Abstract
Honeybees (Apis mellifera L.), which unquestionably play an economically important role in pollination and agricultural production, are at risk of decline. To study changes in gene expression in insects upon exposure to pesticides or other external stimuli, appropriate reference genes are required for data normalization. Since there is no such gene that is absolutely invariable under all experimental conditions, the aim of this study was to identify the most stable targets suitable for subsequent normalization in quantitative experiments based on real-time polymerase chain reaction in honeybee research. Here, we evaluated the expression of fifteen candidate housekeeping genes from three breeding lines of honeybees treated with pyrethroids to identify the most stable genes. The tested insects were exposed to deltamethrin or lambda-cyhalothrin, and then, changes in the accumulation of selected transcripts were assessed, followed by statistical analyses. We concluded that AmRPL32, AmACT and AmRPL13a were the commonly recorded most stable genes in honeybees treated with the selected pyrethroids.
Similar content being viewed by others
Introduction
The importance of honeybees (Apis mellifera L.) as pollinators is unquestionable. However, since intensive crop production is currently strictly dependent on pesticide use, honeybees are exposed to agrochemicals during pollination1, and these chemicals seem to impact the insects.
Pyrethroids are an important group of insecticides used for insect pest management2. They are the most frequently used crop protection products for crops pollinated by honeybees, and their residues are most often found in these insects3,4,5. Pyrethroids target the nervous system of the treated individuals; their active compound binds to voltage-gated sodium channels (VGSCs) in neurons and alters the function of these channels by maintaining the channel opening on axonal membranes. As a result, the neuron membranes cannot repolarize, leaving the axonal membrane permanently depolarized, thereby paralysing the organism6. By performing gene expression analyses, it is possible to understand how honeybees react to pyrethroids at the molecular level. As a result, the data obtained are then relevant for sustainability programmes. For this purpose, several methods can be used, such as next-generation sequencing (RNAseq), microarrays, northern hybridization or quantitative reverse transcription polymerase chain reaction (RT-qPCR). Because of its versatility, low cost and high detection rate, RT-qPCR is now not only a very important tool for a majority of gene expression studies but also a standard for validating results derived from RNAseq7 or microarray analyses8 in which differentially expressed genes are studied. However, similar to other RNA-based quantitative techniques, RT-qPCR experiments need to be carefully designed and performed9. The experimental design is based on, among other factors, the selection of a good internal control, that is, a gene that exhibits stable expression under the experimental conditions being tested. Only by using this approach is it possible to accurately estimate the accumulation of target mRNA molecules. However, it should be noted that there is no universal gene that might be used for RT-qPCR normalization under every experimental condition. The expression of housekeeping genes (HKGs) can be influenced by many factors; therefore, validation of their stability should always be performed before quantitation of mRNA targets10,11. The need of careful selection of reference genes for gene expression studies in insects was widely reviewed previously12.
In this study, we analysed the stability of the expression of fifteen genes, used previously in selection of reference genes in insects12, involved in basic metabolism of the honeybees, namely, actin (AmACT), α-tubulin (AmTUB), glutathione-S-transferase (AmGST1), glyceraldehyde-3-phosphate dehydrogenase (AmGAPDH), porphobilinogen deaminase (AmHMBS), ribosomal protein L32 (AmRPL32), 60S ribosomal protein L13a (AmRPL13a), 40S ribosomal protein S18 (AmRP18S), succinate dehydrogenase (AmSDHA), TATA-box-binding protein (AmTBP), elongation factor 1-alpha (AmEF1α), arginine kinase (AmARGK), chitin synthase 6 (AmCHS6), dorsal (AmDORS), and 18S ribosomal RNA (Am18S), in A. mellifera L. exposed to two types of pyrethroids: deltamethrin and lambda-cyhalothrin. The aim of the study was to identify the HKGs stably expressed in honeybees. We performed experiments to determine the best reference genes (a) for all the experimental conditions tested (namely, for all the breeding lines under treatment with pyrethroids), (b) for a particular breeding line individually, and (c) by focusing on two different active pyrethroid compounds. By utilizing RT-qPCR and four statistical algorithms, we concluded that regardless of the conditions tested, the genes AmRPL32, AmACT or AmRPL13a were commonly found among the most stable genes in honeybees treated with the mentioned pyrethroids. Moreover, by performing pairwise variation analysis (Vn/n+1), we determined that two of the identified reference genes would be sufficient for accurate normalization of RT-qPCR experimental results. Finally, we validated the results and used the selected reference genes to measure the expression of two cytochrome P450 monooxygenase (AmCYP450) genes described previously to be influenced in honeybees treated with insecticides13,14, and therefore the expression of AmCYP6AQ115 and AmCYP305a113 assayed in the research.
