Abstract
We devulcanized ground tire rubber (GTR) in a laboratory microwave oven and an internal mixer, measured the soluble content and the cross-link density of the samples, and then used Horikx’s analysis. The results showed that microwave treatment caused severe degradation of the polymer chains, while in the case of thermomechanical devulcanization, the selective scission of covalent cross-links is more common. Four devulcanized ground tire rubber (dGTR) samples were chosen for further study and three groups of samples were produced: dGTR samples containing vulcanizing agents and different amounts of paraffin oil (dGTR-based mixtures), natural rubber-based rubber mixtures with different dGTR contents and reference rubber mixtures with dGTR-based mixtures (increased vulcanizing agent contents). Cure characteristics showed a plasticizer-like effect of dGTR. Tensile and tear strength decreased drastically with increasing dGTR content; however, elongation at break values did not follow such a trend. Mechanical properties improved with increased vulcanizing agent contents. The examined properties of the samples improved even further with the use of thermomechanically devulcanized samples. Horikx’s analysis showed that this is attributable to moderate polymer chain scission.
Similar content being viewed by others
Introduction
The amount of waste rubber products, especially that of waste tires, is rapidly increasing. The traditional methods of waste tire management have been stockpiling or landfilling, both of which are short-term solutions and pose significant health and environmental problems1. Therefore, many studies focus on the reclamation and recycling of waste rubber products and tires, but reuse and recycling are a challenge due to the 3D cross-linked structure of rubbers. According to the EU waste hierarchy, the reuse of these products is a favorable solution. However, existing approaches, such as the application of waste tires as safety barriers on racetracks and retreading tires, creates new products of reduced quality and functionality.
Another way to use scrap rubber is to burn it in furnaces or convert it to liquid fuel in pyrolysis reactors in an oxygen-free environment. Tires have a high heat value (ca. 32.6 MJ/kg), which is even higher than that of coal (ca. 18.6–27.9 MJ/kg). The end product of pyrolysis is carbon black and an oil-like material, which can be processed like petroleum2,3.
The best way of disposing of waste tires and other rubber products is to turn them into a ground powder (ground tire rubber, GTR)4, which can be produced in different ways: mechanical grinding at ambient or cryogenic temperatures5, and waterjet milling. In waterjet milling, a high-pressure water beam grinds rubber waste. Compared to mechanical grinding, smaller particles can be obtained, and rubber degradation can also be avoided, though the final material needs to be dried.
GTR can be used as an additive without any physical or chemical treatment in asphalt and can be bound together with polyurethane. GTR can be blended with thermoplastic4,5 or thermoset6,7,8,9 polymers with or without compatibilization10,11. However, the volume of these applications cannot meet the ever-increasing need for rubber recycling.
Devulcanization may offer a solution to this problem. This process is suitable to selectively break covalent cross-links in elastomeric materials, keeping the polymer backbone intact. New molecules form, and they can create new bonds on the surface of the GTR particles4,12,13,14,15, enhancing adhesion between devulcanized GTR (dGTR) particles and other polymers. Consequently, the amount of recycled rubber in new rubber products can be increased without compromising their mechanical properties. Conventional devulcanization techniques, such as thermomechanical16,17, thermochemical18, mechanochemical19, and microwave12,13,20,21,22,23 devulcanization, have decades-long history of research24. There are other devulcanization methods utilizing ultrasound25,26, chemolithotrophic bacteria27, and supercritical carbon dioxide28. Each method has its advantages and disadvantages. Microwave-treated GTR has good properties, and the technique has a promise of high productivity. Microwave devulcanization takes advantage of volumetric heating: a fast and uniform rise in temperature can be achieved. The selective scission of sulfuric cross-links is possible with the right parameters (temperature, exposure time, microwave power). The process does not require additional chemicals and is considered an eco-friendly technology. A major drawback of microwave devulcanization is that nonpolar elastomers like NR, styrene-butadiene rubber (SBR) or ethylene propylene diene monomer rubber (EPDM), have low microwave absorbance. However, carbon black, which is often used as a filler in rubber products14, has good microwave absorbance and dissipates its energy in the form of heat29.
