Abstract
CHOP (cyclophosphamide, doxorubicin, vincristine, prednisone) represents standard chemotherapy in non-Hodgkin's lymphoma (NHL) with risk of cardiotoxicity. To define new parameters, such as 3D myocardial deformation, arterial stiffness, and biomarkers for early diagnosis and prediction of cardiotoxicity. 110 NHL patients with LVEF > 50%, scheduled for CHOP, were evaluated at baseline, after third cycle and chemotherapy completion. 3DE assessed LVEF and myocardial deformation: longitudinal (LS), radial, circumferential, area strain. Echo-tracking analysed arterial stiffness: PWV, β index, wave intensity. Troponin I and NT-pro-BNP were measured. After chemotherapy completion, 18 patients (16%) (group I) developed cardiotoxicity (LVEF decrease < 50%, with > 10% from baseline); 92 patients (group II) did not. Significant reduction of 3D LV deformation and increase of arterial stiffness developed starting with third cycle, with greater changes in group I. LS reduction and PWV increase after third cycle were the best independent predictors for LVEF decrease; the association of LS decrease by > 19% and PWV increase by > 27% after third cycle predicted cardiotoxicity after chemotherapy completion (90% sensitivity and 81% specificity). 3D LS and PWV can detect early chemotherapy-induced cardiotoxicity and predict LVEF decline. These parameters should be incorporated in clinical protocols to monitor cardiovascular function during chemotherapy and early intervention.
Similar content being viewed by others
Introduction
Non-Hodgkin’s lymphoma (NHL), the most common hematological neoplasia, represents an important public health problem, due to increased risk of morbidity and mortality1. CHOP regimen (cyclophosphamide, doxorubicin, vincristine, prednisone) is the standard chemotherapy for NHL, providing high rate of cure and reduced recurrence of disease, with good tolerability1. The most severe side effect of these agents, which might determine early withdrawal of therapy, is represented by cardiotoxicity2. Cardiac dysfunction, mainly due to anthracyclines, may be present from asymptomatic forms, with reduction of left ventricular ejection fraction (LVEF), in 57% of patients, to overt heart failure in 16% of patients2,3,4. Cardiac dysfunction induced by chemotherapy is defined by the current guidelines as a LVEF decrease below 50%, with more than 10 percentage points, 2 to 3 weeks after initiation of therapy2. Three-dimensional echocardiography (3DE) is the method recommended for assessing LVEF2. However, 3D LVEF assessment allows only late diagnosis of cardiotoxicity, often irreversible. Thus, description of new and simple parameters of myocardial deformation and arterial stiffness, affected before decrease of LVEF, might diagnose subclinical, early changes of cardiac function induced by CHOP therapy, and might be able to predict cardiotoxicity5,6. Moreover, a role in the early diagnosis of cardiotoxicity can be added by the cardiac biomarkers. Thus, troponin I (TnI), marker of myocardial injury, may increase before occurring of symptomatic cardiotoxicity, while NT-pro-BNP, marker of increased preload, can predict onset of LV dysfunction7.
Our aim was to define new parameters, such as 3D myocardial deformation, arterial stiffness, and biomarkers, in early detection and prediction of cardiovascular toxicity, in NHL patients receiving CHOP chemotherapy.
Results
Baseline characteristics
110 patients were included in the study: 18 patients (16%) developed cardiotoxicity at the end of CHOP chemotherapy (group I), whereas 92 subjects (84%) did not. Baseline characteristics of two groups are presented in Table 1. All patients completed the whole chemotherapeutic treatment without any cardiovascular complications.
Echocardiographic parameters
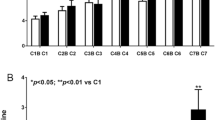
3D echocardiographic parameters at baseline, after third cycle, and at the end of CHOP chemotherapy are shown in Table 2. Baseline 3D LVEF was 62 ± 2%, with no differences between groups; after third cycle of chemotherapy, there was a decrease of 3D LVEF in the study group, with significant difference between the two groups, which persisted after treatment completion (Fig. 1). 3D myocardial deformation parameters were similar at baseline between groups. After third cycle of chemotherapy, there was a significant decrease of LS, CS, RS, and AS in the study group, persistent after final cycle of therapy, with more important reduction in group I (Table 2). Examples of 3D LVEF reduction (a) and 3D LS (b) after third cycle, and at the end of therapy, by comparison with baseline, in a patient from group I, who developed cardiotoxicity, are presented in Fig. 2.
