controls since COPD was also taken into account while calculating the CCI. Nonetheless, the trend of change in the CCI and in the prevalence of certain comorbidities significantly varied between COPD and non-COPD control patients, highlighting and supporting the pathogenic role of COPD in the development of comorbidities, although the mechanistic links between COPD and its comorbidities remain to be elucidated.
The baseline prevalence of comorbidities in our COPD patients was in general comparable to that reported in previous studies3,4,5,8; however, our findings added to the existing knowledge by showing their evolution for the subsequent years. It is not surprising that in this aged population comprised of smokers, either current or former, a growing burden of comorbidities was evidently observed. Of those, the incidence of hypertension, malignancy, diabetes mellitus and peptic ulcer disease were significantly higher in patients with COPD than in non-COPD matched controls, which were consistent with prior literature26,27,28,29. However, the increased risk of heart failure, as seen in our COPD cohort, was not proven in other population studies30,31. On the contrary, it has been shown that COPD is associated with a higher incidence of coronary artery disease and atrial fibrillation32,33,34, but the present study did not disclose such a relationship. The discrepancies may be attributable to one or more factors, such as the study design, case definition and ascertainment, control selection and outcome of interest. No matter what, in view of the interaction of complex networks between COPD and its comorbidities, our study results reinforce the need for a multidisciplinary approach for taking care of COPD patients.
This study has limitations that may limit its generalizability to other COPD populations. First, patients with COPD who lost to follow-up or died during the follow-up period were excluded, so our prevalence and annual incidence of comorbidities are likely to over- or under-estimate the true figures for the entire population with COPD. Second, the proportion of COPD cases who were male (89%) was higher than that seen in most COPD studies12,35,36, which may represent a selection bias. However, the aforementioned limitations would not have influenced the differences that we observed between COPD and matched control patients, and therefore may not affect our main findings and conclusions. Third, chronic bronchitis, another smoking-related respiratory disease, may cause systemic inflammation, and is associated with worse clinical outcomes of COPD patients and with an increased risk of other comorbidities37. Since various definitions of chronic bronchitis have been used in the literature and clinical practice37, we are unable to ascertain its prevalence in both groups of our population. Thus, the impact of chronic bronchitis on our study findings is uncertain and this limitation should be born in mind. Fourth, changing in smoking status and tobacco consumption may also affect the symptoms and lung function in COPD patients and the development of smoking-related comorbidities in smokers38,39. However, given the retrospective design of the study, we could not accurately retrieve the information about the amounts of tobacco consumption and smoking cessation efforts from the medical records of our study population. Thus, these confounders should be considered when interpreting the study results. Last, without systematic diagnostic tests, certain comorbidities, such as coronary artery disease and malignancy, may go undiagnosed at baseline. Nonetheless, this issue should be present in both groups of patients and does not significantly affect our study findings.
In summary, this study provides a more comprehensive view of COPD by showing 5-year trajectories of its pertinent comorbidities. The findings suggest that an increasing comorbidity burden is an unavoidable issue in patients with COPD, and a systematic and holistic approach is often needed to take care of this patient population. In addition, the presence of COPD may be considered a pathogenic factor involved in the development of its comorbidities. Further studies are required to explore the potential benefits and roles of proactive screening of comorbidities in the COPD population.
Data availability
The datasets used and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Singh, D. et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive lung disease: the GOLD science committee report 2019. Eur. Respir. J. 53, 1. https://doi.org/10.1183/13993003.00164-2019 (2019).
Mapel, D. W. et al. Health care utilization in chronic obstructive pulmonary disease: a case-control study in a health maintenance organization. Arch. Intern. Med. 160, 2653–2658. doi:https://doi.org/10.1001/archinte.160.17.2653 (2000).
Hillas, G., Perlikos, F., Tsiligianni, I. & Tzanakis, N. Managing comorbidities in COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 10, 95–109. https://doi.org/10.2147/COPD.S54473 (2015).
Negewo, N. A., Gibson, P. G. & McDonald, V. M. COPD and its comorbidities: impact, measurement and mechanisms. Respirology 20, 1160–1171. https://doi.org/10.1111/resp.12642 (2015).
Putcha, N., Drummond, M. B., Wise, R. A. & Hansel, N. N. Comorbidities and chronic obstructive pulmonary disease: prevalence, influence on outcomes, and management. Semin. Respir. Crit. Care Med. 36, 575–591. https://doi.org/10.1055/s-0035-1556063 (2015).
Sin, D. D., Anthonisen, N. R., Soriano, J. B. & Agusti, A. G. Mortality in COPD: role of comorbidities. Eur. Respir. J. 28, 1245–1257. https://doi.org/10.1183/09031936.00133805 (2006).