The effectiveness of devulcanization can be evaluated with the soluble content of the rubber sample. It can be directly measured via Soxhlet extraction, during which a small amount of organic solvent is repeatedly distilled to dissolve the soluble components of a solid material. Many studies, addressing microwave devulcanization, focused on the effect of different exposure times of GTR to microwaves. The accepted conclusion was that the longer the exposure time, the larger the soluble fraction (sol fraction) of the samples13. Garcia et al.20 devulcanized GTR with microwaves and were able to increase the sol fraction from 14 to 31% after 7 min of treatment. They determined that in addition to the breaking of S–S and C–S bonds, the main chain was also degraded. However, sol fraction on its own is not a sufficient indicator of devulcanization. The cross-link density of dGTR is also an essential factor. The degree of devulcanization can be defined as the percentage decrease in the cross-link density of a sample. De Sousa et al.10 found that the higher the amount of energy absorbed during the treatment, the higher the final temperature of the GTR, hence the higher the decrease in cross-link density. The FTIR analysis of dGTR can also reveal the structural changes that take place during devulcanization13,21. Therefore, FTIR is a suitable supporting method.
Horikx’s analysis30 is a commonly accepted method that establishes a mathematical relationship between the sol fraction and the degree of devulcanization. Ultimately, it can indicate whether the main phenomenon during devulcanization is random scission of the polymer chains or selective scission of the covalent cross-links31. Horikx’s analysis is becoming a widely used method to grade devulcanization. There are many examples of its use in scientific literature: assessing microwave devulcanization of recycled NR12,32, thermomechanical devulcanization of GTR33, thermochemical devulcanization of NR and EPDM34 and microbiological devulcanization of NR35.
In our previous article, we showed the effects of different GTRs (mechanically ground and water-jet milled) with different particle sizes on the devulcanization process and optimized the parameters of microwave devulcanization36. Furthermore, we added dGTR to polypropylene-based thermoplastic dynamic vulcanizates (TDV) to verify our method as a means of GTR recycling. We found that virgin rubber can be replaced with up to 20 wt% devulcanizate without compromising the mechanical properties of the resulting TDV37.
In this study, we compared the applicability of microwave-devulcanized and mechanically devulcanized GTR in virgin NR. First, we selected the ideal devulcanizates for further study based on Horikx’s analysis. We produced NR-based mixtures incorporating various amounts of the four selected dGTR samples. Finally, we tested the mechanical and physical properties of the resulting rubber samples to determine the effectiveness of our recycling processes.
Experimental
Materials
Waterjet-milled crumb rubber was provided by Aquajet Ltd. (Budapest, Hungary). The material originated from the tread area of truck tires. Therefore, this type of GTR is a high-purity material. According to TGA measurements, it contains 50–55 phr of NR, 45–50 phr of synthetic rubber, 4–6 phr of oil, 33–37 phr of carbon black, and 7.5 phr of residual additives. We chose a general-purpose natural rubber for our investigations. Table 1 contains the manufacturers, types, and basic properties of GTR and NR.
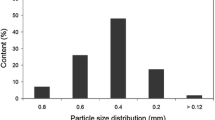
The additives of rubber mixtures and their suppliers were the following: zinc oxide (ZnO, S.C. Werco Metal S.r.l., Zlatna, Romania), stearic acid (Oleon, Ertvelde, Belgium), N772 carbon black (Omsk Carbon Group OOO, Omsk, Russian Federation), paraffin oil (Ipol Lubricants, Mumbai, India), tetramethyl thiuram disulfide (TMTD, Akrochem Corporation, Akron, Ohio, USA), N-cyclohexyl-2-benzothiazole sulfonamide (CBS, Rhein Chemie, Mannheim, Germany) and sulfur (Ningbo Actmix Rubber Chemicals Co., Ltd., Ningbo, China). The particle size distribution of GTR was published in our previous paper36.
Devulcanization of GTR
Microwave devulcanization of GTR was carried out in a BP-125/50 type laboratory microwave oven, produced by Microwave Research Inc. (Carol Stream, Illinois, USA). We heated 50 and 100 g batches of GTR up to 200 °C with a heating rate of 6 °C/min. The microwave power was controlled by a PID controller that used data from a thermocouple that was continuously measuring the temperature of the rubber inside the oven. A motorized stirring system was installed to the microwave oven to insure homogeneous temperature. After the temperature reached 200 °C, the material was taken out and allowed to cool to room temperature. In some cases, the samples were heat-treated at 140 °C in a Venticell LSIS-B2V (MMM Group, Monroe, Louisiana, USA) laboratory oven prior to devulcanization. The parameters of microwave devulcanization, and the nomenclature of the samples can be seen in Table 2.