(a) An example of reduction of 3D left ventricular ejection fraction (LVEF) from baseline (A) to third cycle (B), and after completion of CHOP therapy (C), in a patient who developed cardiotoxicity (from group I). b) Reduction of 3D longitudinal strain (LS) from baseline (A) to third cycle (B), and after completion of CHOP therapy (C), in the same patient who developed cardiotoxicity (from group I).
Arterial stiffness parameters had no significant differences between the two groups at baseline. After third cycle, arterial stiffness increased, being persistently increased at the end of therapy, with higher values in the group with cardiotoxicity (Table 2).
Biomarkers
TnI levels were increased after third cycle and persistent after CHOP completion treatment, with higher values in group I than in group II (Table 2). Although NT-pro-BNP levels increased numerically during chemotherapy, there was no significant difference between the two groups (Table 2).
Prediction models
By univariate analysis, reduction of LVEF at the end of therapy correlated with reduction of myocardial deformation, increase of arterial stiffness, TnI, and doxorubicin dose (Table 3). By stepwise multivariable linear regression analysis, in a model that included variation of 3D myocardial deformation (LS, RS, AS), arterial stiffness (PWV, β index, WI), and doxorubicin dose, LS and PWV after the third cycle of CHOP therapy were the best independent predictors of 3D LVEF decrease after CHOP treatment (R2 = 0.516, p = 0.0001).
ROC analysis showed that a decrease of 3D LS by more than 19% from baseline, after the third cycle, predicted the occurrence of cardiotoxicity at the end of CHOP chemotherapy with a sensitivity of 89% and a specificity of 85%; an increase of PWV by more than 27% after the third cycle comparing with baseline, predicted also the occurrence of cardiotoxicity at the end of chemotherapy with a sensitivity of 88% and a specificity of 83% (Table 4). More than that, by binary logistic regression and ROC curve, the association of 3D LS and PWV identified patients with cardiotoxicity at the end of treatment with a c-statistic (AUC) of 96% (Table 4).
Intraobserver, interobserver, and test–retest variabilities for 3D LV EF, volumes, and myocardial deformation parameters, are shown in Table 5, with a feasibility of > 90% for all myocardial segments.
Discussion
In a study on 110 patients with NHL, we showed that the assessment of 3D myocardial deformation, arterial stiffness, and cardiac biomarkers can detect and predict subclinical cardiotoxicity. From our best knowledge, this is the first study assessing, in a comprehensive way, systolic LV function by EF and myocardial deformation using 3D echocardiography, vascular function by arterial stiffness, and cardiac biomarkers, in a large population diagnosed with NHL, who received a single CHOP-type chemotherapeutic regimen.
CHOP regimen, very effective in achieving complete remission of NHL, has numerous side effects that cause increased morbidity and mortality1,2. Cardiotoxicity remains the most feared side effect of chemotherapy, with growing incidence of 20–30%2,8. Myocardial direct toxic effect of chemotherapy can lead to irreversible cardiomyopathy, with diastolic and/or dysfunction, which progresses to heart failure2,3,4. Using myocardial deformation and cardiac biomarkers, in patients with NHL receiving CHOP therapy, we demonstrated that an early decrease of longitudinal strain with more than 19%, after third cycle of therapy, predicts occurrence of cardiotoxicity after CHOP completion treatment. These results are essential for monitoring patients during chemotherapy and for early detection of patients at risk for heart failure.
Echocardiography is the best method for monitoring patients before, during, and after chemotherapy, being widely available, noninvasive, and cost effective4. LVEF is the standard parameter used to diagnose cardiotoxicity2. Cardiotoxicity is defined as LVEF decrease with more than 10 percentage points, to a value below 50%, evaluated 2–3 weeks after initiation therapy2. To avoid LV geometric assumptions, foreshortening, and inappropriate visualization of apex, we measured LVEF by 3DE, as recommended by current guidelines2. Compared to 2D echocardiography, 3DE has better intra- and inter- observer variability, and test–retest variability, and allows a more accurate assessment of LV volumes and ejection fraction, with better agreement with cardiac magnetic resonance (CMR), considered the “gold standard”2,9. In cancer patients, 3D LVEF is more sensitive and robust in detecting anthracyclines-induced cardiotoxicity in patients with different forms of solid or hematological tumors10,11.