Huber, M. B., Wacker, M. E., Vogelmeier, C. F. & Leidl, R. Comorbid influences on generic health-related quality of life in COPD: a systematic review. PLoS ONE 10, e0132670. https://doi.org/10.1371/journal.pone.0132670 (2015).
Divo, M. et al. Comorbidities and risk of mortality in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 186, 155–161. https://doi.org/10.1164/rccm.201201-0034OC (2012).
Ho, T. W. et al. Metformin use mitigates the adverse prognostic effect of diabetes mellitus in chronic obstructive pulmonary disease. Respir. Res. 20, 69. https://doi.org/10.1186/s12931-019-1035-9 (2019).
Rabe, K. F. et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 176, 532–555. https://doi.org/10.1164/rccm.200703-456SO (2007).
Vestbo, J. et al. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease: GOLD executive summary. Am. J. Respir. Crit. Care Med. 187, 347–365. https://doi.org/10.1164/rccm.201204-0596PP (2013).
Frei, A. et al. Five comorbidities reflected the health status in patients with chronic obstructive pulmonary disease: the newly developed COMCOLD index. J. Clin. Epidemiol. 67, 904–911. https://doi.org/10.1016/j.jclinepi.2014.03.005 (2014).
Whelton, P. K. et al. 2017 ACC/AHA/AAPA/ABC/ACPM/AGS/APhA/ASH/ASPC/NMA/PCNA Guideline for the Prevention, Detection, Evaluation, and Management of high blood pressure in adults: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 71, e127–e248. https://doi.org/10.1016/j.jacc.2017.11.006 (2018).
Grundy, S. M. et al. 2018 AHA/ACC/AACVPR/AAPA/ABC/ACPM/ADA/AGS/APhA/ASPC/NLA/PCNA guideline on the management of blood cholesterol: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 73, e285–e350. https://doi.org/10.1016/j.jacc.2018.11.003 (2019).
Ponikowski, P. et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 37, 2129–2200. https://doi.org/10.1093/eurheartj/ehw128 (2016).
Introduction: Standards of Medical Care in Diabetes-2018. Diabetes Care 41, S1-S2. doi:https://doi.org/10.2337/dc18-Sint01 (2018).
Levine, G. N. et al. 2015 ACC/AHA/SCAI focused update on primary percutaneous coronary intervention for patients with ST-elevation myocardial infarction: an update of the 2011 ACCF/AHA/SCAI guideline for percutaneous coronary Intervention and the 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction. J. Am. Coll. Cardiol. 67, 1235–1250. https://doi.org/10.1016/j.jacc.2015.10.005 (2016).
Rooke, T. W. et al. Management of patients with peripheral artery disease (compilation of 2005 and 2011 ACCF/AHA Guideline Recommendations): a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. J. Am. Coll. Cardiol. 61, 1555–1570. https://doi.org/10.1016/j.jacc.2013.01.004 (2013).
Task Force, M. et al. ESC guidelines on the management of stable coronary artery disease: the Task Force on the management of stable coronary artery disease of the European Society of Cardiology. Eur. Heart J. 34(2949–3003), 2013. https://doi.org/10.1093/eurheartj/eht296 (2013).
Fukui, H. et al. Evidence-based clinical practice guidelines for liver cirrhosis 2015. J. Gastroenterol. 51, 629–650. https://doi.org/10.1007/s00535-016-1216-y (2016).
Smith, M. C. & Wrobel, J. P. Epidemiology and clinical impact of major comorbidities in patients with COPD. Int. J. Chron. Obstruct. Pulmon. Dis. 9, 871–888. https://doi.org/10.2147/COPD.S49621 (2014).
Fabbri, L. M. & Rabe, K. F. From COPD to chronic systemic inflammatory syndrome?. Lancet 370, 797–799. https://doi.org/10.1016/S0140-6736(07)61383-X (2007).
Vanfleteren, L. E. et al. Clusters of comorbidities based on validated objective measurements and systemic inflammation in patients with chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 187, 728–735. https://doi.org/10.1164/rccm.201209-1665OC (2013).
Van Remoortel, H. et al. Risk factors and comorbidities in the preclinical stages of chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 189, 30–38. https://doi.org/10.1164/rccm.201307-1240OC (2014).
Soriano, J. B., Visick, G. T., Muellerova, H., Payvandi, N. & Hansell, A. L. Patterns of comorbidities in newly diagnosed COPD and asthma in primary care. Chest 128, 2099–2107. https://doi.org/10.1378/chest.128.4.2099 (2005).
Stallberg, B. et al. Real-world retrospective cohort study ARCTIC shows burden of comorbidities in Swedish COPD versus non-COPD patients. NPJ Prim. Care Respir. Med. 28, 33. https://doi.org/10.1038/s41533-018-0101-y (2018).