Thermomechanical devulcanization was performed in a Brabender Plasti-corder internal mixer (Brabender GmbH & Co., Duisburg, Germany). The duration of the treatment was 10 min, and the chamber volume was 50 cm3. The parameters of thermomechanical devulcanization and the abbreviations of the samples are listed in Table 3. The GTR was kept at ambient conditions before treatment.
Characterization of GTR and dGTR
GTR and dGTR were characterized by Soxhlet extraction in toluene, according to Eq. (1). The insoluble fraction, or gel fraction of the rubber can be separated from the soluble fraction with this extraction technique. High sol content of a devulcanizate is a good indicator of its processability. It indicates the presence of small polymer molecules ready to be reintegrated into the rubber matrix via curing. These molecules can be effectively separated via Soxhlet extraction. We ran the extraction for 18 h and then dried the samples for 12 h at 80 °C to remove the solvent. We weighed each sample twice: before extraction and after drying.
where Mi and Mf stand for the mass of rubber before and after the extraction, respectively.
The cross-link density of untreated GTR and devulcanizates was determined via swelling tests according to ASTM D 297-15. We calculated the cross-link density values using the Flory-Rehner Eq. (2)38 after equilibrium swelling (72 h followed by drying to constant mass at 80 °C) in toluene.
where \({\nu }_{e}\) is cross-link density (mol/cm3); V1 is the molar volume of the solvent (for toluene: 106.13 cm3/mol); \({\chi }_{1}\) is the rubber-solvent interaction parameter (0.39), and Vr means the volume fraction of rubber in the swollen sample, which can be determined with the Ellis and Welding Eq. (3)31.
where ms is the mass of the swollen rubber sample (g), mr is the mass of the dry rubber sample (g), \({\rho }_{s}\) is the density of the solvent, toluene (0.8669 g/cm3) and \({\rho }_{r}\) is the density of the rubber sample (1.20 g/cm3).
The degree of devulcanization was calculated with Eq. (4)12
where \({\nu }_{f}\) is the cross-link density of the devulcanized sample and \({\nu }_{i}\) is the cross-link density of untreated GTR.
Formulation and preparation of rubbers containing GTR and dGTR
After the evaluation of the devulcanization experiments, we selected four types of dGTR (dGTR_MW_100g_2, dGTR_TM_40/160, dGTR_TM_40/200 and dGTR_TM_120/200) and added vulcanizing agents to them with an internal mixer. The dGTR samples were chosen based on the results of Horikx’s analysis. We investigated the effects of different amounts of vulcanizing agents and paraffin oil. The formulations of rubber mixtures are shown in Table 4.
To assess the usability of dGTR in rubbers, we added different amounts of dGTR and GTR (as reference) to NR-based compounds. The recipes of the rubber compounds are shown in Table 5. We introduced a simplified notation: MW denotes the dGTR_MW_100g_2 microwave-devulcanized sample.
We prepared a reference sample (NR_REF), a reference sample without paraffin oil (NR_REF_WO), and a reference sample where paraffin oil was replaced with dGTR_MW_100g_2 (NR_REF_WO_dGTR_MW). In the abbreviation of the other samples, the number (50, 100, or 185) means the dGTR content in parts per hundred rubber (phr). In the case of samples ending with "A" or "B", mixing consisted of two steps. In the first step, the dGTR was compounded with vulcanizing agents according to Table 4. Then this untreated dGTR mixture was added to the original rubber mixture. Table 6 shows rubber mixtures containing thermomechanically devulcanized GTR. In summary, the dGTR_MW_100g_2 microwave-devulcanized and the dGTR_TM_40/160, dGTR_TM_40/200, dGTR_TM_120/200 thermomechanically devulcanized samples were incorporated in the rubber mixtures.
The rubber ingredients were mixed in a Brabender Plasti-corder internal mixer at 50 °C and 40 rpm. The order of appearance for the components in Tables 4, 5, 6 (left to right) also reflects the order of mixing. The compounds were vulcanized with a Teach-Line Platen Press 200E (Dr. Collin GmbH, Munich, Germany) hot press. The pressure applied was 2.8 MPa, and the temperature was 160 °C. Each compound was cured for t90 (time necessary to reach 90% vulcanization). These time values were obtained from separate rheometer measurements.
Characterization of the rubber mixture and cured rubber sheets
The curing curves of the rubber compounds were recorded with a MonTech Monsanto R100S rheometer (MonTech Werkstoffprüfmaschinen GmbH, Buchen, Germany) in isothermal (T = 160 °C) time sweep mode (1.667 Hz, 1° angle) for 30 min.