Of the four chemotherapeutic agents used in CHOP therapy (cyclophosphamide, doxorubicin, vincristine, and prednisone), doxorubicin is by far the most important for occurrence of heart injury2. Risk for cardiotoxicity is greater when cumulative dose of doxorubicin is high, in association with other anticancer drugs or radiotherapy, or in single injection administration (versus long infusion)2,3. A dose of doxorubicin that exceeds 500 mg/m2 is an important factor in occurrence of cardiotoxicity, while doses below 300 mg/m2 are associated with low risk2. Our study showed a significant reduction of LVEF after a moderate dose of doxorubicin (429 ± 61 mg); however, asymptomatic cardiac dysfunction occurred since third cycle of CHOP, after a low dose of anthracycline (183 ± 42 mg), with reduction of all 3D myocardial deformation parameters. Our findings are consistent to those of Olivieri et al.12, Boyd et al.13, and Yu et al.14, who demonstrated occurrence of cardiotoxicity in 11–35% of patients, with frequent subclinical forms, at low and moderate doses of doxorubicin, between 150 and 450 mg/m2. Cyclophosphamide can induce cardiotoxicity in less than 2% of cases, manifested by arrhythmias and arterial or venous thromboembolism, but the dose used in CHOP regimen is too low to induce cardiac dysfunction by itself2,15; in fact, none of our patients experienced arrhythmia or thromboembolic events. Vincristine can lead to different non-cardiac side effects, but no cardiac side effects have been reported16. Corticosteroids may induce hypertension or atrial fibrillation2, but none of our patients developed atrial fibrillation, while blood pressure remained within the normal limits.
Decrease of myocardial deformation parameters, assessed by speckle-tracking echocardiography, precedes LVEF reduction, and may persist during and after chemotherapy2. Our research group8, similarly with others10,17, identified an early decrease of 2D LS or CS, before reduction of LVEF, in patients with cancers treated with anthracyclines. Although still not used routinely, 3D myocardial deformation had demonstrated its usefulness, feasibility, and superiority versus 2D, with good agreement with CMR strain, through independence from geometry-related deformation, direction, and angle18. We showed a significant decrease of all 3D deformation parameters after third cycle of CHOP, before 3D LVEF reduction by criteria used for diagnosis of cardiotoxicity. Similarly, Mornos et al.19 and Armstrong et al.20 showed reduction of 3D LS, RS, or CS after anthracyclines treatment, associated with increased troponin levels, occurring before decrease of LVEF. Current guidelines define occurrence of cardiac dysfunction during chemotherapy by a reduction of global longitudinal strain with more than 15%2. We also identified longitudinal strain as a powerful independent predictor for LVEF decrease. Reduction of this parameter by more than 19% after third cycle of CHOP therapy was able to identify patients with chemotherapy-induced cardiotoxicity at the end of treatment with a c-statistics (AUC) of 92%.
Besides cardiac effects, chemotherapy determines negative arterial remodeling with increased arterial stiffness, favoring LV hypertrophy and altered ventricular-arterial coupling, worsening preexisting cardiac dysfunction21. In patients with solid or hematological tumors, anthracyclines and adjuvant agents, including CHOP therapy, favor occurrence of arterial stiffness, assessed by increased PWV or WI, persistent after treatment completion6,22,23. Narayan et al. demonstrated that early decrease of myocardial deformation and increased ventriculo-arterial coupling are best independent predictors for LVEF reduction23. These results are similar to our data, by which we demonstrated an early, progressive, and significant increase of arterial stiffness and altered ventriculo-arterial coupling during chemotherapy, with a significant correlation between vascular function and occurrence of cardiotoxicity, suggesting a possible additional mechanism of chemotherapy-induced cardiac dysfunction. We identified PWV and LS as the best independent predictors for 3D LVEF decrease at the end of chemotherapy. An increase of PWV by more than 27% after the third cycle of CHOP therapy may identify patients with cardiovascular toxicity after chemotherapy with c-statistics (AUC) of 90%. Furthermore, the combination of LS decrease by more than 19% and PWV increase by more than 27% after third cycle of CHOP had a greater prediction value, with a c-statistics (AUC) of 96%.
