Abstract
The ‘Out of India’ hypothesis is often invoked to explain patterns of distribution among Southeast Asian taxa. According to this hypothesis, Southeast Asian taxa originated in Gondwana, diverged from their Gondwanan relatives when the Indian subcontinent rifted from Gondwana in the Late Jurassic, and colonized Southeast Asia when it collided with Eurasia in the early Cenozoic. A growing body of evidence suggests these events were far more complex than previously understood, however. The first quantitative reconstruction of the biogeography of Asian forest scorpions (Scorpionidae Latreille, 1802: Heterometrinae Simon, 1879) is presented here. Divergence time estimation, ancestral range estimation, and diversification analyses are used to determine the origins, dispersal and diversification patterns of these scorpions, providing a timeline for their biogeographical history that can be summarized into four major events. (1) Heterometrinae diverged from other Scorpionidae on the African continent after the Indian subcontinent became separated in the Cretaceous. (2) Environmental stresses during the Cretaceous–Tertiary (KT) mass extinction caused range contraction, restricting one clade of Heterometrinae to refugia in southern India (the Western Ghats) and Sri Lanka (the Central Highlands). (3) Heterometrinae dispersed to Southeast Asia three times during India’s collision with Eurasia, the first dispersal event occurring as the Indian subcontinent brushed up against the western side of Sumatra, and the other two events occurring as India moved closer to Eurasia. (4) Indian Heterometrinae, confined to southern India and Sri Lanka during the KT mass extinction, recolonized the Deccan Plateau and northern India, diversifying into new, more arid habitats after environmental conditions stabilized. These hypotheses, which are congruent with the geological literature and biogeographical analyses of other taxa from South and Southeast Asia, contribute to an improved understanding of the dispersal and diversification patterns of taxa in this biodiverse and geologically complex region.
Similar content being viewed by others
Introduction
The ‘Out of India’ hypothesis is often invoked to explain patterns of distribution among Southeast Asian taxa1,2,3,4,5,6,7,8,9,10. According to this hypothesis, the ancestors of Southeast Asian taxa originated in Gondwana, diverged from their Gondwanan relatives when the Indian subcontinent (including present-day India, Bangladesh, Nepal, Pakistan and Sri Lanka) rifted from Gondwana, rafted on the subcontinent, and arrived at Southeast Asia during or after it collided with Eurasia in the early Cenozoic. Support for the ‘Out of India’ hypothesis crosses taxonomic boundaries. Research on plants2,11,12, fish13,14,15, amphibians1,3,4,16,17,18,19,20, birds21, lizards10,22, and arthropods5,8,9,23 suggest an Indian origin for many Southeast Asian taxa. The Indian-Eurasian collision also resulted in colonization of the Indian subcontinent by Eurasian taxa, the so-called ‘Into India’ hypothesis, although there are fewer supporting examples24,25.
The path of the Indian subcontinent on its northward drift, and the timing of its collision with Eurasia have been extensively debated26,27,28,29,30,31,32,33,34,35,36,37,38,39,40,41,42. Many geologists and biogeographers favor an early collision date, occurring 55–50 Ma at the Paleocene–Eocene boundary30,32,33,40,41,42, based largely on geological data including seafloor spreading, tectonic plate movement rates, biostratigraphy, and paleontological data41,42. This ‘traditional’ hypothesis fails to explain why effects of the Indian-Eurasian collision do not appear until 30 Ma, 20 million years after the collision commenced26.
More recent hypotheses account for this discrepancy by suggesting that India experienced several periods of contact with Eurasia, the initial contact occurring as early as 55 Ma, and the final India-Eurasian collision occurring between 35–20 Ma26,27,38. For example, Li et al.19 proposed that rhacophorid frogs first colonized Southeast Asia from India during the Eocene (57–46 Ma). Faunal exchange between India and Southeast Asia ceased during the Middle Eocene but resumed between the Oligocene and Middle Miocene (ca. 34–12 Ma). Although several studies suggest faunal exchange between India and Southeast Asia may have occurred more than once, opinions differ as to how the initial contact occurred. According to Aitchison et al.26 and Ali and Aitchison27, Greater India brushed up against Sumatra and collided with an intra-oceanic island arc, the Dazhuqu Arc, ca. 55 Ma in the Neo-Tethys Ocean. India then moved closer to Eurasia until its final collision, roughly 34 Ma26,27. Two alternative hypotheses were proposed for the location of India between 55–35 Ma, according to which India was situated either very close to Southeast Asia28 or slightly further away29. The hypothesis of Ali and Aitchison27 was supported by a biogeographical analysis of freshwater crabs, which suggested that dispersal from India to Southeast Asia occurred during the Middle Eocene as India brushed up against Southeast Asia before the final collision9. In contrast, Van Hinsbergen et al.38 proposed that initial contact between India and Eurasia occurred when a microcontinent, the Tibetan Himalayan landmass, rifted from northern India and collided with Eurasia approximately 50 Ma. The rest of the Indian subcontinent was isolated following this initial rift until colliding much later (25–20 Ma). Aitchison and Ali35 refuted the hypothesis of Van Hinsbergen et al.38, suggesting the geological data were misinterpreted.
The hypothesis of Gondwanan taxa rafting on the Indian subcontinent is more complex than the number of colonizations of Southeast Asia, however. Approximately 65 Ma, the Deccan Traps, situated in the center of the subcontinent, underwent extreme volcanic activity, coinciding with the meteorite collision in the Chicxulub Crater, Mexico, and lasting less than a million years43,44,45,46,47,48,49,50,51,52,53,54,55. These volcanic eruptions, combined with effects of the Chicxulub impact, resulted in drastic environmental changes across the globe and are thought to have caused the Cretaceous–Tertiary (KT) mass extinction43,45,46,48,51,52,53,54,55. The Western Ghats of India and the Central Highlands of Sri Lanka have been posited as potential refugia during this period of Deccan volcanism56,57,58. Bossuyt et al.17 suggested frog lineages survived in southern India and Sri Lanka during the intense volcanism and subsequently dispersed across Eurasia after India’s collision. Numerous studies identified close relationships between taxa inhabiting the Western Ghats, southern India, and Sri Lanka58,59,60,61,62,63. Conti et al.2 proposed that the angiosperm family Crypteroniaceae de Candolle, 1868 reached Southeast Asia from the Indian subcontinent and explained its absence from India as the result of extinction caused by climatic changes (e.g., associated with volcanism) at the end of the Cretaceous and in the early Tertiary but survived in refugia, including Sri Lanka.
The scorpion family Scorpionidae Latreille, 1802 is distributed across most of Africa (except the Sahara), the Middle East, the Indian subcontinent, and Southeast Asia5,23,64. Asian members of the family, i.e., Heterometrinae Simon, 1879, also known as Asian forest scorpions (Fig. 1), are distributed from Pakistan in the northwest, across India and Sri Lanka to Southeast Asia, reaching Wallace’s Line, between Borneo and Sulawesi, in the east5. The subfamily previously included only a single genus, Heterometrus Ehrenberg, 1828, with five subgenera recognized by some authors23,65,66, but a recent revision identified 41 species and seven reciprocally monophyletic and morphologically distinct clades67, redefined as genera: Chersonesometrus Couzijn, 1978; Deccanometrus Prendini & Loria 67; Gigantometrus Couzijn, 1978; Heterometrus; Javanimetrus Couzijn, 1981; Sahyadrimetrus Prendini & Loria67; Srilankametrus Couzijn, 1981. These fossorial scorpions construct burrows in primary and secondary rainforests, dry deciduous forests, savanna and scrubland68,69.
Representative species of Asian forest scorpions (Scorpionidae: Heterometrinae). (A) Srilankametrus serratus (Pocock, 1900), ♂, Sinharaja Forest Reserve, Sabaragamuwa Province, Sri Lanka. (B) Chersonesometrus madraspatensis (Pocock, 1900), ♀, Gautala Wildlife Sanctuary, Maharashtra, India. (C) Deccanometrus phipsoni (Pocock, 1893), ♂, Achanakmar Tiger Reserve, Chhattisgarh, India. (D) Chersonesometrus tristis (Henderson, 1919), ♂, Kaigal Falls, Andhra Pradesh, India. (E) Javanimetrus cyaneus (C.L. Koch, 1836), ♂, Gunung Kidul, Special Region of Yogyakarta, Indonesia. (F) Heterometrus petersii (Thorell, 1876), ♂, Central Water Catchment, Singapore.
The biogeography of Heterometrinae has received some attention5,23,64,70,71,72,73. According to Couzijn23, the ancestor of Heterometrinae and Pandininae Thorell, 1876 originated in eastern Gondwana during the Triassic (Fig. 2). During the breakup of Gondwana, different lineages of Heterometrinae diverged in isolation on separate fragments of the former supercontinent. Chersonesometrus and Gigantometrus originated on the Indian plate and were carried north as it moved towards the rest of Asia while the ancestor of Javanimetrus was carried north on the Indochinese shelf complex74, which ultimately collided with Southeast Asia (Fig. 2A). After these landmasses arrived at their present locations, by the end of the Cretaceous, an exchange of taxa occurred via the Assam gateway (Fig. 2B). The ancestor of Heterometrus, endemic to Southeast Asia, originated in India, where it shares a common ancestor with the Indian Chersonesometrus, and dispersed east whereas the ancestor of Srilankametrus, endemic to southern India and Sri Lanka, originated in Southeast Asia, where it shares a common ancestor with the Southeast Asian Javanimetrus, and dispersed west73. During the Tertiary, an island chain known as the Luzon Track, which includes Taiwan, the Philippines and Borneo, formed in the Sulu Basin, allowing Heterometrus to disperse to these islands from northern Indochina while other Heterometrus species dispersed south to the Malay Peninsula, Sumatra, Borneo, and Java (Fig. 2C). Srilankametrus meanwhile dispersed from India to Sri Lanka while Javanimetrus dispersed from Sumatra northwest to the Nicobar Islands and southeast to Java (Fig. 2C). During the Pleistocene, the colder and drier climate caused by glaciation in the Himalayas forced some Indian Heterometrinae to disperse to southern India and Sri Lanka, while sea level changes allowed species of Heterometrus to expand their ranges in Southeast Asia (Fig. 2D).