Lee, C. T., Mao, I. C., Lin, C. H., Lin, S. H. & Hsieh, M. C. Chronic obstructive pulmonary disease: a risk factor for type 2 diabetes: a nationwide population-based study. Eur. J. Clin. Invest. 43, 1113–1119. https://doi.org/10.1111/eci.12147 (2013).
Agusti, A. et al. Characterisation of COPD heterogeneity in the ECLIPSE cohort. Respir. Res. 11, 122. https://doi.org/10.1186/1465-9921-11-122 (2010).
Ho, C. H., Chen, Y. C., Wang, J. J. & Liao, K. M. Incidence and relative risk for developing cancer among patients with COPD: a nationwide cohort study in Taiwan. BMJ Open 7, e013195. https://doi.org/10.1136/bmjopen-2016-013195 (2017).
Raymond, I. et al. Prevalence of impaired left ventricular systolic function and heart failure in a middle aged and elderly urban population segment of Copenhagen. Heart 89, 1422–1429. https://doi.org/10.1136/heart.89.12.1422 (2003).
Gottdiener, J. S. et al. Predictors of congestive heart failure in the elderly: the Cardiovascular Health Study. J. Am. Coll. Cardiol. 35, 1628–1637. https://doi.org/10.1016/s0735-1097(00)00582-9 (2000).
Konecny, T. et al. Relation of chronic obstructive pulmonary disease to atrial and ventricular arrhythmias. Am. J. Cardiol. 114, 272–277. https://doi.org/10.1016/j.amjcard.2014.04.030 (2014).
Curkendall, S. M. et al. Cardiovascular disease in patients with chronic obstructive pulmonary disease, Saskatchewan Canada cardiovascular disease in COPD patients. Ann. Epidemiol. 16, 63–70. https://doi.org/10.1016/j.annepidem.2005.04.008 (2006).
Finkelstein, J., Cha, E. & Scharf, S. M. Chronic obstructive pulmonary disease as an independent risk factor for cardiovascular morbidity. Int. J. Chron. Obstruct. Pulmon. Dis. 4, 337–349. https://doi.org/10.2147/copd.s6400 (2009).
Chen, W., FitzGerald, J. M., Sin, D. D. & Sadatsafavi, M. Excess economic burden of comorbidities in COPD: a 15-year population-based study. Eur. Respir. J. 50, 1. https://doi.org/10.1183/13993003.00393-2017 (2017).
Cheng, S. L. et al. COPD in Taiwan: a national epidemiology survey. Int. J. Chron. Obstruct. Pulmon. Dis. 10, 2459–2467. https://doi.org/10.2147/COPD.S89672 (2015).
Kim, V. & Criner, G. J. Chronic bronchitis and chronic obstructive pulmonary disease. Am. J. Respir. Crit. Care Med. 187, 228–237. https://doi.org/10.1164/rccm.201210-1843CI (2013).
Rosenberg, S. R. & Kalhan, R. Chronic bronchitis in chronic obstructive pulmonary disease: magnifying why smoking cessation still matters most. Ann. Am. Thorac. Soc. 13, 999–1000. https://doi.org/10.1513/AnnalsATS.201605-360ED (2016).
Janzon, E., Hedblad, B., Berglund, G. & Engstrom, G. Changes in blood pressure and body weight following smoking cessation in women. J. Intern. Med. 255, 266–272. https://doi.org/10.1046/j.1365-2796.2003.01293.x (2004).
Acknowledgements
We thank the staff of the Eighth Core Lab, Department of Medical Research, National Taiwan University Hospital for technical support during the study and we also thank the staff of the Department of Medical Research, National Taiwan University Hospital for the Integrated Medical Database (NTUH-iMD).
Funding
This study was supported in part by a Grant from the Ministry of Science and Technology, Taiwan (MOST 108-2634-F-002-015).
Author information
Authors and Affiliations
Contributions
C.T.H. made substantial contributions to conception and design, acquisition of data, analysis and interpretation of data, and was involved in drafting the manuscript. T.W.H., Y.J.T., and A.S.Y.L. contributed substantially to the study design, data analysis and interpretation, and the drafting of the manuscript. F.L. contributed substantially to the study design and data interpretation, and was involved in revising it critically for important intellectual content. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ho, TW., Tsai, YJ., Huang, CT. et al. Impact of tobacco-related chronic obstructive pulmonary disease on developmental trajectories of comorbidities in the Taiwan population. Sci Rep 10, 21025 (2020). https://doi.org/10.1038/s41598-020-78325-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-78325-y
- Springer Nature Limited