Hardness was tested according to the ISO 48-4:2018 Shore A method on a Zwick H04.3150.000 hardness tester (Zwick GMBH., Ulm, Germany) on the cured rubber sheets. Ten tests were performed on each compound, followed by the calculation of the average and standard deviation values.
The tensile mechanical properties of the compounds were investigated according to the ISO 37:2017 standard on a Zwick Z250 universal testing machine with a 20 kN load cell (Zwick GmbH, Ulm, Germany). Type 1 specimens with a clamping length of 60 mm were loaded at a crosshead speed of 500 mm/min. Tear tests were performed on the same testing machine, and test speed was according to the ISO 34-1:2015 standard (Type C specimen), with a clamping length of 56 mm. Both tests were run at room temperature. The average and standard deviation of the tensile strength, tear strength, and elongation at break values were calculated based on five tests for each compound.
Results and discussion
Devulcanization of GTR
Table 7 lists the sol content, cross-link density, and the degree of devulcanization of the microwave-devulcanized samples. First, we treated 50 g batches of GTR and observed an increase in sol fraction and a decrease in cross-link density, indicating devulcanization. Later, we scaled up to batch sizes of 100 g to improve productivity. The sol content remained unchanged. The power of the microwave oven was enough to heat the GTR to 200 °C without the need to increase the duration of the treatment. A further increase in batch size was not possible because of the size of the instrument.
An hour-long heat treatment (at 140 °C) before devulcanization did not cause a significant change in the sol content. However, when the samples were treated for two hours, the sol fraction increased significantly. The degree of devulcanization followed a similar trend. In other words, the sol content increased significantly, while the cross-link density did not decrease considerably. That indicates the degradation of the polymer chains. Similar conclusions can be drawn for the GTR_H_2 sample.
Table 8 shows the sol content, cross-link density, and the degree of devulcanization of the samples after thermomechanical devulcanization. The trends are clear; increasing temperature and rotor speed lead to increasing sol content. At the same time, there is a continuous decrease in cross-link density.
We used Horikx’s analysis to determine the relationship between the sol fraction after the degradation of the 3D cross-link structure of rubber and the relative decrease in cross-link density. Horikx derived an extensive method to identify and illustrate whether the degradation of a polymer is dominated by random chain scission or the selective breakdown of cross-links (i.e. devulcanization). He identified two different scenarios: random chain scission and scission of the cross-links. In the case of main chain scission, Eq. (5) shows the relationship between the soluble fraction of the polymer and the relative decrease in the number of elastically active network chains.
where \({v}_{i}\) stands for the initial cross-link density, \({v}_{f}\) stands for cross-link density after degradation, \({s}_{i}\) stands for the initial sol fraction of the polymer and \({s}_{f}\) stands for the sol fraction of the polymer after treatment30,34. Based on Eq. (5) a curve of random scission can be plotted (Fig. 1).
The second scenario involves only cross-link cleavage, with no degradation of the polymer main chain. Equation (6) was formulated based on Horikx’s research30.
where \({\gamma }_{i}\) and \({\gamma }_{f}\) stand for the initial and final cross-linking index, respectively. The cross-linking index tells the average number of cross-link bonds per polymer chain30,34 and it can be determined by Eq. (7)30. This curve contributes to selective cross-link scission (Fig. 1).
where \({\gamma }_{x}\) (−) is the cross-linking index, \({v}_{x}\) (mol/cm3) is the cross-link density, Mn (g/mol) stands for the number-avarege molecular weight of the rubber and \(\rho\) is the rubber density.
In this paper, the initial cross-linking index is approximated by Eq. (8)39.
Based on Eqs. (5) and (6), the relationship between the sol content and the decrease in cross-link density can be plotted (Fig. 1). The two curves represent the scission of the main chain and the selective degradation of the cross-links. Experimental data can be plotted on the graph, and depending on which curve a data point is closer to, it is possible to infer what is the main phenomenon that occurs during the devulcanization process.
dGTR_MW_50g_2 and dGTR_MW_100g_2 samples had high sol contents, coupled with high crosslink density values. It signified a low degree of devulcanization and suggested the degradation of the polymer chains, as shown in Fig. 1, since the corresponding data points are closer to the random scission curve than the selective cross-link scission curve (Table 9). Thermomechanically devulcanized GTR samples showed more promising results as their data points are located closer to the cross-link scission curve of the Horikx's plot.