Couzijn’s23 hypothesis for the evolution and diversification of the Asian forest scorpions (Scorpionidae: Heterometrinae). (A) Gondwana origins and divergence: Heterometrinae diverge from Pandinus (Pandininae) (P) on Gondwana; the Indian subcontinent rifts from Gondwana, carrying Gigantometrus (G) and the ancestor of (Chersonesometrus + Heterometrus); the Indochinese landblock rifts from Gondwana, carrying the ancestor of (Javanimetrus + Srilankametrus). (B) Dispersal across the Assam gateway: Heterometrus (H) disperses east to Southeast Asia; Srilankametrus (S) disperses west to India; Javanimetrus (J) disperses south and southeast to Borneo, the Philippines and the Malay Peninsula; Chersonesometrus (C) disperses north across the Indian subcontinent. (C) Tertiary dispersal: Srilankametrus disperses south to Sri Lanka; Javanimetrus disperses northwest to Sumatra and the Nicobar Islands, and east to Java and Borneo; Heterometrus disperses south to Indochina and the Malay Peninsula, and north to the Philippines. (D) Pleistocene dispersal: Heterometrus disperses more widely across its range; the Indian taxa disperse south due to glaciation in the Himalayas.
Couzijn’s23 biogeographical hypothesis has several implications. The basal phylogenetic position of Gigantometrus, which inhabits southern India and Sri Lanka, suggests Asian scorpionids originated in the southern part of the Indian subcontinent. Couzijn’s23 ‘Out of India’ hypothesis for Heterometrus and ‘Into India’ hypothesis for Srilankametrus imply two dispersal events and two extinction events, as Heterometrus are absent from the Indian subcontinent and Srilankametrus are absent from Southeast Asia. An ‘Out of India’ hypothesis for Heterometrinae was also supported by Prendini’s64 phylogeny of superfamily Scorpionoidea Latreille, 1802, based on morphological data for exemplar species. Prendini64 recovered Scorpionidae as monophyletic, with the Asian Heterometrus sister to the African Pandinus Thorell, 1876, implying that Asian scorpionids diverged from their African relatives when India separated from Africa. Prendini et al.5 corroborated this hypothesis with a phylogeny of Scorpionidae based on morphology and DNA sequence data from five gene loci, for a larger taxon sample, which also recovered two biogeographically distinct clades among the Asian scorpionid exemplars, representing species from the Indian subcontinent and Southeast Asia, respectively.
The first quantitative reconstruction of the biogeography of Asian forest scorpions (Heterometrinae) is presented here. Divergence time estimation, ancestral range estimation, and diversification analyses are used to determine the origins, dispersal and diversification patterns of the subfamily, and place that in the context of modern understanding of the biogeographical history of South and Southeast Asia.
Results
Divergence-time estimate analysis
The divergence-time estimation analysis in BEAST produced a fully dichotomous phylogeny. Posterior probability values were generally high and the monophyly of most genera well supported (≥ 0.99; Fig. 4). Relationships among the genera were also well supported (≥ 0.92) and congruent with the phylogeny based on a more extensive taxon sample (Supplementary Figure).
Ancestral range estimation and phylogenetic diversity
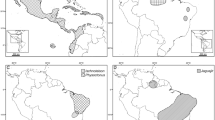
Comparison of the log likelihood, AIC and AICc values of several models identified the DEC + j model as the most appropriate for the dataset (Table 3). Based on this model, the following ranges were recovered as ancestral for each genus: Western Ghats and Sri Lanka for Gigantometrus, Sahyadrimetrus and Srilankametrus and the Greater Indian Subcontinent for Chersonesometrus, Deccanometrus, Heterometrus and Javanimetrus (Table 2; Fig. 3). The Western Ghats and Sri Lanka had the highest phylogenetic diversity (PD) among the six biogeographical areas, whereas the Philippines had the lowest (Table 4). Heterometrus glaucus (Thorell, 1876) had the highest evolutionary distinctiveness (ED) among the species (Fig. 5).
Estimation of ancestral ranges of the Asian forest scorpions (Scorpionidae: Heterometrinae) in six biogeographical regions: Africa (A); Western Ghats and Sri Lanka (B); Greater Indian Subcontinent (C); Indochina (D); Sundaland (E); Philippines (F). Lines on phylogeny indicate periods in the time-stratified analysis: 0–35 Ma, 35–45 Ma, 45–57 Ma, 57–68 Ma, 68–120 Ma. Map produced using ArcGIS v. 10.1 with ESRI (http://www.esri.com) World Countries layer, DeLorme Publishing Company, Inc. (2013).
Diversification analyses
Net diversification rates were as follows: 0.023 for high extinction rate (ε = 0.9) and high number of missing species (n = 50); 0.016 for high extinction rate (ε = 0.9) and low number of missing species (n = 5); 0.040 for low extinction rate (ε = 0) and high number of missing species (n = 50); 0.031 for low extinction rate (ε = 0) and low number of missing species (n = 5). Comparison of AIC values for seven models identified the two-rate Yule model as the best fit for the dataset (Table 5). The gamma statistic was negative (γ = − 2.08) and statistically significant (p = 0.019) suggesting most branching occurred early in the phylogeny. A Monte Carlo Constant Rates (MCCR) test indicated that the gamma statistic was affected by incomplete sampling when at least 28% of the taxa were missing (p > 0.05) (Table 6). A phylorate plot produced using BAMM supported fast speciation rates, occurring early in the phylogeny, and calculation of rate shifts indicated one distinct rate shift occurred (Fig. 5). An LTT plot also supported a declining speciation rate over time (Fig. 5).
Discussion
Asian Scorpionidae originate on Indian subcontinent during Cretaceous
The phylogenetic analyses presented here confirm previous studies5,23,64 in supporting the monophyly of Asian Heterometrinae and their sister group relationship with African Pandininae, represented in the analysis by Pandinus. This relationship implies Scorpionidae originated on the African continent and Heterometrinae diverged from their African relatives following the separation of West Gondwana (comprising Africa and South America) from East Gondwana (comprising the Indian subcontinent, Madagascar, Seychelles, Australia, and Antarctica). The East–West Gondwana rift has been invoked to explain biogeographical patterns among many taxa75,76 but the timing of the complete isolation of the Indian subcontinent from Africa has been debated. Prior to the East–West Gondwana rift, India was connected to Africa via Madagascar and Antarctica77, and it is likely Scorpionidae spread across this area. Land connections between India and Africa began to sever ca. 175 Ma27,29,78. Rifting began in the north, by opening of the Somali Basin between India-Madagascar and present-day Somalia79, and shortly thereafter in the south, between Antarctica and present-day Mozambique, by seafloor spreading in the Mozambique Basin and opening of the Weddell Sea79,80. Antarctica was completely separated from West Gondwana by 135 Ma78,81, and seafloor spreading between Madagascar and Africa had ceased ca. 120 Ma75,82,83, ending the rift between West and East Gondwana. However, despite India being physically separated from West Gondwana by this time, mounting evidence suggests India’s biota was not completely isolated from West Gondwana but rather that connections between India and Africa continued well into the Late Cretaceous, possibly even into the early Paleocene27,84,85,86,87,88,89. For example, several Late Cretaceous–early Paleocene fossil tetrapods, discovered in India, reveal close affinities with African relatives, suggesting connectivity between the two landmasses until ca. 65 Ma88. A divergence time estimation of two extant frog clades, based on DNA and calibrated using fossils, also supports a link between India and Africa until roughly 85 Ma in the Late Cretaceous86.
Several geological explanations have been proposed to explain connections between the faunas of Africa and India in the Late Cretaceous. Chatterjee and Scotese84 proposed a land bridge, connecting northwestern India to eastern Arabia, known as ‘Greater Somalia’. However, this hypothesis was refuted by geological data87,90, which demonstrated that, by the Late Triassic–Early Jurassic, Greater Somalia had already broken into two plates, situated far from the Indian subcontinent. Instead, an alternative explanation was proposed, the ‘Oman-Kohistan-Ladakh Island Arc’ hypothesis, which states that an island chain connected the Indian subcontinent with northern Africa between 80 and 65 Ma, or possibly even later87,91,92,93. A major revision of the plate tectonic and paleogeographical model of the Indian subcontinent27 criticized many plate tectonic models of India proposed by biogeographers for failing to consider geological evidence. Ali and Aitchison27 nevertheless supported the notion that the Indian subcontinent was not totally isolated after the East–West Gondwana rift. Their model suggested biotic exchange could have occurred between India and Antarctica-Australia via the Kerguelen Plateau until 90 Ma, and between India and Africa until 85 Ma with a more limited connection via the Madagascar-Providence Bank-Amirante Ridge-Seychelles block27. According to the model, India had reached its maximum isolation by 68 Ma, making it unlikely that faunal exchange would have occurred during this time, although limited connections to Africa may have existed.
According to the molecular clock calibrations of the present study, Asian scorpionids diverged from their African relatives ca. 113 Ma, supporting hypotheses of earlier biological connectivity between Africa and the Indian subcontinent1. Paleogeographical reconstructions of India for this period27 indicate the landmass comprising India and Madagascar drifting northwards along the eastern side of southeastern Africa (Fig. 4). Prior to 113 Ma, rafting between Africa and India may have been possible via the precursors of Madagascar or the Seychelles, allowing gene flow between Asian scorpionids and their African relatives, until the distance between these landmasses became too great and rafting was impossible. Rafting has been proposed for other scorpion taxa94,95,96 and, given that some Heterometrinae have been found in rotting logs and appear able to withstand habitat disturbance67, this means of dispersal is plausible. More paleontological and phylogenetic studies of African and Indian taxa, as well as geological evidence, are needed to refine the timing and mechanism of divergence between African and Asian scorpionids, however.