Cardiac troponin I is the most sensitive and specific biomarker, used for diagnosis of myocardial injury after chemotherapy. On the contrary, NT-pro-BNP, marker of high filling pressures, has a controversial role in early detection of cardiac dysfunction2. Several studies demonstrated the role of troponin I and NT-pro-BNP in assessing cardiac dysfunction in hematological neoplasia treated with anthracyclines7, however, other studies failed to show contribution of these biomarkers for early detection of cardiotoxicity24. In our study, troponin I increased early, while 3D LVEF maintained within normal ranges, persistent after CHOP completion treatment, and significantly higher in group who developed cardiotoxicity. Even if NT-pro-BNP level increased numerically during treatment, no significant difference was found between groups.
Cardiac assessment of cancer patients should be done at baseline, before starting treatment, during chemotherapy and periodically after its completion. In our study, an early cardiac comprehensive evaluation, after only 3 cycles of CHOP (full treatment had 8 ± 2 CHOP cycles) identified predictive parameters for further cardiotoxicity after chemotherapy ended. Follow-up visits are mandatory, in order to diagnose late chemotherapy-induced cardiovascular toxicity.
Study limitations
First limitation of our study is related to the low number of patients which developed cardiotoxicity (18 out of 110). Comparing with other studies, enrolling between 50 and 70 patients, we enrolled a higher number of oncological patients. However, percentage of those who developed cardiotoxicity was similar in all studies, less than 20%8,11,14,25. Second limitation is that we used only proper echo images, without stitching artifacts or poor quality, taking into consideration that quality of ultrasound images can decrease the accuracy of assessment of 3D LV EF and myocardial deformation parameters. However, feasibility of our data was more than 90% for all myocardial segments. Moreover, we used only troponin I, which is currently provided by our laboratory. High-sensitivity troponin I might have a better accuracy, but is not used routinely for the diagnosis of cardiotoxicity. Finally, follow-up of patients after chemotherapy ended is mandatory, knowing the risk of anthracyclines-induced late cardiotoxicity.
Conclusions
Assessment of 3D longitudinal strain and pulse wave velocity is able to detect early chemotherapy-induced cardiotoxicity, and to predict with good accuracy further decline of 3D LVEF in patients with non-Hodgkin’s lymphoma. Thus, these parameters should be incorporated in clinical protocols, for better monitoring of cardiac function during chemotherapy, and for early intervention.
Methods
Study groups
147 consecutive patients diagnosed with NHL, were prospectively enrolled from a single hematology department between January 2014 and October 2018. Inclusion criteria were: age > 18 years; signed informed consent; 3D LVEF > 50%; sinus rhythm; NHL scheduled to receive CHOP chemotherapy according to hematological guidelines. Exclusion criteria were: history of cardiovascular disease; history of radiotherapy. Study protocol was approved by the local ethics committee and conforms with the principles outlined in the Declaration of Helsinki. All patients gave written inform consent to participate in the study. Of the 147 patients, 8 died during chemotherapy due to aggressive forms of NHL, 11 were lost to follow-up and 18 were excluded because of poor image quality on 3DE. Thus, 110 patients (58 ± 11, 51 men) remained in the study.
CHOP chemotherapy consisted of intravenously administration of cyclophosphamide 750 mg/m2, vincristine 1.4 mg/m2, and doxorubicin 50 mg/m2 on day 1, and orally administration of prednisone 100 mg on days 1–5. Full treatment included 8 ± 2 CHOP cycles, repeated at every 21 ± 3 days. Blood collection, 3DE, and echo-tracking were performed at baseline, one day after completion of third cycle, and at end of CHOP chemotherapy. Cardiotoxicity was defined as 3D LVEF reduction below 50%, with more than 10 percentage points, at any time after initiation of CHOP therapy. According to 3D LVEF value at the end of therapy, two groups were defined: patients who developed cardiotoxicity (group I) and patients who did not fulfill these criteria (group II).