Results
Determination of the specificity of the designed primers
In this study, we evaluated fifteen candidate genes from honeybees to check their stability in insects exposed to pyrethroid treatment (Table 1). The main goal of the research was to identify the most stable genes that could be used as internal controls in experiments based on RT-qPCR to determine or verify differentially expressed genes in honeybees treated with pyrethroids.
First, from the GenBank database, we retrieved cDNA sequences of A. mellifera L. encoding these genes and used the data as input in Primer3 for designing the best primer pairs. By doing so, fifteen primer pairs that matched the implemented parameters were chosen, with the resulting amplicon lengths between 100 and 250 bp and the annealing temperature of the primers set at approximately 60 °C. Then, by performing a pilot experiment (end-point RT-PCR), we tested the designed primers for their specificity. The products of the RT-PCR were resolved on an agarose gel, and after staining, a single DNA band was detected for each tested primer pair (Fig. 1). No amplification products were detected in the no-template control reactions. Moreover, the results from Sanger sequencing of the cloned amplicons verified the sequence specificity of the primers used.
Next, we examined the expression rates of the tested transcripts: after each RT-qPCR, all the CT output data were grouped in a table, and a combined box plot was prepared. All of the fifteen tested genes were amplified by RT-qPCR. The highest expression rate was observed for Am18S rRNA, with a CT value of approximately 5.61. However, we omitted Am18S rRNA in further analyses because of the extremely high accumulation of rRNA in the analysed insects. The CT values of all other candidate genes ranged between 14.33 and 25.62, which were the values for AmEF1α and AmGST, respectively (Fig. 2). Importantly, the expression levels of the analysed genes were similar among the three tested breeding lines (see Supplementary Fig. S1). Next, analysis of melting curves generated during the melting stage of RT-qPCR verified the presence of a single amplification product in each reaction: the generated melting curves were sharp and symmetric (Fig. 3), indicating reaction specificity. The melting temperature of the amplicons ranged from 75.46 to 84.33 °C, which were the values for AmHMBS and Am18S, respectively.
Stability analysis of candidate reference genes
In the present study, we focused on fourteen candidate HKGs of A. mellifera L. treated with two pyrethroids: deltamethrin and lambda-cyhalothrin. Our general goal was to identify the most stable reference genes in honeybees (a) regardless of breeding line and chemical compounds used for treatment and (b) individually for three breeding lines, and (c) for the two pyrethroids used for treating the insects. To achieve this goal, we used the following statistical tools: geNorm, BestKeeper, NormFinder and ΔCT. The comprehensive analysis was performed using RefFinder (https://www.heartcure.com.au/reffinder).
Search for the most stable reference genes for all breeding lines exposed to pyrethroids
geNorm software, installed as a Bioconductor “NormqPCR” package in R software16, was used as the first program to identify the stability of the predicted HKGs, and the resulting values were used to order the HKGs from the most stable (the lowest M value) to the least stable (the highest M value). The AmHMBS gene and the AmSDHA gene had the highest expression stability values (0.0597 and 0.0604 M, respectively), followed by AmTub, AmTBP, and AmRPL32. The five least stable genes were AmRPL13a, AmGST, AmRPL18S, AmGAPDH and AmEF1α (Table 2).
Next, three additional methods were used to calculate the most stable reference genes within the pool of all tested samples. NormFinder analysis classified the AmRPL32, AmHMBS, AmCHS6, AmTub and AmAct genes as the most stable genes, whereas the AmEF1α, AmGST, AmTBP, AmGAPDH and AmDORS genes were indicated as being the least stable. The ΔCT analysis showed that AmRPL32, AmHMBS, AmTub, AmCHS6 and AmAct had the highest expression stability in comparison to AmEF1α, AmGST, AmTBP, AmGAPDH and AmDORS, which exhibited unstable expression. Then, based on the standard deviation (SD) of the CT measurements, the stability values for the expression of fourteen candidate reference genes were calculated using the BestKeeper program, which showed slight differences compared to previous algorithms. The AmRPL32, AmSDHA, AmCHS6, AmHMBS, and AmRPL13a genes were identified as the most stable genes, and the AmTBP, AmEF1α, AmDORS, AmRP18S and AmGST genes were identified as the least stable genes.
