Abstract
The aim was to assess the role of Metabolic Syndrome (MetS) diagnostic markers, recommended by three different guidelines, in the prediction of hyperglycemia in pregnancy. This cross-sectional cohort study included 506 non-diabetic women, with a singleton pregnancy, who underwent a diagnostic test for hyperglycemia at 24–28 weeks. Clinical, anthropometric, and laboratory data were obtained. The relationship between MetS markers and the risk of hyperglycemia was evaluated by backward stepwise logistic regression analysis (OR, 95% CI). The limit of statistical significance was 95% (p < 0.05). Triglycerides (TG) ≥ 150 mg/dL, blood pressure (BP) ≥ 130/85 mmHg, fasting glucose (FG) ≥ 100 mg/dL, and waist circumference (WC) > 88 cm were identified as independent risk factors for hyperglycemia in pregnancy. These results might help the selective screening of hyperglycemia in pregnancy.
Similar content being viewed by others
Introduction
Metabolic syndrome (MetS) is a clustering of clinical and laboratory abnormalities that include central obesity, insulin resistance, hypertension, hyperglycemia and dyslipidemia (elevated triglycerides and reduced HDL-cholesterol levels). MetS represents a high socioeconomic burden as it significantly increases the risk of cardiovascular disease (CVD), and metabolic disorders such as type 2 diabetes (DM2)1,2,3. In 2008, Bartha et al.4 proposed cutoff values for the diagnosis of MetS during pregnancy.
Gestational diabetes mellitus (GDM) is defined as any degree of glucose intolerance diagnosed in the second and third trimesters of pregnancy with different degrees of maternal hyperglycemia5. GDM is the most common metabolic disorder of pregnancy, and it shows facets of MetS including obesity, insulin resistance, and dyslipidemia6. Additionally, GDM is associated with adverse short- and long-term effects on both the mother and offspring7,8, and increased risk for DM2, MetS and CVD9.
In milder forms of GDM, which do not fully meet diagnostic criteria, hyperglycemia and adverse effects to the mother and offspring are also present10. In the Perinatal Diabetes Research Centre (PDRC), Botucatu Medical School—UNESP (Sao Paulo State University), Brazil, women with a glycemic profile indicative of hyperglycemia and a normal response to glucose tolerance testing have been identified as having Mild Gestational Hyperglycemia (MGH) and offered the same treatment given to those with GDM for over two decades11,12. Today, women with MGH account for 17.3% of our cases13.
Current diagnostic guidelines distinguish diabetes mellitus during pregnancy that is diagnosed before 20 weeks of pregnancy from GDM, which is identified during the second and third trimesters. Most frequently, universal screening is performed by initially measuring FG during the initial visit for prenatal care (before 20 weeks of pregnancy). When neither diabetes in pregnancy (FG ≥ 126 mg/dL) nor GDM (FG ≥ 92 and < 126 mg/dL) is detected, a 75 g-OGTT is offered at 24–28 weeks5,7,14,15,16. However, the cost-effectiveness of this strategy, as well as its effects on short- or long-term maternal and offspring outcomes remain unknown and evidence supporting its use is still insufficient17.
Recognized risk factors for GDM include advanced maternal age, family history of diabetes, history of GDM, history of macrosomia, non-Caucasian race or ethinicity, excessive weight gain or obesity during pregnancy, and smoking (actively or passively). However, as the prevalence of such risk factors among women with GDM is low (at most 50%)18,19 and their predictive accuracy is poor, this may lead to unnecessary testing17,20.
The association of MetS with maternal hyperglycemia has been documented since the past decade21, and MetS has been shown to be more frequent in women with GDM4,21. Studies of BMI, waist circumference (WC), and lipid profile components as independent predictors of GDM have yielded conflicting results22,23,24,25. However, to the best of our knowledge, the relationship between the MetS markers recommended by different sets of guidelines2,3,4 and risk of MGH or GDM remains uninvestigated. Thus, the objective of this study was to assess the role of the MetS diagnostic markers proposed in three different sets of guidelines—International Diabetes Federation (IDF)2, National Cholesterol Education Program’s Adult Treatment Panel III (NCEP-ATP III)3, and Bartha et al.4, in the prediction of hyperglycemia (MGH or GDM) in pregnancy.