Echocardiography
All ultrasound examinations were performed with a commercially available system equipped with a 4 V probe for 3DE (Vivid E9 Dimension, GE Medical Systems, Horten, Norway). Before every recording, blood pressure and heart rate were measured; one-lead electrocardiogram was used during examination. Images were obtained in left lateral decubitus position, during breath holding, performed by the same investigator and taken in accordance with our previously reported protocol and current guidelines16,26. Three cardiac cycles were achieved at each recording. Digital achieved data were analyzed offline using a dedicated software package (EchoPac version BT 12 for PC; GE Medical Systems) with 4D auto-LVQ system. 3DE full-volume LV data sets were achieved in six consecutive electrocardiographically gated subvolumes, with good visualization of all segments, excluding any stitching artifacts. Endocardial border tracing was initiated manually, by identifying two points in 4-chamber view (middle of mitral annulus and LV apex) at end-diastole and end-systole. Then, the software generated semiautomated complete endocardial contour that included papillary muscles and LV outflow tract for end-diastolic and end-systolic volumes27; manual edits were performed if needed. 3D strain analysis was the last step of the 4D auto LVQ software. Strain region of interest was automatically generated at end-systole, and had two borders: endocardial, the same used for 3D volumes assessment, and epicardial, generated in previous stage; both contours were adjusted, if necessary. Deformation parameters (longitudinal strain LS, circumferential strain CS, radial strain RS, and area strain AS) were automatically calculated for each of 17 LV segments after tracking was confirmed visually8.
Arterial stiffness
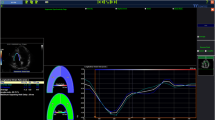
Echo-tracking system (Aloka Prosound α10, Tokyo, Japan) was used to determine arterial stiffness at right common carotid artery, using our protocol published previously21,28. Following parameters of arterial stiffness were assessed: pulse wave velocity (PWV), augmentation index (AIX), beta index, Young modulus of stiffness (Ep), arterial compliance (AC), and wave intensity (WI) (Fig. 3) (detailed definitions and measurements are given in the Supplemental Appendix). WI records two positive peaks: first peak (compression wave), occurs in early systole and depends on LV contractility and second peak (expansion wave), occurs at the end of ejection period and is influenced by LV capacity to stop aortic blood flow. Between the two positive peaks is defined a new parameter, negative area, which represents reflections from the cerebral circulation28.
Echo-tracking principle used to measure pulse wave velocity (PWV), augmentation index (AIX), β index, Young modulus of stiffness (Ep), arterial compliance (AC), and Wave Intensity (WI) at the right common carotid artery level, based on the arterial wave obtained from the differences between the arterial diameter in systole and diastole and specific formulas. Modified from21.
Troponin I and NT-pro-BNP
Blood was collected in EDTA tubes and TnI and NT-pro-BNP concentrations were measured by CLEIA method with Pathfast Synttergy 2005 analyzer (Mitsubishi Kagaku Iatron, Inc, Tokyo, Japan). The reported value for the 99th percentile for TnI was < 0.02 ng/ml, and less than 112 pg/ml for NT-pro-BNP29.
Reproducibility
For 3D LV EF and deformation parameters, intraobserver, interobserver, and test–retest variability (repeatability) were assessed in our laboratory for 20 consecutive patients, by two observers with same experience. For arterial stiffness and ventriculo-arterial coupling, reproducibility in our laboratory has been reported previously28.
Statistical analysis
SPSS version 19.0 (SPSS, Inc, Chicago, Illinois) was used for data analysis. Results are presented as mean ± standard deviation (SD) or as percentages (%). P < 0.05 was considered significant. Differences between variables of two groups were quantified with independent t-test (for means) and χ2 test (for proportions). Comparisons of parameters within each group at baseline, after third cycle, and after CHOP completion, were assessed with one-way analysis of variance (ANOVA); Scheffé test was used for subgroup analysis. Association between two variables and influence of a parameter on LVEF reduction below 50% at the end of chemotherapy were evaluated by univariate Pearson correlation and multiple linear stepwise regression analysis. Receiver operating characteristic (ROC) curves were obtained for parameters that were predictive for occurrence of cardiotoxicity after the last cycle of CHOP treatment. Intraobserver, interobserver, and repeatability were calculated as 2SD/√2 and reported as percentages from mean value and coefficient of variation30.