Estimation of divergence times (A) in evolution of the Asian forest scorpions (Scorpionidae: Heterometrinae) with paleogeographical reconstructions (B) indicating tectonic positions of India at various geological time periods, following Ali and Aitchison27 and Acton28. Continental outlines indicate past and present plate boundaries and colored regions indicate ancestral ranges for clades in the phylogeny (A). ‘X’ marks the south (166 Ma, 120 Ma, 100 Ma) and north poles (55 Ma, 45 Ma, 35 Ma). Only relevant landmasses are modeled27 in the 55 Ma, 45 Ma and 35 Ma paleogeographical reconstructions. Geological time periods on the phylogeny include Permian (Per), Triassic, Jurassic, Cretaceous, Paleocene (Pal), Eocene, Oligocene (Oli), Miocene (Mio), Pliocene (P) and Quaternary (Q). Circled numbers on phylogeny denote three dispersal events (55 Ma, 45 Ma and 35 Ma) of Heterometrinae from India to Southeast Asia. Ages (in millions of years) plotted on phylogeny refer to paleomap ages not node ages. Timeline for the origins, dispersal and diversification of Heterometrinae (B) as follows. 166 Ma: Africa begins to rift from India. 120 Ma: Heterometrinae (Het) diverge from sister group, the African Pandininae (P) as India becomes isolated from Africa. 100 Ma: Heterometrinae splits into two clades: one confined to southern India and Sri Lanka (I) and the ancestor of Heterometrus (H), widely distributed across the Indian subcontinent. 55 Ma: The ancestor of Heterometrus glaucus (Hg) disperses to Sundaland as the Indian subcontinent brushes up against the western coast of Sumatra; Srilankametrus (Sr) diverges and later diversifies across the Western Ghats and Sri Lanka. 45 Ma: The ancestor of Javanimetrus (J) disperses to Sundaland; Sahyadrimetrus (Sa) diverges and later diversifies across the Western Ghats and Sri Lanka. 35 Ma: The ancestor of a clade comprising most of the extant species of Heterometrus disperses to Indochina and Sundaland; the ancestor of the clade comprising Chersonesometrus (C), Deccanometrus (D) and Gigantometrus (G) disperses northwards across the Indian subcontinent.
Range contraction of Indian Heterometrinae around KT boundary
The phylogeny suggests that, after diverging from their African relatives, the Asian scorpionids diverged into two major clades. One clade includes Chersonesometrus, Deccanometrus, Gigantometrus and Sahyadrimetrus from the Indian subcontinent, and the monotypic genus, Javanimetrus, known from the Nicobar Islands, peninsular Thailand, and several Indo-Pacific islands. The other comprises Heterometrus, widely distributed throughout Southeast Asia including the Andaman and Nicobar Islands, Indochina, the Thai-Malay Peninsula and many Indo-Pacific islands to the west of Wallace’s Line. The diversification time and ancestral range analyses estimate this divergence occurred approximately 92 Ma in the Late Cretaceous.
At the end of the Cretaceous, starting 67.4 Ma, the Deccan Traps erupted, and India experienced a period of intense volcanism that occurred in three phases, interrupted by nonvolcanic phases, over a short timespan lasting less than 1 million years51. During these eruptions, between 1.2 and 1.5 km3 of lava extruded across India, especially the present-day states of Gujarat, Madhya Pradesh, and Maharashtra, with the longest lava flow known in the world reaching as far south as the Krishna-Godavari Basin in Andhra Pradesh49,51. Large amounts of volcanic gases were also released with estimates of 15,000–35,000 Gt of carbon dioxide (CO2) and 6800–17,000 Gt of sulphur dioxide (SO2) entering the atmosphere during the most intense phase of volcanic eruption47,51. Despite the severity of this volcanic activity, the role of the Deccan eruptions in the KT mass extinction has been debated. Indian paleontological records reveal that many terrestrial organisms survived the eruptions50. Consequently, the meteorite impact at the end of the Late Cretaceous, which created the Chicxulub Crater off the Caribbean coast of Mexico, is favored as the primary cause for mass extinctions across the globe, and many studies argue that the effects of Deccan volcanism were only accessory to the environmental changes caused by the meteorite impact45,53. Some paleontological and geological data suggest the effects of Deccan volcanism were not insignificant, however, leading several researchers to argue that the Deccan eruptions were the primary cause of the global KT mass extinction49,51,54,55. For example, Schoene et al.54 used uranium-lead geochronology to demonstrate that the environmental conditions, which led to mass extinction, initiated during the main phase of eruptions, prior to the Chicxulub meteorite impact, suggesting volcanism was a major contributor to extinction. Palynological data from the Deccan region also indicate a shift in composition of the floral community after these eruptions. The flora was more diverse, and included an abundance of gymnosperms, before the eruptions, whereas angiosperms and pteridophytes dominated afterwards48,50. Examination of exposed marine beds on India demonstrate a major extinction of planktonic foraminifera with nearly all species disappearing after the last megaflow due to ocean acidification caused by volcanic gases51,52.
Although the contribution of the Deccan eruptions to the KT mass extinction, relative to the Chicxulub meteorite impact, remains unclear, several studies suggest diverse taxa were confined to the southern part of the Indian subcontinent, specifically the Western Ghats of India and the Central Highlands of Sri Lanka, at the end of the Late Cretaceous1,2,17,57. Consequently, these areas have been posited as potential refugia during the KT mass extinction. The results presented here support the hypothesis that the Western Ghats and Central Highlands of Sri Lanka were refugia and suggest Asian scorpionids experienced range contraction during the KT mass extinctions due to stresses caused by the Deccan eruptions, the Chicxulub meteorite impact, or both. The phylogeny and ancestral range estimation analyses support this hypothesis as the ancestor of the largely Indian clade, comprising Chersonesometrus, Deccanometrus, Gigantometrus, Javanimetrus, Sahyadrimetrus and Srilankametrus, was confined to the Western Ghats and Sri Lanka during this period. Sahyadrimetrus and Srilankametrus, endemic to the Western Ghats and Sri Lanka, are basal in the Indian clade, whereas Chersonesometrus, Deccanometrus and Gigantometrus, more widespread on the Indian subcontinent, are more distal. No new lineages of Heterometrinae arose between 92 and 56 Ma (Fig. 4).
Three dispersal events follow Indian collision with Eurasia
The ‘Out of India’ hypothesis, according to which India acted as a raft carrying the ancestors of Asian taxa from Gondwana to Eurasia on its northward journey, has been invoked to explain the biogeographical history of many taxa, including Asian forest scorpions1,2,3,4,5,6,7,8,9,10,23,73,97. The present study confirms previous suggestions that the ‘Out of India’ hypothesis best explains the origin of Southeast Asian Heterometrinae, given their sister-group relationship with the African Pandininae, and the absence of Heterometrinae or Pandininae between the Middle East and the Indian subcontinent. However, prior to the present study, the pattern and timing of dispersal to Southeast Asia, including the question as to whether this involved a single colonization event, were unknown. As noted above, the timing and manner in which India collided with Eurasia, have been intensely debated. The analyses presented here are consistent with the model which proposes that India could have been connected to Southeast Asia as early as 57 Ma26,28 and gradually moved northwards until its final collision with Eurasia 35 Ma, and demonstrate that Heterometrinae dispersed into Southeast Asia on three independent occasions.
The earliest dispersal event occurred within Heterometrus. As India moved northward, it collided with an intra-oceanic island arc, the Dazhuqu Arc, ca. 55 Ma, at a time when its northern edge was subaerially exposed and close to Sumatra26,27,28. At around 56 Ma, according to the biogeographical analysis, one lineage of Heterometrus, the ancestor of H. glaucus, dispersed and diversified into Sundaland, just as the northern edge of Greater India and its island arc were approaching Southeast Asia, representing the first dispersal event within Heterometrinae (Fig. 4). At 44 Ma, when eastern India was adjacent to Sumatra28, a second dispersal event occurred in another genus when the ancestor of Javanimetrus dispersed from the Indian subcontinent to Sundaland (Fig. 4). The third dispersal event again involved Heterometrus, when the ancestor of the clade comprising all species of the genus except H. glaucus dispersed from the Indian subcontinent to Indochina and Sundaland ca. 37 Ma (Fig. 4). According to the models of Ali and Aitchison27 and Acton28, India was adjacent to Indochina around this time and this also constitutes the final collision event between India and Eurasia.
After the final dispersal event, ca. 37 Ma, Heterometrus appears to have followed a northwest to southeast pattern of dispersal as the species from Indochina are more basal in the phylogeny than those from Sundaland and the Philippines. The number of lineages also rose rapidly between 56 and 37 Ma, suggesting Asian scorpionids were diversifying into the new habitats encountered in Southeast Asia. This supports other studies which suggested accelerated biotic interchange for plants, vertebrates and various arthropods between Asia and India since the Eocene98. These findings are also congruent with a biogeographical analysis of rhacophorid frogs, which reconstructed an initial dispersal event into Southeast Asia, occurring 57 Ma, and several other dispersal events, occurring later19.
Scorpionids disperse and diversify northwards across India
The phylogeny suggests the ancestors of Chersonesometrus, Deccanometrus and Gigantometrus, widespread across the interior of present-day India, dispersed across the subcontinent after the colonization of Southeast Asia. The collision of India with Eurasia resulted in the uplift of the Tibetan Plateau and the closure of the Paratethys Sea which, in turn, caused the shift from a zonal to monsoon climate on the Indian subcontinent, in the Miocene25,99. Although the precise timing of the onset of the Indian monsoons is debated, the effects of a monsoon climate were evident, with India significantly drier, by 8 Ma98. The seasonally drier climate changed the flora and fauna by reducing or eliminating the tropical evergreen forests of the lowlands and the moist mixed deciduous-pine forests of the highlands, allowing C4 plants, typical of savanna-grasslands, to dominate100. Fossil vertebrates from this time suggest a shift from forest-dwelling mammals to herbivores associated with open savanna100,101.
Environmental changes occurring across the Indian subcontinent during the Miocene also allowed Indian scorpionids to take advantage of new habitats, resulting in the diversification of Chersonesometrus, Deccanometrus and Gigantometrus. Dispersal into more open, arid habitats was associated with ecomorphological adaptations. For example, Deccanometrus latimanus (Pocock, 1894) and Deccanometrus xanthopus (Pocock, 1897), which inhabit the savanna and scrub of Pakistan and India, respectively, exhibit reddish-brown coloration, robust pedipalps, metasoma and telson, and short, pale legs unlike the closely related species, Deccanometrus bengalensis (C.L. Koch, 1841) and Deccanometrus phipsoni (Pocock, 1893), which inhabit forests and exhibit uniformly blackish or dark brown coloration, more slender pedipalps, metasoma and telson, and longer legs102. According to the LTT plot (Fig. 5), the speciation rate reached a plateau by the beginning of the Middle Miocene (ca. 10 Ma) suggesting all new niches were occupied and diversification into new environments was complete.
Diversification and evolutionary distinctiveness of the Asian forest scorpions (Scorpionidae: Heterometrinae): (A) Speciation rate. (B) Lineage through time plot. (C) Extinction rate. (D) Evolutionary distinctiveness per species. (E) Net diversification rate. (F) Phylorate plot indicating speciation rates among lineages.