Finally, to prepare a general ranking of most stable/unstable genes, a comprehensive analysis was performed with the support of RefFinder. According to the recommended comprehensive ranking, the AmRPL32, AmAct, AmHMBS, AmTub, and AmCHS6 genes were identified as the five most stable genes, and the AmRP18S, AmDORS, AmTBP, AmGAPDH, and AmEF1α genes were identified as the five least stable genes.
Analysis of HKG stability among the three breeding lines
Next, the mentioned calculating methods were implemented to check the most stable HKGs in honeybees with regard to their breeding origin (breeding line) separately.
For the Kortówka line (Table 3), the five most stable genes were as follows: AmGST, AmRPL32, AmTub, AmARGK and AmAct (according to geNorm); AmAct, AmARGK, AmRPL13a, AmCHS6 and AmSDHA (according to NormFinder); AmGST, AmARGK, AmRPL32, AmTub and AmAct (according to BestKeeper); AmAct, AmARGK, AmRPL13a, AmCHS6 and AmSDHA (according to the ΔCT method).
For the Kortówka breeding line, the RefFinder method ordered the most stable genes as follows: AmAct, AmARGK, AmRPL13a, AmGST and AmGAPDH.
For the Alpejka line (Table 4), the most stable genes were AmTBP, AmDORS, RPL32, AmHMBS and AmRP18S (according to geNorm); AmDORS, AmRP18S, AmEF1α, AMHMBS and AmRPL13a (according to NormFinder); AmDORS, AmRP18S, AmRPL32, AmGST and AmRPL13a (according to BestKeeper); and AmDORS, AmRP18S, AmEF1α, AmHMBS and AmRPL13a (according to the ΔCT method).
On the basis of the abovementioned results, the RefFinder analysis identified AmDORS, AmRP18S, AmRPL13a, AmGAPDH, and AmEF1α as the most stable genes for the Alpejka breeding line.
For the Nieska line (Table 5), the most stable genes were AmTBP, AmDORS, AmRPL32, AmHMBS, AmRP18S (according to geNorm); AmARGK, AmTub, RPL13a, AmCHS6 and AmTBP (according to NormFinder); AmRPL32, AmRP18S, AmRPL13a, AmAct and AmGST (according to BestKeeper); and AmARGK, AmTub, AmRPL13a, AmRP18S and AmRPL32 (according to the ΔCT method).
Thus, the following genes were selected as the most stable genes according RefFinder calculations for the Nieska line: AmARGK, AmRPL32, AmRP18S, AmRPL13a and AmTub (Table 5).
Analysis of the HKG stability with regard to the active substance of pyrethroid insecticide
Analysis of the influence of each insecticide used for insect treatment on HKG stability was also performed (Table 6). All calculations were performed as stated above using geNorm, NormFinder, BestKeeper, ΔCT and RefFinder. In insects treated with deltamethrin, the rank order of the most stable genes was as follows: AmSDHA, AmTub, AmHMBS, AmTBP and AmRPL32 (according to the geNorm method); AmCHS6, AmRPL32, AmARGK, AmHMBS and AmRPL13a (according to NormFinder); AmRPL32, AmRPL13a, AmGST, AmAct and AmRP18S (according to the BestKeeper); AmCHS6, AmRPL32, AmTub, AmHMBS and AmARGK (according to the ΔCT method).
The comprehensive analysis performed by RefFinder ranked the most stable genes in the following order for deltamethrin treatment: AmRPL32, AmCHS6, AmTub, AmARGK and AmHMBS.
The same calculations were performed to select HKGs stably expressed in A. mellifera L. exposed to lambda-cyhalothrin. The CT data obtained after RT-qPCR were grouped in a table and subjected to subsequent calculations. The geNorm method indicated the following genes as being the most stable: AmTub, AmTBP, AmHMBS, AmSDHA and AmCHS6. Next, the list of the five most stable genes calculated by NormFinder included AmRPL32, AmRPL13a, AmHMBS, AmAct and AmTub. BestKeeper selected AmGST, AmRPL32, AmRPL13a, AmRP18S and AmAct as the most stable genes. AmRPL32, AmRPL13a, AmHMBS, AmAct and AmTub were identified as the most stable genes using the ΔCT method.