Methods
Study setting and design
This cross-sectional cohort study, undertaken from March 2014 to December 2016, included women with a singleton pregnancy who underwent a 75 g-Oral Glucose Tolerance Test (75 g-OGTT) and Glucose Profile (GP) testing between 24 and 28 weeks of pregnancy. The Mets components were evaluated in the inclusion of the study and previously to diagnostic tests. Women with a previous diagnosis of type 1 or type 2 DM, as well as those diagnosed with overt diabetes or GDM before 20 weeks of pregnancy were excluded5.
The study was conducted in the Botucatu Public Health Network (the primary care) and in the PDCR–Botucatu Medical School/UNESP, Brazil, our referral center (tertiary care). The study protocol was approved by the Human Subject Research Ethics Committee of Botucatu Medical School/Unesp (# 3900-2011; Of. No. 244/11). All methods were performed in accordance with the principles of the Brazilian National Heath Council (Resolution CNS 466/12) in compliance with local/institutional guidelines and regulations in all stages of this study.
Sample size
The sample size was based on the prevalence of maternal hyperglycemia between 15 and 20%13,17, the risk of 4.21 for hyperglycemia when pre-BMI ≥ 25 kg/m222, a type 2 error of 20%, and a 95% confidence level. According to the assumed prevalence, the minimum sample size resulted in 258 and 194, respectively. A total of 506 pregnant women were included, 283 with gestational age < 24 and 223 with gestational age ≥ 24 weeks.
Data collection
At enrollment, all participants were asked to answer a specific structured questionnaire for the collection of epidemiological and clinical data. These included information on prepregnancy weight, used for the calculus of the prepregnancy BMI, weight gain, and family and personal obstetric risk factors for GDM5,18,26. Incomplete or missing information was recovered from the participant’s prenatal care chart. Also at enrollment, were collected data on blood pressure, height and current weight, to calculate the gestational BMI, and waist circumference27, besides blood collection for the analysis of FG, glycated hemoglobin (HbA1c), basal insulin and complete lipid profile (LDL and HDL-cholesterol, total cholesterol and TG).
Variables
The maternal characteristics assessed included: self-reported race (white and non-white), age in complete years (categorized as < 19 years, 19–35 years, and > 35 years); number of pregnancies including current (categorized as 1 and ≥ 2 pregnancies), physical activity (No, Yes, < 150 min/week, and ≥ 150 min/week28), smoking status (yes); presence of risk factors for DM18,26, and gestacional age at enrollment (< 24 weeks, and ≥ 24 weeks).
The risk factors were defined by MetS diagnostic markers, recommended by IDF, NCEP-ATP III, and Bartha et al. guidelines2,3,4, presented follow:
MetS componentes | IDF3 | NCEP-ATP III2 | Bartha et al.4 |
|---|---|---|---|
Central obesity: waist circumference (WC; cm) OR pregestational BMI (Kg/m2) | Central obesity: WC ≥ 80 OR pregest BMI ≥ 30 | > 88 | Abdominal obesity, given as WC > 2 S.D. for gestational age in the first half of pregnancy OR pregest BMI > 30 |
Triglycerides (mg/dL) | ≥ 150 | ≥ 150 | ≥ 2 S.D. for gestational age |
HDL-cholesterol (mg/dL) | < 50 | < 50 | < 2 S.D. for gestational age |
Blood pressure (mmHg) | ≥ 130 / ≥ 85 | ≥ 130 / ≥ 85 | ≥ 130 / ≥ 85 |
Fasting glicose (mg/dL) | ≥ 100 OR T2DM | ≥ 110 | ≥ 105 |
MetS criteria | Central obesity plus any two of the four factors | Any three or more factors | Any three of the five factors |
GDM and MGH diagnoses
All pregnant women included in the study underwent 75-g oral glucose tolerance test (75 g-OGTT) and glucose profile (GP) tests between 24 and 28 weeks of pregnancy.
GDM was diagnosed if there was one abnormal value (92, 180 and 153 mg/dL for fasting, one-hour and two-hour postglucose load, respectively), after a 75 g-OGTT5,7,14,15,16.