Ethical approval
Our research was approved by Ethics Committee of the University and Emergency Hospital of Bucharest
Abbreviations
- 3DE:
-
3-Dimensional echocardiography
- 4D autoLVQ:
-
4-Dimensional auto left ventricular quantification
- AC:
-
Arterial compliance
- AIX:
-
Augmentation index
- ANOVA:
-
One-way analysis of variance
- AS:
-
Area strain
- CHOP:
-
Cyclophosphamide, hydroxydaunorubicin (doxorubicin), vincristine, prednisone
- CLEIA:
-
Chemo-luminescence enzyme immuno-assay
- CMR:
-
Cardiac magnetic resonance
- CS:
-
Circumferential strain
- EDTA:
-
Ethylenediamine tetra-acetic acid
- Ep:
-
Young modulus of stiffness
- GE:
-
General electrics
- LS:
-
Longitudinal strain
- LVEDV:
-
Left ventricular end-diastolic volume
- LVEF:
-
Left ventricular ejection fraction
- LVESV:
-
Left ventricular end-systolic volume
- NHL:
-
Non-Hodgkin’s lymphoma
- NT pro-BNP:
-
N-terminal pro-brain natriuretic peptide
- PWV:
-
Pulse wave velocity
- ROC:
-
Receiver operating curve
- RS:
-
Radial strain
- SD:
-
Standard deviation
- Tn I:
-
Troponin I
- WI:
-
Wave intensity
References
Vidal, L. et al. CHOP-like-14 compared to CHOP-like-21 for patients with aggressive lymphoma: a meta-analysis of randomized controlled trials. Acta Oncol. 55, 77–84 (2016).
Zamorano, J. L. et al. 2016 ESC Position Paper on cancer treatments and cardiovascular toxicity developed under the auspices of the ESC Committee for Practice Guidelines: the Task Force for cancer treatments and cardiovascular toxicity of the European Society of Cardiology (ESC). Eur Heart J. 37, 2768–2801 (2016).
Mihalcea, D. J., Florescu, M. & Vinereanu, D. Mechanisms and genetic susceptibility of chemotherapy-induced cardiotoxicity in patients with breast cancer. Am. J. Ther. 24, e3-11 (2017).
Plana, J. et al. Expert consensus for multimodality imaging evaluation of adult patients during and after cancer therapy: a report from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Eur. Heart J. 27, 911–939 (2014).
Lorenzini, C. et al. Reliability of left ventricular ejection fraction from three-dimensional echocardiography for cardiotoxicity onset detection in patients with breast cancer. J. Am. Soc. Echocardiogr. 30, 1103–1110 (2017).
Mozos I., Borzak G., Caraba A. & Mihaescu R. Arterial stiffness in hematologic malignancies. Onco. Targets Ther. 10, 1381–1388 (2017).
Cardinale, D., Biasillo, G., Salvatici, M., Sandri, M. T. & Cipolla, C. M. Using biomarkers to predict and to prevent cardiotoxicity of cancer therapy. Expert Rev. Mol. Diagn. 17, 245–256 (2017).
Florescu, M., Magda, L. S., Enescu, O. A., Jinga, D. & Vinereanu, D. Early detection of epirubicin-induced cardiotoxicity in patients with breast cancer. J. Am. Soc. Echocardiogr. 27, 83–92 (2014).
Tamborini G., et al. Feasibility and accuracy of automated software for transthoracic three-dimensional left ventricular volume and function analysis: comparisons with two-dimensional echocardiography, three-dimensional transthoracic manual method, and cardiac magnetic resonance imaging. J. Am. Soc. Echocardiogr. 30, 1049–1058 (2017).
Santoro, C. et al. 2D and 3D strain for detection of subclinical anthracycline cardiotoxicity in breast cancer patients: a balance with feasibility. Eur. Heart J. Cardiovasc. Imaging. 18, 930–936 (2017).
Tarr, A. et al. Early detection of cardiotoxicity by 2D and 3D deformation imaging in patients receiving chemotherapy. Echo Res. Pract. 2, 81–88 (2015).
Olivieri, J. et al. Modern management of anthracycline-induced cardiotoxicity in lymphoma patients: low occurrence of cardiotoxicity with comprehensive assessment and tailored substitution by nonpegylated liposomal doxorubicin. Oncologist 22, 422–431 (2017).
Boyd, A. et al. Anthracyclines induce early changes in left ventricular systolic and diastolic function: a single centre study. PLoS ONE 12, e0175544 (2017).
Yu, A. F. et al. Two-dimensional speckle tracking echocardiography detects subclinical left ventricular systolic dysfunction among adult survivors of childhood, adolescent, and young adult cancer. Biomed Res Int. 2016, 9363951 (2016).
Iqubal A, et al. Molecular mechanism involved in cyclophosphamide-induced cardiotoxicity: old drug with a new vision. Life Sci. 218, 112–131 (2019).