Alternative hypotheses
The analyses presented here, as well as evidence from other phylogenetic and biogeographical studies, refute Couzijn’s23 hypothesis for the origins, dispersal and diversification of Heterometrinae (Fig. 2). Couzijn23 suggested Heterometrinae diverged from their African relatives during the breakup of Gondwana and different lineages were transported to Asia on separate landmasses, the ancestors of Gigantometrus, Chersonesometrus and Heterometrus traveling on the Indian landmass, and the ancestor of Javanimetrus and Srilankametrus traveling on an Indochinese landmass. According to recent geological and biogeographical studies, however, the Indochinese landmass arrived at Southeast Asia in the Jurassic103, contradicting the timeline proposed by Couzijn23. Furthermore, Couzijn’s23 phenetic hypothesis of relationships among the genera recognized today, i.e., (Gigantometrus ((Javanimetrus + Srilankametrus) (Chersonesometrus + Heterometrus))), is contradicted by the phylogenetic reconstruction of Heterometrinae presented here and by Loria and Prendini102, i.e., (Heterometrus (Srilankametrus (Javanimetrus (Sahyadrimetrus (Deccanometrus (Chersonesometrus + Gigantometrus)))))).
Alternative hypotheses have been proposed to explain the origins and distribution patterns of other Gondwanan scorpions. Although Monod and Prendini96 used the Africa-India rift to explain the origins of an Indian clade of Hormuridae Laurie, 1896, the ‘Eurogondwana’ hypothesis104 was invoked to account for the distribution of an Indo-Pacific hormurid clade. According to this hypothesis, Hormuridae originated in Gondwana during the Paleozoic and the ancestors of this Indo-Pacific hormurid clade diverged in isolation from the ancestors of the African and South American hormurid genera, when the Apulian microplate rifted from Africa in the Early Cretaceous105. The Indo-Pacific hormurid clade subsequently arrived in Eurasia when the Apulian microplate collided into Europe and dispersed across the Palearctic region, eventually reaching Southeast Asia and Australasia. No hormurids presently occur in the Palearctic, hence Monod and Prendini96 suggested that the ‘icehouse’ climate in the Neogene and the ‘hothouse’ climate in the late Paleocene–early Eocene may have led to the extinction of these mostly tropical or subtropical, humidity-dependent scorpions.
The Eurogondwana hypothesis is a less likely explanation for the distribution patterns of Asian scorpionids. It implies the absence of scorpionids from the Palearctic, which is contradicted by the presence of Scorpio L., 1758 in northern Africa, the Arabian Peninsula and the Middle East (including part of Iran), suggesting scorpionids were able to survive severe climatic changes such as global warming and cooling. On the other hand, the ‘Out of India’ hypothesis is consistent with the geographical distribution of Pandininae, the sister group of Heterometrinae, which extends from Senegambia across western and central Africa to the Red Sea and the Gulf of Aden, and southward as far as the Zambezi River5,67,95. As discussed above, evidence exists that biotic exchange was still occurring between central Africa and India well into the Late Cretaceous and early Paleocene.
Sharma et al.106 produced a chronogram for scorpions, using crown and stem group ages for five arachnid taxa, including the scorpion family Buthidae C.L. Koch, 1837, estimated from fossil data, according to which superfamily Scorpionoidea began to diversify at the end of the Cretaceous, roughly 90 Ma. A biogeographical explanation for the early diversification of Scorpionoidea was not offered. Using five molecular markers and phylogenomic dating, Santibáñez et al.107 recovered the divergence between Heterometrus and Pandinus at the end of the Jurassic (145 Ma), but later108 suggested this divergence occurred in the Late Cenozoic (ca. 20 Ma). Whereas a Jurassic divergence is plausible, based on the present study and the geological literature, a Cenozoic divergence between Heterometrus and Pandinus is unlikely.
Clouse109 proposed a Cimmerian continent origin for the Southeast Asian harvestman family Stylocellidae Hansen and Sørensen, 1904. This scenario seems less plausible for Heterometrinae given that Cimmeria rifted from Gondwana ca. 290 Ma, and the dated tree presented here suggests that Heterometrinae diverged from the African Pandininae ca. 115 Ma.
Conclusions
Understanding the biogeographical patterns of South and Southeast Asian taxa is confounded by the complex geological history of the region. Despite decades of research, there remains little consensus regarding how and when the Indian subcontinent collided with Eurasia or the events that unfolded during its northward journey. The analyses presented here provide a plausible reconstruction of the origins, dispersal and diversification patterns of another group of taxa, the Asian forest scorpions (Heterometrinae), which can be summarized into four major events. (1) Heterometrinae diverged from other Scorpionidae on the African continent after the Indian subcontinent became separated in the Cretaceous. (2) Environmental stresses during the KT mass extinction resulted in range contraction, restricting Heterometrinae to refugia in southern India (the Western Ghats) and Sri Lanka (the Central Highlands). (3) Heterometrinae dispersed to Southeast Asia three times during India’s collision with Eurasia, the first dispersal event occurring as the Indian subcontinent brushed up against the western side of Sumatra, and the other two events occurring as India moved closer to Eurasia. (4) Indian Heterometrinae, confined to southern India and Sri Lanka during the KT mass extinction, recolonized the Deccan Plateau and northern India, diversifying into new, more arid habitats after environmental conditions stabilized.
Methods
Taxon sampling
An extensive reconstruction of the phylogeny of Asian forest scorpions, based on 186 morphological characters and ca. 4200 DNA nucleotides from three mitochondrial and two nuclear gene loci for 132 terminals (Supplementary Figure) is published elsewhere102. The dataset was reduced to one terminal per species for the biogeographical analyses presented here, resulting in 35 terminals, representing all seven genera and 31 (76%) species of Heterometrinae, and an outgroup comprising exemplar species of three genera, representing each of the other subfamilies of Scorpionidae, from Africa and the Middle East (Supplementary Table 1): Pandinus imperator (C. L. Koch, 1841), representing Pandininae; Scorpio fuscus (Ehrenberg, 1829), representing Scorpioninae Latreille, 1802; and Opistophthalmus capensis (Herbst, 1800), representing Opistophthalminae Rossi, 2016. The tree was rooted on Nebo hierichonticus (Simon, 1872), representing Nebinae Kraepelin, 1905, the basal clade of Diplocentridae Karsch, 1880, the putative sister group of Scorpionidae5,64.
DNA extraction and sequencing
Muscle tissue was dissected from the legs of specimens preserved in 95% ethanol, and DNA isolated using the DNEasy Blood and Tissue Extraction Kit (Qiagen, Hilden, Germany). Three mitochondrial gene loci, Cytochrome c Oxidase Subunit I (COI), 16S rDNA (16S) and 12S rDNA (12S), and two nuclear gene loci, 18S rDNA (18S) and 28S rDNA (28S), were sequenced. Previous research demonstrated this combination of loci was informative for resolving relationships at deep to shallow levels in scorpion phylogeny5,110,111,112,113. The COI and 18S loci were sequenced in two and three fragments, respectively, whereas all other loci were sequenced in single fragments102. Each locus was sequenced in both the forward and reverse directions. Sequence lengths were calculated using the gc_calculator.py script in Biopython114.
DNA sequence alignment
Sequences were aligned using Mafft v. 7.429115,116 by auto-aligning with the ‘leavegappyregion’ option selected. The L-INS-I method, an iterative refinement method which uses a local pairwise alignment with an affine gap cost, was selected as the best alignment for all loci117. The aligned sequences were 1761 base-pairs (bp) in length for 18S, 515 bp for 28S, 341 bp for 12S, 493 bp for 16S, and 1078 bp for COI, summing to a total of 4188 bp for the concatenated dataset (Table 1). Gaps representing insertion/deletion events were present in the aligned 12S (17 gaps), 16S (12 gaps) and 28S (1 gap). Average percent A, C, G, T content was calculated for the aligned loci (Table 1) using MEGA v. 7118.
Divergence time estimation
A divergence time estimation analysis was performed in BEAST v. 1.10.4119. No fossil data exist for Heterometrinae hence a relaxed lognormal molecular clock was used to date the phylogeny with the following parameters in BEAUTi v. 1.10.4119. The diplocentrid, N. hierichonticus, was used as outgroup and a clade comprising Heterometrinae and Pandinus was constrained to be monophyletic based on evidence that the clade including Pandinus is the sister group of Heterometrinae5,64. Relationships among the ingroup were constrained to match the larger phylogenetic analysis of Heterometrinae102 (Supplementary Figure). The dataset was partitioned by loci with molecular clocks and substitution models unlinked across all partitions. An uncorrelated relaxed molecular clock was applied for each partition and models for individual loci selected using the Bayesian Information Criterion (BIC) in JModeltest v. 2120 on the CIPRES Science Gateway (Table 1)121. Trees were linked across all partitions and a birth–death tree prior implemented. Clock rates for the mitochondrial COI and 16S loci have been published for scorpion taxa from families Buthidae, Vaejovidae Thorell, 1876 and Bothriuridae Simon, 1880122,123,124,125,126,127,128,129,130,131. A normal clock rate prior was applied to the COI and 16S loci with the following settings: μ = 0.007, σ = 0.00146 for COI and μ = 0.005, σ = 0.00270 for 16S, such that 95% of the normal distribution included minimum and maximum values of the COI and 16S loci in scorpions131. Rates for all other loci were estimated using uniform priors with the following constraints: 12S, ucld.min = 0.002 and ulcd.max = 0.5; 18S and 28S, ucld.min = 0.0001 and ulcd.max = 0.01127. Two independent Bayesian analyses were conducted with the following settings: mcmc; ngen = 500,000,000; print frequency = 10,000; sample frequency = 10,000. Effective sample sizes for all parameters were above 200 and convergence between independent runs was assessed using Tracer v. 1.7.1132. Log Combiner v. 1.10.4 was used to combine tree files from the independent runs with burnin set to 25%. After burnin samples were removed, Tree Annotator v. 1.10.4 was used to compile a 50% majority rule consensus tree from the independent runs.