For MGH diagnosis, a GP and a 75 g-OGTT were performed during a 1-day hospital stay with the woman on a 2840 kcal-diet fractionated in five meals. Plasma glucose was measured every two hours, from 8 AM to 6 PM. The thresholds used were 90 mg/dL for fasting (8 h) and 130 mg/dL for any postprandial level. MGH was confirmed when response to 75 g-OGTT was normal and one GP measure was equal or greater than threshold values11,12.
Follow-up
Non-diabetic women were followed up at their original primary care center. Women with MGH or GDM were followed up at the PDCR–Botucatu Medical School/UNESP, a tertiary center. In both MGH and GDM cases, maternal hyperglycemia control was performed according to the protocol established in our center as recommended by ADA5,14.
Statistical analyses
Statistical analyses were performed using Statistical Analysis System-9.3.
The results expressed in mean (m) and standard deviation (sd) with symmetric distribution were compared by one-way ANOVA followed by the Tukey test (75 g-OGTT and GP tests, and anthropometric measures); the Gamma test followed by the Wald test (asymmetric distribution) were used to compare means values relative to glucose and lipid profiles. Likewise, Chi-square or Exact Fisher tests (if applicable) were used to test association with MetS criteria according to the guidelines evaluated2,3,4. Two by two proportions were compared using a comparison proportion test based on the normal distribution (similar to chi-square test) by two groups (ND, MGH, and GDM).
The logistic regression model (using backward stepwise) was fitted to identify the independent risk factors for maternal hyperglycemia. It was conducted by two different approaches—one considering all 506 pregnant women included, regardless of the gestational age at enrollment, and another based on metabolic phases of pregnancy, that is < 24 and ≥ 24 weeks in the study inclusion. Here, MGH and GDM were assessed as a unique condition—maternal hyperglycemia as the response variable in function of the MetS diagnostic markers2,3,4. The odds ratio (OR) and 95% confidence intervals (95% CI) were calculated for each MetS diagnostic markers and adjusted for all other variables within the respective guideline. The initial model included all variables/MetS markers, and the variables were excluded one by one until reaching the final model, defined by the impossibility to exclude any other variable without significant loss in adjustment. The backward elimination criterion was 5%.
Ethical considerations
The study protocol was approved by the Human Subject Research Ethics Committee of Botucatu Medical School/Unesp (# 3900-2011; Of. No. 244/11). All subjects signed an informed consent form before inclusion in the present study.
Consent for publication
All authors approved the current version and agreed to submit for publication.
Results
According to 75 g-OGTT response, study participants were assigned to three groups: Non-diabetic (ND, normal 75 g-OGTT and GP); MGH (normal 75 g-OGTT + abnormal GP); and GDM (abnormal 75 g-OGTT + abnormal GP).
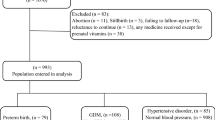
The study flowchart (Fig. 1) shows the number of pregnant women included (N = 517), excluded (N = 3), and withdrawn from the study (N = 8), as well as the number of participants in each group—ND (N = 430), MGH (N = 30) and GDM (N = 46). Of the 506 women assessed, 283 (55.9%) were included before 24 weeks and 223 (44.1%) at 24–30 weeks of pregnancy.
The characteristics of the 506 study participants are shown in Table 1. A personal and obstetric history of hypertension (6.5%) and macrossomia (5.1%), and a family history of GDM (58.1%) and hypertension (63.2%) were the most common.
Table 2 shows that the glucose levels in response to diagnostic tests were different among groups, with higher values in the GDM group. Maternal height and the levels of total cholesterol and HDL-c were similar in all groups. The remaining anthropometric and metabolic variables were lower in ND than in the other groups.
MetS frequency, as evaluated using NCEP-ATP III2 criteria, was 7.0% in ND, 16.7% in MGH, and 21.7% in GDM (p = 0.0011).
Regarding the MetS diagnostic markers defined by IDF3, the proportion of women with HDL-cholesterol < 50 mg/dL and WC ≥ 80 cm did not differ among groups. The proportion of women with TG ≥ 150 mg/dL, BP ≥ 130/85 mmHg, FG ≥ 100 mg/dL and pregestational BMI ≥ 30 kg/m2 was statiscally different among groups with higher values in GDM.