Webre, C., Shonka, N., Smith, L., Liu, D. & De Groot, J. PC or PCV, that is the question: primary anaplastic oligodendroglial tumors treated with procarbazine and CCNU with and without vincristine. Anticancer Res. 35, 5467–5472 (2015).
Tsai, H. R. et al. Left ventricular function assessed by two-dimensional speckle tracking echocardiography in long-term survivors of Hodgkin’s lymphoma treated by mediastinal radiotherapy with or without anthracycline therapy. Am. J. Cardiol. 107, 472–477 (2011).
Zhou, X. et al. Feasibility of automated three-dimensional rotational mechanics by real-time volume transthoracic echocardiography: preliminary accuracy and reproducibility data compared with cardiovascular magnetic resonance. J. Am. Soc. Echocardiogr. 29, 62–73 (2016).
Mornoş, C. et al. The value of left ventricular global longitudinal strain assessed by three-dimensional strain imaging in the early detection of anthracycline mediated cardiotoxicity. Hellenic J. Cardiol. 55, 235–244 (2014).
Armstrong, G. T. et al. Comprehensive echocardiographic detection of treatment-related cardiac dysfunction in adult survivors of childhood cancer: results from the St. Jude Lifetime Cohort Study. J. Am. Coll. Cardiol. 65, 2511–2522 (2015).
Mihalcea, D. J. et al. Comparison of pulse wave velocity assessed by three different techniques: arteriograph, complior, and echo-tracking. Heart Vessels 31, 568–577 (2016).
Grover, S. et al. Early and late changes in markers of aortic stiffness with breast cancer therapy. Intern. Med. J. 45, 140–147 (2015).
Narayan, H. K. et al. Noninvasive measures of ventricular-arterial coupling and circumferential strain predict cancer therapeutics-related cardiac dysfunction. JACC 9, 1131–1141 (2016).
Ylänen, K., Poutanen, T. & Savukoski, T. Cardiac biomarkers indicate a need for sensitive cardiac imaging among long-term childhood cancer survivors exposed to anthracyclines. Acta Paediatr. 104, 313–319 (2015).
Okuma, H. et al. Impact of persistent left ventricular regional wall motion abnormalities in childhood cancer survivors after anthracycline therapy: assessment of global left ventricular myocardial performance by 3D speckle-tracking echocardiography. J. Cardiol. 70, 396–401 (2017).
Lang, R. M. et al. EAE/ASE recommendations for image acquisition and display using three-dimensional echocardiography. Eur. Heart J. Cardiovasc Imaging. 13, 1–46 (2012).
Muraru, D. et al. Comprehensive analysis of left ventricular geometry and function by three-dimensional echocardiography in healthy adults. J. Am. Soc. Echocardiogr. 26, 618–628 (2013).
Magda, S. L., Ciobanu, A. O., Florescu, M. & Vinereanu, D. Comparative reproducibility of the noninvasive ultrasound methods for the assessment of vascular function. Heart Vessels. 28, 143–150 (2013).
Kurihara, T. et al. Evaluation of cardiac assays on a benchtop chemiluminescent enzyme immunoassay analyser, PATHFAST. Anal. Biochem. 375, 144–146 (2008).
Bland, J. M. & Altman, D. G. Statistical methods for the assessing agreement between two methods of clinical measurement. Lancet 1, 307–310 (1986).
Author information
Authors and Affiliations
Contributions
D.M., M.F., A.M.V. and D.V. designed the study; D.M., M.F., R.B., N.P. performed the protocol examinations. D.M., M.F., R.B., N.P. and D.V. analyzed and interpreted data. D.M., A.M.V. and D.V. wrote the main manuscript text. All authors reviewed and approved the version to be submitted.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Mihalcea, D., Florescu, M., Bruja, R. et al. 3D echocardiography, arterial stiffness, and biomarkers in early diagnosis and prediction of CHOP-induced cardiotoxicity in non-Hodgkin’s lymphoma. Sci Rep 10, 18473 (2020). https://doi.org/10.1038/s41598-020-75043-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-75043-3
- Springer Nature Limited
This article is cited by
-
3D-based strain analysis and cardiotoxicity detection in cancer patients received chemotherapy
BMC Cancer (2023)
-
Clinical roles of vascular function assessment in cancer care
Supportive Care in Cancer (2022)
-
Antineoplastics
Reactions Weekly (2021)