Ancestral range estimation and phylogenetic diversity
Ancestral range reconstruction for the time-calibrated phylogenetic tree was implemented using the BioGeoBEARS v. 1.1.1 package133,134,135 in R v. 4.0.0136. Outgroups were excluded except for Pandinus, the sister group of Heterometrinae, using the drop.tip function from the R package ape147 and the tree was forced to be ultrametric using force.ultrametic from the R package phytools148. Six biogeographical areas were defined based on previous studies60,137,138: (A) Africa; (B) Western Ghats and Sri Lanka; (C) Greater Indian Subcontinent; (D) Indochina; (E) Sundaland; (F) Philippines (Fig. 3). The Isthmus of Kra was designated as the boundary between Indochina and Sundaland137. Each terminal taxon was scored as present or absent in each area. The maximum number of areas a species can occupy was assumed to be four. The analysis was time-stratified into five periods based on geological events27: 0–35 Ma, 35–45 Ma, 45–57 Ma, 57–68 Ma, 68–120 Ma (Supplementary Tables 2–4). Areas allowed and areas adjacent matrices (Supplementary Tables 3, 4) were included as well as a dispersal multiplier matrix with a probability of 1 for adjacent areas, 0.5 for areas with intermediate connections, and 1e−07 for areas with unlikely connection due to separation by large geographical distance or an ocean barrier (Supplementary Table 2). The models Dispersal-Extinction-Cladogenesis, DIVALIKE, and BAYAREALIKE were compared, and an additional parameter (j), which allows a founder speciation event, tested for each (Table 3). Statistical analyses were used to determine the likelihood of the dataset given each model and identify the most appropriate model for the dataset. Faith’s139 phylogenetic diversity (PD) index was applied to all ingroup areas (Table 4) using ten thousand randomizations of the ‘trial swap’ null model140 with the R package picante141. The evolutionary distinctiveness (ED) for each species was also calculated using the ‘fair proportions’142 and ‘equal splits’143 methods in picante.
Diversification analyses
Outgroups were removed from the divergence time tree and the tree forced to be ultrametric as described above. A series of analyses were performed on the ingroup tree, to understand diversification patterns within Heterometrinae, using the R package laser v. 2.4-1144. The Akaike Information Criterion (AIC) was calculated to determine the best fitting model for the dataset. The command fitdAICrc was applied for the pure birth, birth–death, exponential density-dependent (DDX), linear density-dependent (DDL) and two-rate Yule models (Table 5). The commands fitSPVAR and fitEXVAR were applied for the variable speciation (SPVAR) and variable extinction (EXVAR) models145. Net diversification rates were calculated for a low extinction rate (ε = 0) and a high extinction rate (ε = 0.9), assuming low (n = 5) and high (n = 50) numbers of missing species, respectively. The gamma statistic, which assumes complete sampling, was calculated to determine whether the diversification rate is constant over time, using the gamStat (γ)146 function in the R package ape147. A Monte Carlo constant rates test, conducted using the function mccrTest, was also used to assess whether the observed gamma rate was affected by incomplete sampling, assuming ten different clade sizes (Table 6). A lineage-through-time (LTT) plot was generated, using the R package phytools148, to visualize lineage diversification in Heterometrinae, with 10,000 trees generated under a pure birth (Yule) model for comparison. Speciation rates, extinction rates, net diversification rates, and number of rate shifts were calculated by running Bayesian Analysis of Macroevolutionary Mixtures (BAMM) v. 2.5.0149 with the settings nchains = 4, ngen = 10,000,000 and initial priors selected using BAMMtools v. 2.1.6150 in R. The statistical significance of rate shifts was calculated using Bayes factors151. Results were visualized using BAMMtools.
References
Bossuyt, F. & Milinkovitch, M. C. Amphibians as indicators of Early Tertiary “Out-of-India” dispersal of vertebrates. Science 292, 93–95 (2001).
Conti, E., Eriksson, T., Schönenberger, J., Sytsma, K. J. & Baum, D. A. Early Tertiary Out-of-India dispersal of Crypteroniaceae: Evidence from phylogeny and molecular dating. Evolution 56, 1931–1942 (2002).
Gower, D. J. et al. A molecular phylogeny of ichthyophiid caecilians (Amphibia: Gymnophiona: Ichthyophiidae): Out of India or out of South East Asia? Proc. R. Soc. Lond. B Biol. Sci. 269, 1563–1569 (2002).
Wilkinson, M., Sheps, J. A., Oommen, O. V. & Cohen, B. L. Phylogenetic relationships of Indian caecilians (Amphibia: Gymnophiona) inferred from mitochondrial rRNA gene sequences. Mol. Phylogenet. Evol. 23, 401–407 (2002).
Prendini, L., Crowe, T. M. & Wheeler, W. C. Systematics and biogeography of the family Scorpionidae (Chelicerata: Scorpiones), with a discussion on phylogenetic methods. Invertebr. Syst. 17, 185–259 (2003).
Karanth, K. P. Out-of-India Gondwana origin of some tropical Asian biota. Curr. Sci. 90, 789–792 (2006).
Datta-Roy, A. & Karanth, K. P. The Out-of-India hypothesis: What do molecules suggest? J. Biosci. 34, 687–697 (2009).
Svenson, G. J. & Whiting, M. F. Reconstructing the origins of praying mantises (Dictyoptera, Mantodea): The roles of Gondwanan vicariance and morphological convergence. Cladistics 25, 468–514 (2009).
Klaus, S., Schubart, C. D., Streit, B. & Pfenninger, M. When Indian crabs were not yet Asian—biogeographic evidence for Eocene proximity of India and Southeast Asia. BMC Evol. Biol. 10, 287. https://doi.org/10.1186/1471-2148-10-287 (2010).
Okajima, Y. & Kumazawa, Y. Mitochondrial genomes of acrodont lizards: Timing of gene rearrangements and phylogenetic and biogeographic implications. BMC Evol. Biol. 10, 141. https://doi.org/10.1186/1471-2148-10-141 (2010).
Meimberg, H., Wistuba, A., Dittrich, P. & Heubl, G. Molecular phylogeny of Nepenthaceae based on cladistic analysis of plastid trnK intron sequence data. Plant Biol. 3, 164–175 (2001).
Dutta, S. et al. Eocene out-of-India dispersal of Asian dipterocarps. Rev. Palaeobot. Palynol. 166, 63–68 (2011).
Kumazawa, Y. & Nishida, M. Molecular phylogeny of osteoglossoids: A new model for Gondwanian origin and plate tectonic transportation of the Asian arowana. Mol. Biol. Evol. 17, 1869–1878 (2000).
Li, X., Musikasinthorn, P. & Kumazawa, Y. Molecular phylogenetic analyses of snakeheads (Perciformes: Channidae) using mitochondrial DNA sequences. Ichthyol. Res. 53, 148–159 (2006).
Inoue, J. G., Kumazawa, Y., Miya, M. & Nishida, M. The historical biogeography of the freshwater knifefishes using mitogenomic approaches: A Mesozoic origin of the Asian notopterids (Actinopterygii: Osteoglossomorpha). Mol. Phylogenet. Evol. 51, 486–499 (2009).
Duellman, W. E. & Trueb, L. Biology of Amphibians. 670 (McGraw-Hill, New York, 1986).
Bossuyt, F., Brown, R. M., Hillis, D. M., Cannatella, D. C. & Milinkovitch, M. C. Phylogeny and biogeography of a cosmopolitan frog radiation: Late Cretaceous diversification resulted in continent-scale endemism in the family Ranidae. Syst. Biol. 55, 579–594 (2006).
Nishikawa, K. et al. Molecular phylogeny and biogeography of caecilians from Southeast Asia (Amphibia, Gymnophiona, Ichthyophiidae), with special reference to high cryptic species diversity in Sundaland. Mol. Phylogenet. Evol. 63, 714–723 (2012).
Li, J.-T. et al. Diversification of rhacophorid frogs provides evidence for accelerated faunal exchange between India and Eurasia during the Oligocene. Proc. Natl. Acad. Sci. U.S.A. 110, 3441–3446 (2014).
Pyron, R. A. Biogeographic analysis reveals ancient continental vicariance and recent Oceanic dispersal in amphibians. Syst. Biol. 63, 779–797 (2014).
Cooper, A. et al. Complete mitochondrial genome sequences of two extinct moas clarify ratite evolution. Nature 409, 704–707 (2001).
Macey, J. R. et al. Evaluating trans-Tethys migration: An example using acrodont lizard phylogenetics. Syst. Biol. 49, 233–256 (2000).
Couzijn, H. W. C. Revision of the genus Heterometrus Hemprich & Ehrenberg (Scorpionidae, Arachnidea). Zool. Verhand. Leiden 184, 1–196 (1981).
Köhler, F. & Glaubrecht, M. Out of Asia and into India: On the molecular phylogeny and biogeography of the endemic freshwater gastropod Paracrostoma Cossmann, 1900 (Caenogastropoda: Pachychilidae). Biol. J. Linn. Soc. 91, 627–651 (2007).
Van Bocxlaer, I., Biju, S. D., Loader, S. P. & Bossuyt, F. Toad radiation reveals into-India dispersal as a source of endemism in the Western Ghats-Sri Lanka biodiversity hotspot. BMC Evol. Biol. 9, 131. https://doi.org/10.1186/1471-2148-9-131 (2009).
Aitchison, J. C., Ali, J. R. & Davis, A. M. When and where did India and Asia collide? J. Geophys. Res. Solid Earth 112, B05423. https://doi.org/10.1029/2006JB004706 (2007).
Ali, J. R. & Aitchison, J. C. Gondwana to Asia: Plate tectonics, paleogeography and the biological connectivity of the India sub-continent from the Middle Jurassic through latest Eocene (166–35 Ma). Earth Sci. Rev. 88, 145–166 (2008).
Acton, G. D. Apparent polar wander of India since the Cretaceous with implications for regional tectonics and true polar wander. In The Indian Subcontinent and Gondwana: A Paleomagnetic and Rock Perspective. (eds. Radhakrishna, T. & Piper, J. D. A.). Mem. Geol. Soc. India 44, 129–175 (1999).
Schettino, A. & Scotese, C. R. Apparent polar wander paths for the major continents (200 Ma to the present day): A paleomagnetic reference frame for global plate tectonic reconstructions. Geophys. J. Int. 163, 727–759 (2005).
Garzanti, E. Comment on “When and where did India and Asia collide?” by Johnathan C. Aitchison, Jason R. Ali, and Aileen M. Davis. J. Geophys. Res. Solid Earth 113, B04411. https://doi.org/10.1029/2007JB005276 (2008).
Aitchison, J. C., Ali, J. R. & Davis, A. M. Reply to comment by Eduardo Garzanti on “When and where did India and Asia collide?” J. Geophys. Res. Solid Earth 113, B04412. https://doi.org/10.1029/2007JB005431 (2008).
Najman, Y. et al. Timing of India-Asia collision: Geological biostratigraphic, and palaeomagnetic constraints. J. Geophys. Res. Solid Earth 115, B12416. https://doi.org/10.1029/2010JB007673 (2010).