According to NCEP-ATP III2 MetS protocol, only the frequency of HDL-cholesterol < 50 mg/dL did not differ among groups, while the proportion of women with TG ≥ 150 mg/dL, BP ≥ 130/85 mmHg, FG ≥ 110 mg/dL and WC > 88 cm was always higher in GDM. Similar results were obtained with the markers recommended by Bartha et al.4, adapted for pregnancy (Table 3).
Considering all 506 pregnant women, the logistic regression analysis revealed that TG ≥ 150 mg/dL, BP ≥ 130/85 mmHg, FG ≥ 100 mg/dL and WC > 88 cm are independent risk factors for MGH and GDM, whereas HDL-c < 50 mg/dL and pregestational BMI ≥ 30 kg/m2 are not associated with MGH or GDM risk. In contrast, gestational BMI ≥ 30 kg/m2, was an independent risk factor for MGH and GDM (OR = 2.796; 95% CI 1.213–6.446) (Table 4).
According to the gestational age at enrollment, the logistic regression analysis indicated only WC > 88 cm (OR = 2.720; 95% CI 1.113–6.647) as an independent risk factor for MGH and GDM before 24 weeks of pregnancy. At ≥ 24 weeks, TG ≥ 150 mg/dL, BP ≥ 130/85 mmHg and FG ≥ 105 mg/dL (OR = 20.889; 95% CI 1.580–276.104) or ≥ 100 mg/dL (OR = 19.845; 95% CI 1.845–213.493) were identified as independent risk factors for hyperglycemia during pregnancy. In this gestational age, no woman showed WC < 88 or 80 cm (Table 4).
Discussion
This study evaluated the role of MetS diagnostic markers recommended by different sets of guidelines2,3,4 in the prediction of MGH and GDM risk. In the comparison among groups, the proportion of women with TG ≥ 150 mg/dL, BP ≥ 130/85 mmHg, FG ≥ 100 mg/dL, WC > 88 cm, and pregestational BMI ≥ 30 kg/m2 were larger in GDM than in ND group. In the MGH group, these proportions were statistically comparable to those seen in ND and GDM groups. The thresholds HDL-c > 30.54 or 50 mg/dL2,3 and CC ≥ 80 cm3 did not differentiate the proportion of pregnant women with hyperglycemia from those without. These findings reinforce the proportional relation between MetS and glucose status, as previously reported by our team21,29.
Considering all 506 pregnant women, the logistic regression analysis indicate that TG ≥ 150 mg/dL2,3, BP ≥ 130/85 mmHg2,3, FG ≥ 100 mg/dL2 and WC > 88 cm2 were identified as independent predictors of MGH or GDM (Table 4). Therefore, according to the physiopathology that points the insulin resistance as the common base to GDM and MetS3, these values seem adequate for the diagnosis of MetS during pregnancy. On the other hand, HDL-c < 502,3 or < 30.6 mg/dL4, pre-BMI ≥ 30 kg/m23,4 and WC ≥ 80 cm3 or > 126.1 cm4 showed no predictive value to maternal hyperglycemia. However, news values of HDL-c and pregestational BMI should be proposed to MetS criteria in pregnancy.
Before 24 weeks, only WC > 88 cm and TG ≥ 300 mg/dL were independently predictive of risk for MGH or GDM. At 24–30 weeks, TG ≥ 150 mg/dL, BP ≥ 130/85 mmHg and FG ≥ 100 mg/dL were identified as independent predictors of MGH or GDM risk. On the other hand, HDL-c < 50 mg/dL and pregestational BMI ≥ 30 kg/m2 showed no predictive value at any gestational age.
Some of the markers evaluated in this study have been previously investigated, but individually and independently from MetS diagnostic guidelines2,3,4. Nonetheless, according to these previous studies, pregestational BMI ≥ 25 kg/m2 and WC ≥ 88 cm are predictive of GDM risk22; MetS occurrence is directly associated with the level of glucose intolerance21,22,23,24,25,26,27,28,29; obesity (pregestational BMI ≥ 30Kg/m2) is associated with MGH or GDM risk, and gestational HbA1c ≥ 6.5%23, TG ≥ 137 and 182 mg/dL increase the risk of GDM30.