Table 9 shows the vertical distance (in percentage) of experimental data points from the selective cross-link scission curve. If the value is zero, then the data point is on the selective cross-link scission curve. Based on these results, the best sample is dGTR_TM_40/160, and the worst is dGTR_MW_100g_2. But we need to evaluate these results with the degree of devulcanization to get a complete overview. If we look at these two samples, it is easy to determine which method is better, because both samples have almost the same cross-link density; they significantly differ only in sol content. We chose four samples for further study: dGTR_MW_100g_2, dGTR_TM_40/160, dGTR_TM_40/200 and dGTR_TM_120/200.
Cure characteristics of the rubber compounds
Figures 2 and 3 show the recorded vulcanization curves of the samples. Table 10 shows the cure characteristics of the dGTR-based rubber mixtures.
First, we tried to revulcanize neat dGTR (dGTR_MW_100g_2 sample) without any vulcanizing agents, but curing did not occur (Fig. 2a). The recorded torque values (S′) showed a continuous decrease with time. It can be inferred that the microwave treatment removed all active sulfur from the sample, inhibiting the formation of new cross-links. During devulcanization, sulfur-based cross-link bonds break. There are very few active sulfur atoms that can take part in the vulcanization process later. The sulfur atoms stay in the system in an inactive form or exit from it, generating sulfur dioxide or hydrogen sulfide. dGTR_MW_100g_2_0.5A and dGTR_MW_100g_2_A samples, containing additional curing agents according to Table 4, vulcanized like conventional rubber, and we were able to determine the main characteristics of vulcanization (Table 10). With devulcanization, active molecules were generated, capable of creating new bonds, therefore cross-links formed during vulcanization with the help of sulfur. We were able to produce a solid homogeneous rubber sheet via hot pressing. S′max values increased because of the extra vulcanizing agents added to dGTR but there is no significant effect of the oil content in dGTR mixtures (A and B samples). Traditional vulcanization curves were recorded in the case of all samples containing vulcanizing agents.
In the case of the NR_REF samples (Fig. 2b), paraffin oil and dGTR had similar plasticizing effects, and dGTR also accelerated curing. For the other samples (Fig. 2c), the trend is clear: S′max values decreased with increasing dGTR content. dGTR has a strong plasticizing effect on the mixtures. Vulcanization time also decreased with dGTR, but independently of its amount. The S′max values also decreased in GTR100 samples, but not as much as in the samples containing 100 phr of dGTR. The S′min values were almost twice as high as in the case of the other mixtures. This behavior is the result of the presence of hard GTR particles, and hence we can observe that dGTR has a stronger plasticizing effect than GTR. In the case of the tested samples with thermomechanically devulcanized rubber content, the lower their cross-link densities were, the lower the respective maximum torque values were (Fig. 2c).
Figure 3 shows the vulcanization curves of NR samples containing microwave devulcanized GTR with extra vulcanization agents (Fig. 3a) and NR samples containing thermomechanically devulcanized GTRs with extra vulcanization agents (Fig. 3b) (samples ending with “A” or “B”). The S′max values increased because of the extra vulcanizing agents added to dGTR; the extra vulcanizing agents increased the number of cross-links in the samples during curing.
Table 10 also shows the hardness of the samples; both GTR and dGTR content decreased the hardness of the compounds.
Mechanical properties of the cured rubber compounds
We were able to perform tensile tests on samples dGTR_MW_100g_2_A (tensile strength: 2.3 ± 0.3 MPa, elongation at break: 85 ± 12%) and dGTR_MW_100g_2_0.5A (tensile strength: 2.1 ± 0.2 MPa, elongation at break: 78 ± 10%). Even though hot pressing yielded homogeneous, rubber-like sheets, their mechanical properties were quite poor. It is necessary to combine NR with dGTR (the applied curing systems can be seen in Table 4).
Figure 4a shows the tensile strength of the NR-based samples containing different amounts of dGTR. There is no significant difference in tensile strength in the NR_REF samples; paraffin oil and dGTR have a similar effect. dGTR significantly reduced the tensile strength of the samples (samples containing 50, 100, and 185 phr of dGTR_MW_100g_2). The tensile strength values of samples containing thermomechanically devulcanized GTR are higher than those of the samples containing microwave-devulcanized GTR. Based on Horikx’s analysis, the polymer backbone of thermomechanically devulcanized GTRs suffered less severe degradation than that of microwave-devulcanized GTR. Additional vulcanizing agents in dGTR helped recover tensile strength because of the more significant number of cross-links generated compared with other samples (Fig. 4b).