Clementz, M. et al. Early Eocene warming events and the timing of terrestrial faunal exchange between India and Asia. Geol. Soc. Am. 39, 15–18 (2011).
Van Hinsbergen, D. J. J. et al. Restoration of Cenozoic deformation in Asia and the size of Greater India. Tectonics 30, TC5003. https://doi.org/10.1029/2011TC002908 (2011).
Aitchison, J. C. & Ali, J. R. India-Asia collision timing. Proc. Natl. Acad. Sci. U.S.A. 109, E2645. https://doi.org/10.1073/pnas.1207859109 (2012).
Aitchison, J. C. & Ali, J. R. Comment on “Restoration of Cenozoic deformation in Asia and the size of Greater India” by D. J. J. van Hinsbergen et al. Tectonics 31, TC4006. https://doi.org/10.1029/2011TC003091 (2012).
Van Hinsbergen, D. J. J. et al. Reply to comment by Ali and Aitchison on “Restoration of Cenozoic deformation of in Asia, and the size of Greater India”. Tectonics 31, TC4007. https://doi.org/10.1029/2012TC003144 (2012).
Van Hinsbergen, D. J. J. et al. Greater India Basin hypothesis and a two-stage Cenozoic collision between India and Asia. Proc. Natl. Acad. Sci. U.S.A. 109, 7659–7664 (2012).
Van Hinsbergen, D. J. et al. Reply to Aitchison and Ali: Reconciling Himalayan ophiolite and Asian magmatic arc records with a two-stage India-Asia collision model. Proc. Natl. Acad. Sci. U.S.A. 109, E2646. https://doi.org/10.1073/pnas.1208836109 (2012).
Hu, X. et al. The timing of India-Asia collision onset—Facts, theories, controversies. Earth Sci. Rev. 160, 264–299 (2016).
Beck, R. A. et al. Stratigraphic evidence for an early collision between northwest India and Asia. Nature 373, 55–58 (1995).
Rust, J. et al. Biogeographic and evolutionary implications of a diverse paleobiota in amber from the early Eocene in India. Proc. Natl. Acad. Sci. U.S.A. 107, 18360–18365 (2010).
Courtillot, V. et al. Deccan flood basalts at the Cretaceous/Tertiary boundary? Earth Planet. Sci. Lett. 80, 361–374 (1986).
Thewissen, J. G. M. & McKenna, M. C. Paleobiogeography of Indo-Pakistan: A response to Briggs, Patterson, and Owen. Syst. Biol. 41, 248–251 (1992).
Wilf, P., Johnson, K. R. & Huber, B. T. Correlated terrestrial and marine evidence for global climate changes before mass extinction at the Cretaceous-Paleogene boundary. Proc. Natl. Acad. Sci. U.S.A. 100, 599–604 (2003).
Chenet, A.-L., Fluteau, F., Courtillot, V., Gérard, A. & Subbarao, K. V. Determination of rapid Deccan eruptions across the Cretaceous-Tertiary boundary using paleomagenetic secular variation: Results from a 1200-m-thick section in the Mahabaleshwar escarpment. J. Geophys. Res. Solid Earth 113, B04101. https://doi.org/10.1029/2006JB004635 (2008).
Chenet, A.-L. et al. Determination of rapid Deccan eruptions across the Cretaceous-Tertiary boundary using paleomagnetic secular variation: 2. Constraints from analysis of eight new sections and synthesis for a 3500-m-thick composite section. J. Geophys. Res. Solid Earth 114, B06103. https://doi.org/10.1029/2008JB005644 (2009).
Samant, B. & Mohabey, D. M. Palynoflora from Deccan volcano-sedimentary sequence (Cretaceous-Palaeogene transition) of central India: Implications for spatio-temporal correlation. J. Biosci. 34, 811–823 (2009).
Bajpai, S. Biotic perspective of the Deccan volcanism and India–Asia collision: Recent advances. In Current Trends in Science, Platinum Jubilee Special Publication (ed. Mukunda, N.) 505–516 (Indian Academy of Sciences, Bangalore, 2009).
Prasad, G. V. & Sahni, A. Vertebrate fauna from the Deccan volcanic province: Response to volcanic activity. Geol. Soc. Am. Spec. Pap. 505, 193–211 (2014).
Keller, G. Deccan volcanism, the Chicxulub impact, and the end-Cretaceous mass extinction: Coincidence? Cause and effect. Geol. Soc. Am. Spec. Pap. 505, 57–89 (2014).
Punekar, J., Mateo, P. & Keller, G. Effects of Deccan volcanism on paleoenvironment and planktic foraminifera: A global survey. Geol. Soc. Am. Spec. Pap. 505, 91–116 (2014).
Brusatte, S. L. et al. The extinction of the dinosaurs. Biol. Rev. 90, 628–642 (2015).
Schoene, B. et al. U–Pb geochronology of the Deccan Traps and relation to the end-Cretaceous mass extinction. Science 347, 182–184 (2015).
Petersen, S. V., Dutton, A. & Lohmann, K. C. End-Cretaceous extinction in Antarctica linked to both Deccan volcanism and meteorite impact via climate change. Nat. Commun. 7, 12079. https://doi.org/10.1038/ncomms12079 (2016).
Kamei, R. G. et al. Discovery of a new family of amphibians from northeast India with ancient links to Africa. Proc. R. Soc. Lond. B Biol. Sci. 279, 2396–2401 (2012).
Joshi, J. & Karanth, P. Did southern Western Ghats of peninsular India serve as refugia for its endemic biota during the Cretaceous volcanism? Ecol. Evol. 3, 3275–3282 (2013).
Toussaint, E. F. A., Fikáček, M. & Short, A. E. Z. India-Madagascar vicariance explains cascade beetle biogeography. Biol. J. Linn. Soc. 118, 982–991 (2016).
Wallace, A. R. The Geographical Distribution of Animals with a Study of the Relations of Living and Extinct Faunas as Elucidating the Past Changes of the Earth’s Surface. Vol. 1. 503 (MacMillan, London, 1876).
Myers, N., Mittermeler, R. A., Mittermeler, C. G., da Fonseca, G. A. B. & Kent, J. Biodiversity hotspots for conservation priorities. Nature 403, 853–858 (2000).
Naggs, F. & Raheem, D. Sri Lankan snail diversity: Faunal origins and future prospects. Rec. West. Aust. Mus. Suppl. 68, 11–29 (2005).
Maduwage, K., Silva, A., Manamendra-Arachchi, K. & Pethiyagoda, R. A taxonomic revision of South Asian hump-nosed pit vipers (Squamata: Viperidae: Hypnale). Zootaxa 2232, 1–28 (2009).
Van Bocxlaer, I. et al. Mountain-associated clade endemism in an ancient frog family (Nyctibatrachidae) on the Indian subcontinent. Mol. Phylogenet. Evol. 62, 839–847 (2012).
Prendini, L. Phylogeny and classification of the superfamily Scorpionoidea Latreille 1802 (Chelicerata, Scorpiones): An exemplar approach. Cladistics 16, 1–78 (2000).
Tikader, B. K., & Bastawade, D. B. Fauna of India. Vol. 3. Scorpions (Scorpionida: Arachnida). Zoological Survey of India. 671 (Sangam Press, Pune, 1983).
Fet, V. Family Scorpionidae Latreille, 1802. In Catalog of the Scorpions of the World (1758–1998) (Fet, V., Sissom, W. D., Lowe, G. & Braunwalder, M. E.) 427–486 (The New York Entomological Society, New York, 2000).
Prendini, L. & Loria, S. F. Systematic revision of the Asian forest scorpions (Heterometrinae Simon, 1879), revised suprageneric classification of Scorpionidae Latreille, 1802, and revalidation of Rugodentidae Bastawade et al., 2005. Bull. Am. Mus. Nat. Hist. 442, 1–480 (2020).
Tahir, H. M. & Prendini, L. Redescription of Heterometrus latimanus and confirmation of the genus Heterometrus (Scorpiones: Scorpionidae) in Pakistan. Am. Mus. Novit. 3805, 1–23 (2014).
Prendini, L. Substratum specialization and speciation in southern African scorpions: The Effect Hypothesis revisited. In Scorpions 2001. In Memoriam Gary A. Polis (eds. Fet, V. & Selden, P. A.) 113–138 (British Arachnological Society, Burnham Beeches, Bucks, U.K., 2001).
Pocock, R. I. Scorpions and their geographical distribution. Nat. Sci. 4, 353–364 (1894).
Kraepelin, K. Die geographische Verbreitung der Skorpione. Zool. Jahrb. Abt. Syst. Geog. Biol. Tiere 22, 321–364 (1905).
Werner, F. Scorpiones, Pedipalpi. In Klassen und Ordnungen des Tierreichs. (ed. Bronn, H. G.) 5, IV, 8, Lief. 1–2, Scorpiones, 1–316 (Akademische Verlaggesellschaft, Leipzig, 1934).
Sissom, W. D. Systematics, biogeography and paleontology. In The Biology of Scorpions (ed. Polis, G. A.) 64–160 (Stanford University Press, Stanford, CA, 1990).
Mani, M. S. (ed.) Ecology and Biogeography in India. 400 (Dr. W. Junk, The Hague, 1974).
Vences, M., Freyhof, J., Sonnenberg, R., Kosuch, J. & Veith, M. Reconciling fossils and molecules: Cenozoic divergence of cichlid fishes and the biogeography of Madagascar. J. Biogeogr. 28, 1091–1099 (2001).
Toon, A. et al. Gondwanan radiation of the Southern Hemisphere crayfishes (Decapoda: Parastacidae): Evidence from fossils and molecules. J. Biogeogr. 37, 2275–2290 (2010).
Norton, I. O. & Sclater, J. G. A model for the evolution of the Indian Ocean and the breakup of Gondwanaland. J. Geophys. Res. Solid Earth 84, 6803–6830 (1979).
Masters, J. C., De Wit, M. J. & Asher, R. J. Reconciling the origins of Africa, India and Madagascar with vertebrate dispersal scenarios. Folia Primatol. 77, 399–418 (2006).
McLoughlin, S. The breakup history of Gondwana and its impact on pre-Cenozoic floristic provincialism. Aust. J. Bot. 49, 271–300 (2001).
Jokat, W., Boebel, T., König, M. & Meyer, U. Timing and geometry of early Gondwana breakup. J. Geophys. Res. Solid Earth 108, 2428. https://doi.org/10.1029/2002JB001802 (2003).