Our findings are consistent with those reported in the literature, but our data regarding FG, BMI and HDL-c are worth of note. The FG values ≥ 100, 105 and 110 mg/dL established in the guidelines for MetS diagnosis2,3,4 are beyond the limit of 92 mg/dL recommended for the diagnosis of GDM5,7,14,15,16. Thus, these results have no clinical application and indicate that FG values should be recalculated and adapted for the diagnosis of MetS during gestation.
In our study, pregestational BMI ≥ 30 kg/m2 was not found to be independently predictive of MGH or GDM risk. This was an unexpected finding as it contradicts previous studies17,20,22,23. Obesity has been progressively increasing worldwide and, as a result, a larger number of pregnant women has elevated pregestational BMI3,31. In our study population, ¼ of ND participants had pregestational BMI ≥ 30 kg/m2. Additionally, mean BMI indicated overweight (≥ 25 kg/m2) in ND and obesity (≥ 30 kg/m2) in MGH and GDM groups. This might have influenced this marker’s ability to predict MGH and GDM and points to the necessity of investigating new threshold limits to prepregnancy BMI.
Among our study participants, HDL-c < 50 mg/dL2,3,4 was not predictive of MGH or GDM. In general, total cholesterol, TG, LDL-c and VLDL-c increase in the second trimester, and are even more greatly increased in the third trimester, whereas HDL-c levels remain unchanged in the second trimester and are decreased in the third trimester. In women with GDM, reduction in lipid measures is even more accentuated24,25,32. A recent metanalysis corroborates these results and highlights the progressive increase in TG and significant reduction in HDL-c that occur in the second and third trimesters of pregnancy in women with GDM33.
In this study, mean HDL-c levels were higher than the threshold limit of 30.5 and 50 mg/dL proposed with no association with maternal glycemic status. The dynamics of lipid profile during pregnancy and the HDL-c levels observed in our population might explain the inability to predict MGH or GDM, and underscore the need for investigating new HDL-c values to be used in the diagnosis of MetS during pregnancy.
Pregestational BMI and WC have been previously identified as predictors of maternal hyperglycemia risk in Brazil22,23 and in other countries17,20,34,35,36,37,38. However, the establishment of TG ≥ 150 mg/dL as an independent predictor of risk of MGH and GDM from 24 weeks of pregnancy onward, seem to be contributions unique to this study. The literature shows conflicting results. While some studies do consider TG an independent risk predictor, but only in the first half of pregnancy, others have not associated it with GDM risk24,30,39. Nonetheless, the physiological events that take place in the second half of pregnancy—insulin resistance due to the action of the placental hormones, maternal catabolism by rising fetal demands, and a greater increase in TG levels during the third trimester33,40 seem to support our findings.
Study limitations
Although the sample size was enough, the results of the current study should be limited to its setting and population, and future studies in different centers are necessary before clinical application. In addition, gestational age at enrollment, which indicated the timing of MetS markers assessment, ranged from 5 to 30 weeks (total of 26 weeks). Before 24 weeks, gestational age at enrollment ranged from 5 to 23 weeks (total of 19 weeks), and after 24 weeks it ranged from 24 to 30 weeks (total of 7 weeks). Thus, in order to equalize the time periods, the group gestational age < 24 weeks should have been split into 5–12, 13–18, and 19–23 weeks. However, as shown in Table 2, this would reduce the number of samples in each gestational age group and weaken statistical power. In contrast, the novelty in testing the SM markers, defined by three different protocols, in the prediction of the GDM represents the strength of the our study.
Clinical implications
Our results showed that some MetS markers were identified as independent risk factors for hyperglycemia in pregnancy. So, both in the first and second half of pregnancy, TG ≥ 150 mg/dL, BP ≥ 130/85 mmHg, FG ≥ 100 mg/dL and WC > 88 cm are independent risk factors for MGH and GDM; HDL-c < 50 mg/dL and pregestational BMI ≥ 30 kg/m2 are not associated with MGH or GDM risk. In contrast, gestational BMI ≥ 30 kg/m2, was an independent risk factor for MGH and GDM.