The elongation at break of the samples (Fig. 5) decreased slightly when dGTR was used because of the intense plasticizing effect of the GTR particles softened by devulcanization. The tensile strength of the mixture containing GTR (NR_GTR_100) did not decrease as much as that of the mixture containing dGTR. The samples with GTR and dGTR became more rigid; their elongation at break values were lower than those of samples prepared by two-step mixing.
Tear strength decreased significantly because of the effect of dGTR and GTR (Fig. 6a). However, with additional vulcanizing agents (samples with a code ending with “A” and “B”), tear strength reached and exceeded the values of those of the NR_REF sample (Fig. 6b).
Devulcanized GTR samples suffered degradation, chain scission occurred and the mechanical properties, especially tear strength, dropped significantly when these dGTRs were introduced into NR samples. Extra vulcanization agents helped recover mechanical properties, but the increase was modest because of degraded dGTR particles. However, the shorter and more mobile molecules that formed during microwave devulcanization with the aid of extra vulcanization agents generated more cross-links between the rubber matrix and the surface of the dGTR particles. The improved adhesion between the phases and the different load mode of the tear test caused excellent tear strength in these samples.
Figure 7 shows the scanning electron microscopic images of the fracture surface of two tear specimens. Figure 7a shows the relatively smooth tear surface of the NR_GTR100 sample, containing untreated GTR. While, several vertical cracks can be seen in Fig. 7b, indicating the border of dGTR particles (marked with white arrows). It can be inferred that, because of the better adhesion between NR and dGTR (compared to NR and GTR), crack propagation in the dGTR-containing sample required a larger force. In the NR_GTR100 sample, the low interphase adhesion did not allow the GTR component to carry the tensile load. Consequently, GTR particles did not get deformed during the test, hence the smooth appearance of the sample.
Conclusions
We devulcanized ground tire rubber (GTR) with microwaves in a laboratory oven and thermomechanically in an internal mixer with different rotor speeds and temperatures. Then we characterized the devulcanized GTR (dGTR) samples by Soxhlet extraction and swelling tests to determine their soluble content and cross-link density and performed Horikx’s analysis. In the case of microwave-devulcanized samples, cross-link density was considerably reduced while sol content was high, which suggests that the devulcanization process was dominated by the random degradation of polymer chains. Horikx’s analysis showed that these samples suffered severe degradation. In the case of the thermomechanically devulcanized samples at low temperature and rotor speed settings, the main phenomenon was selective cross-link scission. At higher temperatures and rotor speeds, degradation of the main chains occurred along with cross-link cleavage. Based on Horikx’s analysis, four devulcanized GTR samples were chosen and mixed with NR. dGTR content reduced the tensile strength of the samples drastically, but elongation at break did not follow this trend. Curing curves showed that dGTR has a plasticizing effect on rubber mixtures. The tensile strength of samples containing different dGTRs reflects the results of Horikx’s analysis. The samples containing 100 phr of thermomechanically devulcanised GTR had the same tensile strength as the samples with 50 phr of microwave-devulcanized GTR. Two-step mixing (first adding vulcanization agents to dGTR, then mixing it with the reference rubber mixture) helped recover mechanical properties, especially tear strength.
References
Ali, S. A., Hasan, F., Shah, Z., Kanwal, N. & Zeb, S. Biodegradation of natural and synthetic rubbers: A review. Int. Biodeterior. Biodegrad. 83, 145–157 (2013).
Adhikari, B., De, D. & Maiti, S. Reclamation and recycling of waste rubber. Prog. Polym. Sci. 25, 909–948 (2000).
Liu, S., Yu, J., Bikane, K., Chen, T., Ma, C., Wang, B. & Sun, L. Rubber pyrolysis: Kinetic modeling and vulcanization effects. Energy 155, 215–225 (2018).
Karger-Kocsis, J., Mészáros, L. & Bárány, T. Ground tyre rubber (GTR) in thermoplastics, thermosets, and rubbers. J. Mater. Sci. 48, 1–38 (2013).
Hejna, A., Klein, M., Saeb, M. R. & Formela, K. Towards understanding the role of peroxide initiators on compatibilization efficiency of thermoplastic elastomers highly filled with reclaimed GTR. Polym. Testing 73, 143–151 (2019).
Lu, X., Wang, W. & Yu, L. Waste ground rubber tire powder/thermoplastic vulcanizate blends: Preparation, characterization, and compatibility. J. Appl. Polym. Sci. 131, 9 (2014).