Jokat, W., Ritzmann, O., Reichert, C. & Hinz, K. Deep crustal structure of the continental margin off the Explora Escarpment and in the Lazarev Sea, East Antarctica. Mar. Geophys. Res. 25, 283–304 (2004).
Rabinowitz, P. D., Coffin, M. F. & Falvey, D. The separation of Madagascar and Africa. Science 220, 67–69 (1983).
Cracraft, J. Avian evolution, Gondwana biogeography and the Cretaceous-Tertiary mass extinction event. Proc. R. Soc. Lond. B Biol. Sci. 268, 459–469 (2001).
Chatterjee, S. & Scotese, C. R. The breakup of Gondwana and the evolution and biogeography of the Indian plate. Proc. Indian Natl. Sci. Acad. 65, 397–426 (1999).
Briggs, J. C. The biogeographic and tectonic history of India. J. Biogeogr. 30, 381–388 (2003).
Van Bocxlaer, I., Roelants, K., Biju, S. D., Nagaraju, J. & Bossuyt, F. Late Cretaceous vicariance in Gondwanan amphibians. PLoS ONE 1, e74. https://doi.org/10.1371/journal.pone.0000074 (2006).
Chatterjee, S. & Scotese, C. R. The wandering Indian plate and its changing biogeography during the Late Cretaceous-Early Tertiary period. In New Aspects of Mesozoic Biodiversity (ed. Bandopadhyay, S.) 105–126 (Springer, Berlin, 2010).
Prasad, G. V. et al. First mammal evidence from the Late Cretaceous of India for biotic dispersal between India and Africa at the KT transition. C.R. Palevol 9, 63–71 (2010).
Yuan, Z.-Y. et al. Natatanuran frogs used the Indian Plate to step-stone disperse and radiate across the Indian Ocean. Natl. Sci. Rev. 6, 10–14 (2019).
Sharland, P. R., Casey, D. M., Davies, R. B., Simmons, M. D. & Sutcliffe, O. E. Arabian plate sequence stratigraphy–revisions to SP2. GeoArabia 9, 199–214 (2004).
Chatterjee, S., Goswami, A. & Scotese, C. R. The longest voyage: Tectonic, magmatic, and paleoclimatic evolution of the Indian plate during its northward flight from Gondwana to Asia. Gondwana Res. 23, 238–267 (2013).
Chatterjee, S., Scotese, C. R. & Bajpai, S. The restless Indian Plate and its epic voyage from Gondwana to Asia: Its tectonic, paleoclimatic, and paleobiogeographic evolution. Geol. Soc. Am. 529, 1–147 (2017).
Chatterjee, S. India’s northward drift from Gondwana to Asia during the Late Cretaceous-Eocene. Proc. Indian Natl. Sci. Acad. 82, 479–487 (2016).
Newlands, G. Zoogeographical factors involved in the trans-Atlantic dispersal pattern of the genus Opisthacanthus Peters (Arachnida; Scorpionidae). Ann. Transv. Mus. 28, 91–98, pl. 1: 1–7 (1973).
Prendini, L. Scorpion diversity and distribution in southern Africa: Pattern and process. In African Biodiversity: Molecules, Organisms, Ecosystems. Proceedings of the 5th International Symposium on Tropical Biology, Museum Alexander Koenig, Bonn (eds. Huber, B. A., Sinclair, B. J. & Lampe, K.-H.) 25–68 (Springer, New York, 2005).
Monod, L. & Prendini, L. Evidence for Eurogondwana: The roles of dispersal, extinction, and vicariance in the evolution and biogeography of Indo-Pacific Hormuridae (Scorpiones: Scorpionoidea). Cladistics 31, 71–111 (2015).
Manchester, S. R., Kapgate, D. K. & Wen, J. Oldest fruits of the grape family (Vitaceae) from the Late Cretaceous Deccan cherts of India. Am. J. Bot. 100, 1849–1859 (2013).
Klaus, S., Morley, R. J., Plath, M., Zhang, Y.-P. & Li, J.-T. Biotic interchange between the Indian subcontinent and mainland Asia through time. Nat. Commun. 7, 12132. https://doi.org/10.1038/ncomms12132 (2016).
Guo, Z. T. et al. A major reorganization of Asian climate regime by the Early Miocene. Clim. Past Discus. 4, 535–584 (2008).
Beerling, D. J. & Osborne, C. P. The origin of the savanna biome. Global Change Biol. 12, 2023–2031 (2006).
Jacobs, B. F., Kingston, J. D. & Jacobs, L. L. The origin of grass-dominated ecosystems. Ann. MO Bot. Gard. 86, 590–643 (1999).
Loria, S. F. & Prendini, L. Burrowing into the forest: Phylogeny of the Asian forest scorpions (Scorpionidae: Heterometrinae) and the evolution of ecomorphotypes. Cladistics. https://doi.org/10.1111/cla.12434 (2020).
Metcalfe, I. Tectonic framework and Phanerozoic evolution of Sundaland. Gondwana Res. 19, 3–21 (2011).
Ezcurra, M. D. & Agnolín, F. L. A new global palaeobiogeographical model for the Late Mesozoic and Early Tertiary. Syst. Biol. 61, 553–566 (2012).
Bosellini, A. Dinosaurs “re-write” the geodynamics of the eastern Mediterranean and the paleogeography of the Apulia Platform. Earth Sci. Rev. 59, 211–234 (2002).
Sharma, P. P. et al. A revised dated phylogeny of scorpions: Phylogenomic support for ancient divergence of the temperate Gondwanan family Bothriuridae. Mol. Phylogenet. Evol. 122, 37–45 (2018).
Santibáñez-López, C. E., Kriebel, R. & Sharma, P. P. eadem figura manet: Measuring morphological convergence in diplocentrid scorpions (Arachnida: Scorpiones: Diplocentridae) under a multilocus phylogenetic framework. Invertebr. Syst. 31, 233–248 (2017).
Santibáñez-López, C. E. et al. Integration of phylogenomics and molecular modeling reveals lineage specific diversification of toxins in scorpions. PeerJ 6, e5902. https://doi.org/10.7717/peerj.5902 (2018).
Clouse, R. M. & Giribet, G. When Thailand was an island—the phylogeny and biogeography of mite harvestmen (Opiliones, Cyphophthalmi, Stylocellidae) in Southeast Asia. J. Biogeogr. 37, 1114–1130 (2010).
Santibáñez-López, C. E., Francke, O. F. & Prendini, L. Phylogeny of the North American scorpion genus Diplocentrus Peters, 1861 (Scorpiones: Diplocentridae) based on morphology, nuclear and mitochondrial DNA. Arthropod Syst. Phylo. 72, 257–279 (2014).
Ceccarelli, F. S. et al. Andean uplift drives diversification of the bothriurid scorpion genus Brachistosternus. J. Biogeogr. 43, 1942–1954 (2016).
Ojanguren-Affilastro, A. A. et al. Phylogeny, species delimitation and convergence in the South American bothriurid scorpion genus Brachistosternus Pocock, 1893: Integrating morphology, nuclear, and mitochondrial DNA. Mol. Phylogenet. Evol. 94, 159–170 (2016).
Esposito, L. A., Yamaguti, H. Y., Pinto-da-Rocha, R. & Prendini, L. Plucking with the plectrum: Phylogeny of the New World buthid scorpion subfamily Centruroidinae Kraus, 1955 (Scorpiones: Buthidae) reveals evolution of three pecten-sternite stridulation organs. Arthropod Syst. Phylo. 76, 87–122 (2018).
Cock, P. J. A. et al. Biopython: Freely available Python tools for computational molecular biology and bioinformatics. Bioinformatics 25, 1422–1423 (2009).
Katoh, K., Misawa, K., Kuma, K. & Miyata, T. MAFFT: A novel method for rapid multiple alignment based on fast Fourier transform. Nucleic Acids Res. 30, 3059–3066 (2002).
Katoh, K. & Standley, D. M. MAFFT multiple sequence alignment software version 7: Improvements in performance and usability. Mol. Biol. Evol. 30, 772–780 (2013).
Katoh, K. & Toh, H. Recent developments in the MAFFT multiple sequence alignment program. Brief. Bioinform. 9, 286–298 (2008).
Kumar, S., Stecher, G. & Tamura, K. MEGA7: Molecular evolutionary genetics analysis version 7.0 for bigger datasets. Mol. Biol. Evol. 33, 1870–1874 (2016).
Drummond, A. J., Suchard, M. A., Xie, D. & Rambaut, A. Bayesian phylogenetics with BEAUti and the BEAST 1.7. Mol. Biol. Evol. 29, 1969–1973 (2012).
Darriba, D., Taboada, G. L., Doallo, R. & Posada, D. jModelTest 2: More models, new heuristics and parallel computing. Nat. Methods 9, 772. https://doi.org/10.1038/nmeth.2109 (2012).
Miller, M., Pfeiffer, W. & Schwartz, T. Creating the CIPRES Science Gateway for inference of large phylogenetic trees. Proceedings of the Gateway Computing Environments Workshop (GCE), New Orleans, LA, 14 November 2010, 1–8 (2010).
Gantenbein, B. & Largiadèr, C. R. Mesobuthus gibbosus (Scorpiones: Buthidae) on the island of Rhodes—hybridization between Ulysses’ stowaways and native scorpions? Mol. Ecol. 11, 925–938 (2002).
Gantenbein, B., Fet, V., Gantenbein-Ritter, I. A. & Balloux, F. Evidence for recombination in scorpion mitochondrial DNA (Scorpiones: Buthidae). Proc. R. Soc. Lond. B Biol. Sci. 272, 697–704 (2005).
Graham, M. R., Stoev, P., Akkari, N., Blagoev, G. & Fet, V. Euscorpius sicanus (Scorpiones: Euscorpiidae) from Tunisia: DNA barcoding confirms ancient disjunctions across the Mediterranean Sea. Serket 13, 16–26 (2012).
Graham, M. R., Jaeger, J. R., Prendini, L. & Riddle, B. R. Phylogeography of the Arizona hairy scorpion (Hadrurus arizonensis) supports a model of biotic assembly in the Mojave Desert and adds a new Pleistocene refugium. J. Biogeogr. 40, 1298–1312 (2013).