In the clinical practice, and at any gestational age, TG ≥ 150 mg/dL, BP ≥ 130/85 mmHg, WC > 88 cm, and BMI ≥ 30 kg/m2 can be used as screeners associated with the selective diagnostic protocol of MGH or GDM. Ultimately, they would be interpreted as warning signs for hyperglycemia in pregnancy. Considering the cut-off (FG ≥ 92 mg/dL) in the current GDM diagnostic protocol (IADPSG, 2010; ADA, 2011), FG ≥ 100 mg/dL would not useful. The same argument could be done with the results obtained in the first (< 24 weeks) and the second (≥ 24 weeks) half of the pregnancy.
Research implications
Our results were defined after two different approaches, one based on any gestational age at enrollment, and another according to metabolic phases of pregnancy. Although the sample size was enough for both strategies, the gestational age variation among the subjects (Table 1) may have influenced some results. Moreover, these results are limited to a population of pregnant women with characteristics of their own. Therefore, further studies are needed to (1) reevaluate the threshold limits defined by the MetS diagnostic guidelines; (2) assess the uselfulness of these markers in the prediction of MGH or GDM risk using more restricted gestational age ranges; (3) assess the repeatability of these markers in different populations; (4) to determine the actual role of lipid profile in the physiopathology of hyperglycemia in pregnancy.
Finally, some MetS diagnostic markers recommended by different guidelines can independently predict the risk of MGH and GDM. These findings have important clinical implications as they might help to identify women at risk in the selective screening for hyperglycemia in pregnancy. However, other cut-off points were unable to predict this condition and news studies are necessary to adapt them to metabolic changes of pregnancy.
Data availability
The data sets generated and analyzed in the current study may be made available by the corresponding author if requested.
References
Kassi, E., Pervanidou, P., Kaltsas, G. & Chrousos, G. Metabolic syndrome: definitions and controversies. BMC Med. 9, 48 (2011).
Expert Panel on Detection, Evaluation and Treatment of High Blood Cholesterol in Adults. Executive Summary of the third report of the National Cholesterol Education Program (NCEP) Expert Panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III). JAMA. 18, 126–32 (2001).
International Diabetes Federation (IDF). The IDF consensus worldwide definition of the metabolic syndrome [Internet]. Brussels:IDF (2006).
Bartha, J. L. et al. Metabolic syndrome in normal and complicated pregnancies. Eur. J. Obstet. Gynecol. Reprod. Biol. 137, 178–184 (2008).
American Diabetes Association. Classification and diagnosis of diabetes. Diabetes Care 40, S11-24 (2017).
Fasshauer, M., Blüher, M. & Stumvoll, M. Adipokines in gestational diabetes. Lancet Diabetes Endocrinol. 2, 488–499 (2014).
International Association of Diabetes and Pregnancy Study Groups. Recommendations on the diagnosis and classification of hyperglycemia in pregnancy. Diabetes Care 33, 676–682 (2010).
O'Sullivan, E.P., Avalos, G., O'Reilly, M., Dennedy, M.C., Gaffney, G., Dunne. F. Atlantic DIP Collaborators. Atlantic DIP: the prevalence and consequences of gestational diabetes in Ireland. Ir. Med. J. 105, 13–5 (2012).
Vrachnis, N. et al. Previous gestational diabetes mellitus and markers of cardiovascular risk. Int. J. Endocrinol. https://doi.org/10.1155/2012/458610 (2012).
Rice, M.M., Landon, M.B. What we have learned about treating mild gestational diabetes mellitus. Semin. Perinatol. 40, 298–302. https://doi.org/10.1053/j.semperi.2016.03.006(2016).
Rudge, M. V., Peraçoli, J. C., Berezowski, A. T., Calderon, I. M. & Brasil, M. A. The oral glucose tolerance test is a poor predictor of hyperglycemia during pregnancy. Braz. J. Med. Biol. Res. 23, 1079–1089 (1990).
Rudge, M. V. C. et al. Hiperglicemia materna diária diagnosticada pelo perfil glicêmico: um problema de saúde pública materno e perinatal. Rev. Bras. Ginecol. Obstet. 27(11), 691–697 (2005).