Bagheri, R., Williams, M. A. & Pearson, R. A. Use of surface modified recycled rubber particles for toughening of epoxy polymers. Polym. Eng. Sci. 37, 245–251 (1997).
Lamminmäki, J., Li, S. & Hanhi, K. Feasible incorporation of devulcanized rubber waste in virgin natural rubber. J. Mater. Sci. 41, 8301–8307 (2006).
Abang Ismawi Hassim, D. H., Abraham, F., Summerscales, J. & Brown, P. The effect of interface morphology in waste tyre rubber powder filled elastomeric matrices on the tear and abrasion resistance. Express Polym. Lett. 13, 248–260 (2019).
Formela, K., Korol, J. & Saeb, M. R. Interfacially modified LDPE/GTR composites with non-polar elastomers: From microstructure to macro-behavior. Polym. Testing 42, 89–98 (2015).
Sonnier, R., Leroy, E., Clerc, L., Bergeret, A., Lopez-Cuesta, J.-M., Bretelle, A.-S. & Ienny, P. Compatibilizing thermoplastic/ground tyre rubber powder blends: Efficiency and limits. Polym. Testing 27, 901–907 (2008).
de Sousa, F. D. B., Scuracchio, C. H., Hu, G.-H. & Hoppe, S. Devulcanization of waste tire rubber by microwaves. Polym. Degrad. Stab. 138, 169–181 (2017).
Colom, X., Faliq, A., Formela, K. & Cañavate, J. FTIR spectroscopic and thermogravimetric characterization of ground tyre rubber devulcanized by microwave treatment. Polym. Testing 52, 200–208 (2016).
Scuracchio, C. H., Waki, D. A. & da Silva, M. L. C. P. Thermal analysis of ground tire rubber devulcanized by microwaves. J. Therm. Anal. Calorim. 87, 893–897 (2007).
Movahed, S. O., Ansarifar, A. & Estagy, S. Review of the reclaiming of rubber waste and recent work on the recycling of ethylene propylene diene rubber waste. Rubber Chem. Technol. 89, 54–78 (2016).
Formela, K. & Cysewska, M. Efficiency of thermomechanical reclaiming of ground tire rubber conducted in counter-rotating and co-rotating twin screw extruder. Polimery 59, 231–238 (2014).
Hassan, M. M., Aly, R. O., Aal, S. E. A., El-Masry, A. M. & Fathy, E. S. Styrene butadiene-based blends containing waste rubber powder: Physico-mechanical effects of mechanochemical devulcanization and gamma irradiation. J. Ind. Eng. Chem. 19, 1735–1742 (2013).
Sripornsawat, B., Saiwari, S., Pichaiyut, S. & Nakason, C. Influence of ground tire rubber devulcanization conditions on properties of its thermoplastic vulcanizate blends with copolyester. Eur. Polym. J. 85, 279–297 (2016).
Ghorai, S., Bhunia, S., Roy, M. & De, D. Mechanochemical devulcanization of natural rubber vulcanizate by dual function disulfide chemicals. Polym. Degrad. Stab. 129, 34–46 (2016).
Garcia, P. S., de Sousa, F. D. B., de Lima, J. A., Cruz, S. A. & Scuracchio, C. H. Devulcanization of ground tire rubber: Physical and chemical changes after different microwave exposure times. Express Polym. Lett. 9, 1015–1026 (2015).
Aoudia, K., Azem, S. Hocine, N. A., Gratton, M., Pettarin, V. & Seghar, S. Recycling of waste tire rubber: Microwave devulcanization and incorporation in a thermoset resin. Waste Manag. 60, 471–481 (2017).
Molanorouzi, M. & Mohaved, S. O. Reclaiming waste tire rubber by an irradiation technique. Polym. Degrad. Stab. 128, 115–125 (2016).
Formela, K., Hejna, A., Zedler, Ł, Colom, X. & Cañavate, J. Microwave treatment in waste rubber recycling—Recent advances and limitations. Express Polym. Lett. 13, 565–588 (2019).
Isayev, A. I. Recycling of rubbers. In Science and Technology of Rubber (eds Mark, J. E. et al.) 697–764 (Elsevier, Amsterdam, 2013).
Isayev, A. I., Chen, J. & Tukachinsky, A. Novel ultrasonic technology for devulcanization of waste rubbers. Rubber Chem. Technol. 68, 267–280 (1995).