Graham, M. R., Jaeger, J. R., Prendini, L. & Riddle, B. R. Phylogeography of Beck’s desert scorpion, Paruroctonus becki, reveals Pliocene diversification in the eastern California Shear Zone and postglacial expansion in the Great Basin Desert. Mol. Phylogenet. Evol. 69, 502–513 (2013).
Bryson, R. W. Jr., Riddle, B. R., Graham, M. R., Smith, B. T. & Prendini, L. As old as the hills: Montane scorpions in southwestern North America reveal ancient associations between biotic diversification and landscape history. PLoS ONE 8, e52822. https://doi.org/10.1371/journal.pone.0052822 (2013).
Bryson, R. W. Jr., Prendini, L., Savary, W. E. & Pearman, P. B. Caves as microrefugia: Pleistocene phylogeography of the troglophilic North American scorpion Pseudouroctonus reddelli. BMC Evol. Biol. 14, 9. https://doi.org/10.1186/1471-2148-14-9 (2014).
Bryson, R. W. Jr., Savary, W. E. & Prendini, L. Biogeography of scorpions in the Pseudouroctonus minimus complex (Vaejovidae) from south-western North America: Implications of ecological specialization for pre-Quaternary diversification. J. Biogeogr. 40, 1850–1860 (2013).
Graham, M. R., Wood, D. A., Henault, J. A., Valois, Z. J. & Cushing, P. E. Ancient lakes, Pleistocene climates and river avulsions structure the phylogeography of a large but little-known rock scorpion from the Mojave and Sonoran deserts. Biol. J. Linn. Soc. 122, 133–146 (2017).
Graham, M. R., Myers, E. A., Kaiser, R. C. & Fet, V. Cryptic species and co-diversification in sand scorpions from the Karakum and Kyzylkum deserts of Central Asia. Zool. Scr. 48, 801–812 (2019).
Rambaut, A., Suchard, M. A., Xie, D. & Drummond, A. J. Tracer 1.6. Available at http://beast.bio.ed.ac.uk/tracer (2014).
Matzke, N. J. BioGeoBEARS: BioGeography with Bayesian (and Likelihood) Evolutionary Analysis in R Scripts. R package, version 0.2.1. Available at http://CRAN.R-project.org/package=BioGeoBEARS (2013).
Matzke, N. J. Probabilistic historical biogeography: New models for founder-event speciation, imperfect detection, and fossils allow improved accuracy and model-testing. Front. Biogeogr. 5, 242–248 (2013).
Matzke, N. J. Model selection in historical biogeography reveals that founder-event speciation is a crucial process in island clades. Syst. Biol. 63, 951–970 (2014).
R Core Team. R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. Available at https://www.R-project.org (2019).
Woodruff, D. S. Biogeography and conservation in Southeast Asia: How 2.7 million years of repeated environmental fluctuations affect today’s patterns and the future of the remaining refugial-phase biodiversity. Biodivers. Conserv. 19, 919–941 (2010).
Bird, M. I. et al. A long record of environmental change from bat guano deposits in Makangit Cave, Palawan, Philippines. Earth Environ. Sci. Trans. R. Soc. Edinb. 98, 59–69 (2007).
Faith, D. P. Systematics and conservation: On predicting the feature diversity of subsets of taxa. Cladistics 8, 361–373 (1992).
Miklós, I. & Podani, J. Randomization of presence–absence matrices: Comments and new algorithms. Ecology 85, 86–92 (2004).
Kembel, S. W. et al. Picante: R tools for integrating phylogenies and ecology. Bioinformatics 26, 1463–1464 (2010).
Isaac, N. J. B., Turvey, S. T., Collen, B., Waterman, C. & Baillie, J. E. M. Mammals on the EDGE: Conservation priorities based on threat and phylogeny. PLoS ONE 2, e296. https://doi.org/10.1371/journal.pone.0000296 (2007).
Redding, D. W. & Mooers, A. Ø. Incorporating evolutionary measures into conservation prioritization. Conserv. Biol. 20, 1670–1678 (2006).
Rabosky, D. L. & Schliep, K. Laser: Likelihood analysis of speciation/extinction rates from phylogenies, 2.4-1. R package version, Available at https://cran.r-project.org/src/contrib/Archive/laser (2013).
Rabosky, D. L. & Lovette, I. J. Density-dependent diversification in North American wood warblers. Proc. R. Soc. Lond. B Biol. Sci. 275, 2363–2371 (2008).
Pybus, O. G. & Harvey, P. H. Testing macro-evolutionary models using incomplete molecular phylogenies. Proc. R. Soc. Lond. B Biol. Sci. 267, 2267–2272 (2000).
Paradis, E., Claude, J. & Strimmer, K. APE: Analyses of phylogenetics and evolution in R language. Bioinformatics 20, 289–290 (2004).
Revell, L. J. phytools: An R package for phylogenetic comparative biology (and other things). Methods Ecol. Evol. 3, 217–223 (2012).
Rabosky, D. L. Automatic detection of key innovations, rate shifts, and diversity-dependence on phylogenetic trees. PLoS ONE 9, e89543. https://doi.org/10.1371/journal.pone.0089543 (2014).
Rabosky, D. L. et al. BAMM tools: An R package for the analysis of evolutionary dynamics on phylogenetic trees. Methods Ecol. Evol. 5, 701–707 (2014).
Kass, R. E. & Raftery, A. E. Bayes factors. J. Am. Stat. Assoc. 90, 773–795 (1995).
Acknowledgments
S.F.L. was financially supported by a fellowship from the AMNH Richard Gilder Graduate, a National Science Foundation (NSF) Graduate Research Fellowship, and NSF grant DEB 1655050. Aspects of this research were funded by the following awards: NSF Doctoral Dissertation Improvement grant DEC 1310855 to L.P. and S.F.L.; NSF grants EAR 0228699, DEB 0413453 and DEB 1655050 to L.P.; U.S.-Israel Binational Science Foundation grant 2014046 to L.P.; a grant from the Richard Lounsbery Foundation to L.P.; two Constantine S. Niarchos Expedition grants from the Stavros Niarchos Foundation to L.P.; a grant from the Exploration Fund of the Explorer’s Club to S.F.L.; a Young Explorers Grant 9816-15 from the National Geographic Society to S.F.L. Travel by L.P. to Israel was funded by the Israel Taxonomy Initiative; to Pakistan by the Visiting Scholar’s Program of the Higher Education Commission, Pakistan; to collections in South Africa by a grant from the JRS Biodiversity Foundation; and to collections in the U.K. by The Systematics Association and Oxford University. The Habitat Foundation funded S.F.L. to participate in the BioBlitz on Penang Island, Malaysia. The authors acknowledge the following friends and colleagues for assistance with fieldwork and/or donations of material used in the study: M. Ahsan, A. Ang, S. M. Aye, M. Ayyub, M. Azhar, S. Basi, S. P. Benjamin, T. L. Bird, B. T. Chin, S. H. Chit, R. Datta, L. A. Esposito, E. Gefen, A. Goncharov, C. E. Griswold, M. S. Harvey, P. Horsley, S. Huber, J. Huff, M. Hussain, M. Irfan, P. Jäger, A. Khan, S. Kangnavong, P. Kanyavong, T. Kroes, E. Leong, M. M. Locke, Y. Lubin, P. Menon, L. Ngo, J. Ove Rein, F. Paul, C. Rahmadi, M. Roppo, P. Schwendinger, M. Seiter, M. Shahid, F. Somma, H. M. Tahir, M. Teo, A. Tietz, A. Ullrich, R. C. West, R. Wickramarachchi, and the late J. Visser; the following colleagues and agencies for facilitating permissions to collect and export material: V. Khem and T. Chea (Cambodia), C. Rahmadi (Indonesia), B. T. Chin, P. C. Ee, C. Lee and N. A. B. Umar (Malaysia), T. Win and officials at Hlawga National Park (Myanmar), H. M. Tahir (Pakistan), S. Lai (Singapore), S. P. Benjamin (Sri Lanka), N. Warrit (Thailand), D.-S. Pham (Vietnam); the following curators and collections managers for loaning material from the collections in their care and/or assistance during our visits: J. Beccaloni and E. McAlister (BMNH), L. A. Esposito, C. E. Griswold and D. Ubick (CAS), P. Sierwald and C. Maier (FMNH), W. Wang (LKC), M. Tavano (MCSNG), G. Giribet and L. Leibensperger (MCZ), M. Judson, C. Rollard and E.-A. Leguin (MNHN), W. Dekoninck (MRHNB), C. Hörweg and M. Seiter (NHMW), D. Herbert, B. Muller and C. Stoffels (NM), C. Jonsson (NMG), T. Kronestedt, J. Ferrer and G. Lindberg (NRS), J. Goud (RMNH), S. van Noort, M. Cochrane, D. Larsen and A. Mayekiso (SAM), P. Jäger and J. Altmann (SMF), A. Ndaba and the late M. Krüger (TM), J. Coddington, H. Wood and D. DeRoche (USNM), M. S. Harvey (WAM), J. Dunlop (ZMB), D. Harms and N. Dupérré (ZMH), N. Scharff and J. Pedersen (ZMUC); P. Colmenares, O. Delgado, P. Horsley, J. Huff, M. M. Locke and L. Sorkin for logistical support with collections at AMNH; D. Sherwood and R. Botero-Trujillo for assistance locating type material at BMNH; the following for generating DNA sequence data used in the study: D. Casellato (supported by the Brazil Scientific Mobility Program of the Institute for International Education), D. Chin (supported by NSF grant DEB 1655050), O. Delgado, E. Goetz and S. Reiter (supported by the AMNH Science Research Mentoring Program), V. Ehrenthal, P. Rubi and T. Sharma; iNaturalist (https://www.inaturalist.org), S. Averroes Supartha, Z. Mirza, S. K. Yung, J. Shanks, P. Raja and Karthik S. for the use of live habitus photos; S. Thurston for preparing the plates for this contribution; S. Perkins, D. Grimaldi, and S. Ceccarelli for assistance with analyses and/or comments on preliminary results of the project; two reviewers for comments on a draft of the manuscript.
Author information
Authors and Affiliations
Contributions
S.F.L. and L.P. conceived the project and obtained the samples, S.F.L. performed most of the labwork and conducted the analyses, and S.F.L. and L.P. interpreted the results and wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Loria, S.F., Prendini, L. Out of India, thrice: diversification of Asian forest scorpions reveals three colonizations of Southeast Asia. Sci Rep 10, 22301 (2020). https://doi.org/10.1038/s41598-020-78183-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-78183-8
- Springer Nature Limited