Sirimarco, M.P. et al. Diagnostic protocol for gestational diabetes mellitus (GDM) (IADPSG/ADA, 2011): influence on the occurrence of GDM and mild gestational hyperglycemia (MGH) and on the perinatal outcomes. Diabetol. Metab. Syndr. 9, 1. https://doi.org/10.1186/s13098-016-02002 (2017).
American Diabetes Association. Standards of medical care in diabetes: 2011. Diabetes Care 34, S11-61. https://doi.org/10.2337/dc11-S011 (2011).
World Health Organization. Diagnostic criteria and classification of hyperglycaemia first detected in pregnancy (WHO, Geneva, 2013).
American College of Obstetricians and Gynecologists. Practice Bulletin No. 137: diabetes mellitus gestacional. Obstet. Gynecol. Clin. 122, 406–16. https://doi.org/10.1097/01.AOG.0000433006.09219.f1 (2013).
Farrar, D. Hyperglycemia in pregnancy: prevalence, impact, and management challenges. Int. J. Women’s Health. 8, 519–527 (2016).
Zhang, C., Rawal, S. & Chong, Y. Risk factors for gestational diabetes: is prevention possible?. Diabetologia 59, 1385–1390. https://doi.org/10.1007/s00125-016-3979-3 (2016).
Bao, W. et al. Parental smoking during pregnancy and the risk of gestational diabetes in the daughter. Int. J. Epidemiol. 45, 160–169. https://doi.org/10.1093/ije/dyv334 (2016).
Farrar, D. et al. Risk factor screening to identify women requiring oral glucose tolerance testing to diagnose gestational diabetes: a systematic review and meta-analysis and analysis of two pregnancy cohorts. PLoS ONE 12, e0175288. https://doi.org/10.1371/journal.pone.0175288 (2017).
Negrato, C. A. et al. Mild Gestacional hyperglycemia as a risk factor for metabolic syndrome in pregnancy and adverse perinatal outcomes. Diabetes Metab. Res. Rev. 24, 324–330. https://doi.org/10.1002/dmrr.815 (2008).
Bolognani, C. V., Reis, L. B. S. M., Dias, A., Rudge, M. V. C. & Calderon, I. M. P. Waist circumference in predicting gestational diabetes mellitus. J. Matern. Fetal Neonatal. Med. 27, 9438. https://doi.org/10.3109/14767058.2013.847081 (2014).
Vernini, J. et al. Maternal and fetal outcomes in pregnancies complicated by overweight and obesity. Reprod. Health. 13, 100. https://doi.org/10.1186/s12978-016-0206-0 (2016).
Ghodke, B., Pusukuru, R. & Mehta, V. Association of lipid profile in pregnancy with preeclampsia, gestational diabetes mellitus, and preterm delivery. Cureus. 9, e1420. https://doi.org/10.7759/cureus.1420 (2017).
Pusukuru, R. et al. Evaluation of lipid profile in second and third trimester of pregnancy. J. Clin. Diagn. Res. 10, QC12–6. DOI: https://doi.org/10.7860/JCDR/2016/17598.7436 (2016).
Sociedade Brasileira de Diabetes. Diretrizes da Sociedade Brasileira de Diabetes (2015–2016). Diabetes mellitus gestacional: diagnóstico, tratamento e acompanhamento pós-gestação. São Paulo: A.C. Farmacêutica. 69–73 (2016).
Ministério da Saúde (BR). Orientações para coleta e análise de dados antropométricos em serviços de saúde: norma técnica do sistema de Vigilância Alimentar e Nutricional - SISVAN. Brasília: Ministério da Saúde; Série G. Estatística e Informação em Saúde (2011).
ACOG Committee Opinion No. 650: physical activity and exercise during pregnancy and the postpartum period. Obstet Gynecol. 126, e135–e142. https://doi.org/10.1097/AOG.0000000000001214 (2015).
Negrato, C. A. et al. Association between different levels of dysglycemia and metabolic syndrome in pregnancy. Diabetol Metab Syndr. 26, 3. https://doi.org/10.1186/1758-5996-1-3 (2009).