Mangili, I., Lasagni, M., Huang, K. & Isayev, A. I. Modeling and optimization of ultrasonic devulcanization using the response surface methodology based on central composite face-centered design. Chemom. Intell. Lab. Syst. 144, 1–10 (2015).
Ghavipanjeh F., Ziaei Rad Z. & Pazouki M. Devulcanization of ground tires by different strains of bacteria: Optimization of culture condition by Taguchi method. J. Polym. Environ. 26, 3168–3175 (2018).
Meysami, M., Tzoganakis, C., Mutyala, P., Zhu, S. H. & Bulsari, M. Devulcanization of scrap tire rubber with supercritical CO2: A study of the effects of process parameters on the properties of devulcanized rubber. Int. Polym. Proc. 32, 183–193 (2017).
Jones, D. A., Lelyveld, T. P., Mavrofidis, S. D., Kingman, S. W. & Miles, N. J. Microwave heating applications in environmental engineering—A review. Resour. Conserv. Recycl. 34, 75–90 (2002).
Horikx, M. M. Chain scissions in a polymer network. J. Polym. Sci. 19, 445–454 (1956).
Ellis, B. & Welding, G. N. Estimation, from swelling, of the structural contribution of chemical reactions to the vulcanization of natural rubber. Part II. Estimation of equilibrium degree of swelling. Rubber Chem. Technol. 37, 571–575 (1964).
de Sousa, F. D. B., Zanchet, A. & Scuracchio, C. H. Influence of reversion in compounds containing recycled natural rubber: In search of sustainable processing. J. Appl. Polym. Sci. 134, 45325 (2017).
Formela, K., Cysewska, M. & Haponiuk, J. T. Thermomechanical reclaiming of ground tire rubber via extrusion at low temperature: Efficiency and limits. J. Vinyl Addit. Technol. 22, 213–221 (2016).
Verbruggen, M. A., Van Der Does, L., Noordermeer, J. W., Van Duin, M. & Manuel, H. J. Mechanisms involved in the recycling of NR and EPDM. Rubber Chem. Technol. 72, 731–740 (1999).
Tatangelo, V., Mangili, I., Caracino, P., Anzano, M., Najmi, Z., Bestetti, G., Collina, E., Franzetti, A. & Lasagni, M. Biological devulcanization of ground natural rubber by Gordonia desulfuricans DSM 44462T strain. Appl. Microbiol. Biotechnol. 100, 8931–8942 (2016).
Simon, D. Á., Pirityi, D., Tamás-Bényei, P. & Bárány, T. Microwave devulcanization of ground tire rubber and applicability in SBR compounds. J. Appl. Polym. Sci. 137, 48351 (2020).
Simon, D. Á, Halász, I., Karger-Kocsis, J. & Bárány, T. Microwave devulcanized crumb rubbers in polypropylene based thermoplastic dynamic vulcanizates. Polymers 10, 767 (2018).
Flory, P. J. & Rehner Jr, J. Statistical mechanics of cross-linked polymer networks II. Swelling. J. Chem. Phys. 11, 521–526 (1943).
Verbruggen, M. A. L., van der Does, L., Dierkes, W. K. & Noordermeer, J. W. M. Experimental validation of the Charlesby and Horikx models applied to de-vulcanization of sulfur and peroxide vulcanizates of NR and EPDM. Rubber Chem. Technol. 89, 671–688 (2016).
Acknowledgements
This work was supported by the National Research, Development and Innovation Office, Hungary (OTKA K115949), and by BME Nanotechnology and Materials Science TKP2020 IE grant of NKFIH Hungary (BME IE-NAT TKP2020).
Author information
Authors and Affiliations
Contributions
Conceptualization, D.Á.S., and T.B.; Methodology, D.Á.S., and D.Z.P.; Validation, D.Á.S., D.Z.P., and T.B.; Investigation, D.Á.S.; Resources, T.B.; Data Curation, D.Á.S., and D.Z.P.; Writing–Original Draft Preparation, D.Á.S.; Writing, Review & Editing, D.Á.S., D.Z.P., and T.B.; Visualization, D.Á.S.; Supervision, T.B.; Project Administration, T.B.; Funding Acquisition, T.B.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Simon, D.Á., Pirityi, D.Z. & Bárány, T. Devulcanization of ground tire rubber: microwave and thermomechanical approaches. Sci Rep 10, 16587 (2020). https://doi.org/10.1038/s41598-020-73543-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-73543-w
- Springer Nature Limited