Kumru, P. et al. Prediction of gestational diabetes mellitus at first trimester in low-risk pregnancies. Taiwan J. Obstet. Gynecol. 55, 815–820. https://doi.org/10.1016/j.tjog.2016.04.032 (2016).
American Diabetes Association. Diagnosis and classification of diabetes mellitus. Diabetes Care 34, S62–S69. https://doi.org/10.2337/dc11-S062 (2011).
Jamil, A. A. T. et al. Assessment of lipid profile in sudanese pregnant women. Key. Res. J. Biotechnol. 1, 4–15 (2013).
Ryckman, K. K., Spracklen, C. N., Smith, C. J., Robinson, J. G. & Saftlas, A. F. Maternal lipid levels during pregnancy and gestational diabetes: a systematic review and meta-analysis. BJOG 122, 643–651. https://doi.org/10.1111/1471-0528.13261 (2015).
Torloni, M. R. et al. Prepregnancy BMI and the risk of gestational diabetes: a systematic review of the literature with meta-analysis. Obes. Rev. 10, 194–203. https://doi.org/10.1111/j.1467789X.2008.00541.x (2009).
Teede, H. J., Harrison, C. L., Teh, W. T., Paul, E. & Allan, C. A. Gestational diabetes: development of an early risk prediction tool to facilitate opportunities for prevention. Aust. N Z J. Obstet. Gynaecol. 51, 499–504. https://doi.org/10.1111/j.1479-828X.2011.01356.x (2011).
Van Leeuwen, M. et al. Estimating the risk of gestational diabetes mellitus: a clinical prediction model based on patient characteristics and medical history. BJOG 117, 69–75. https://doi.org/10.1111/j.1471-0528.2009.02425.x (2010).
Savona-Ventura, C., Vassallo, J., Marre, M. & Karamanos, B. G. A com- posite risk assessment model to screen for gestational diabetes mellitus among Mediterranean women. Int. J. Gynaecol. Obstet. 120, 240–244. https://doi.org/10.1016/j.ijgo.2012.10.016 (2013).
Theriault, S., Forest, J. C., Masse, J. & Giguere, Y. Validation of early risk-prediction models for gestational diabetes based on clini- cal characteristics. Diabetes Res. Clin. Pract. 103, 419–425. https://doi.org/10.1016/j.diabres.2013.12.009 (2014).
Vitoratos, N. et al. Maternal homocysteine levels and plasma lipids in gestational diabetes: is there any relationship?. J. Obstet. Gynaecol. 22, 366–369 (2002).
Herrera, E. & Ortega-Senovilla, H. Disturbances in lipid metabolism in diabetic pregnancy—are these the cause of the problem?. Best Pract. Res. Clin. Endocrinol. Metab. 24, 515–525. https://doi.org/10.1016/j.beem.2010.05.006 (2010).
Acknowledgements
The authors are thankful to the Research Support Center (GAP) of Botucatu Medical School, Unesp, for assisting with statistical analyses. To the São Paulo Research Foundation for providing a research Grant [FAPESP Grant 12/51257-9 CNPq-PPSUS]. To National Council for Scientific and Technological Development (CNPq), for the researcher scholarship [PQ-CNPq #304771/2015-5].
Author information
Authors and Affiliations
Contributions
I.M.C. conceived, designed and coordinated the study. J.M.V., B.F.N., and M.A.A. collected data; J.M.V. wrote and discussed the first manuscript version. J.E.C. was responsible for the statistical analysis. B.F.N., R.A.A.C., C.G.M., S.A.M.L., M.V.R., and I.M.C. contributed to the discussion and reviewed/edited the manuscript. All authors read and approved the final manuscript version.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vernini, J.M., Nicolosi, B.F., Arantes, M.A. et al. Metabolic syndrome markers and risk of hyperglycemia in pregnancy: a cross-sectional cohort study. Sci Rep 10, 21042 (2020). https://doi.org/10.1038/s41598-020-78099-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-78099-3
- Springer Nature Limited
This article is cited by
-
Early pregnancy metabolic syndrome and risk for adverse pregnancy outcomes: findings from Rajarata Pregnancy Cohort (RaPCo) in Sri Lanka
BMC Pregnancy and Childbirth (2023)