Finally, comprehensive analysis (by RefFinder) indicated AmRPL32, AmCHS6, AmTub, AmARGK and AmHMBS as the most stable genes under lambda-cyhalothrin exposure.
Determination of the minimum number of reference genes necessary for normalization
Pairwise variation analysis (Vn/n+1) performed using the geNorm method17 indicated that the expression of the target gene in each considered experimental variant needs to be normalized using two selected reference genes. This was indicated by pairwise variation (V) with the threshold value set at 0.1517. In all tested experimental variants, the V2/3 value was lower than 0.15 (Fig. 4).
Optimal number of reference genes for various conditions. The geNorm algorithm was used to determine the pairwise variation (V) between the reference genes for treatments with pyrethroids together (Carnolian honeybees) or separately (deltamethrin or lambda-cyhalothrin). The effect of pyrethroid treatments on three breeding lines was also indicated (Kortówka, Alpejka and Nieska). The threshold for adequate normalization was V ≤ 0.15.
Validation of reference genes
To validate the obtained results (the indicated stable HKGs for each experimental condition), we performed an analysis of the expression of two cytochrome P450 monooxygenase (AmCYP450) genes in honeybees exposed to pyrethroid treatment. CYP450s are known to be involved in xenobiotic detoxification in insects14. Importantly, it was described previously that the expression of AmCYP6AQ115 and AmCYP305a113 was influenced by insecticides. Validation experiments aimed at normalization of the expression of both AmCYP450 genes were performed in the following contexts: first, for the entire set of tested Carnolian honeybees exposed to pyrethroid treatment; second, for testing the effect of pyrethroid treatment on the expression of AmCYP450 in each breeding line separately; and finally, for analysing the expression of AmCYP450 separately in deltamethrin- or lambda-cyhalothrin-treated insects.
As indicated earlier in the manuscript, using two reference genes is sufficient for accurate normalization of genes in pyrethroid-treated insects. For normalization of AmCYP450 expression in Carnolian honeybees, the two following HKGs were used: AmRPL32 and AmHMBS.
The results showed (Fig. 5) that expression of the AmCYP6AQ1 gene increased slightly in honeybees treated with deltamethrin (1 h and 24 h after treatment) and lambda-cyhalothrin (24 h after treatment) with a 1.35-fold change, 1.28-fold change and 1.47-fold change (all with p < 0.01), respectively. On the other hand, the expression of AmCYP305a1 in pyrethroid-treated honeybees increased over time, reaching a 4.91-fold change 24 h after treatment (p > 0.05) in the insects exposed to lambda-cyhalothrin (Fig. 5).
Expression of the two AmCYP450 genes AmCYP6AQ1 and AmCYP305a1 in Apis mellifera L. treated with either deltamethrin (A) or lambda-cyhalothrin (B) normalized against the indicated reference genes (AmRPL32 and AmHMBS). Blue bars: 1 h post treatment, orange bars: 24 h post treatment. Error bars represent the standard deviation. The Mann–Whitney U-test was used. **p < 0.01, *p < 0.05.
Next, the expression of AmCYP450 genes was validated in each breeding line individually (Fig. 6). For the Kortówka line, the AmAct and AmARGK genes were chosen; for Alpejka, AmDORS and AmRP18S were used for normalization; and for the Nieska line, the AmRPL32 and AmRPL13a genes were selected as the best normalizers for expression of the mentioned AmCYP450s.
Expression of the two AmCYP450 genes (AmCYP6AQ1 and AmCYP305a1) in three breeding lines of Apis mellifera L., namely, Alpejka (A), Kortówka (B) and Nieska (C), treated with either deltamethrin or lambda-cyhalothrin, normalized against the indicated reference genes: (A) AmDORS and AmRP18S; (B) AmAct and AmARGK; (C) AmRPL32 and AmRPL13a). Blue bars—1 h post treatment, orange bars—24 h post treatment. Error bars represent the standard deviation. Error bars represent the standard deviation. The Mann–Whitney U-test was used. **p < 0.01, *p < 0.05.
When considering the expression of AmCYP450s in honeybees treated with pyrethroids, we observed that the expression of AmCYP6AQ1 and AmCYP305a1 in insects belonging to the Alpejka breeding line changed slightly. On the other hand, in the Kortówka breeding line, the level of expression of the AmCYP305a1 gene increased slightly 1 h and 24 h after deltamethrin treatment. For the Nieska breeding line, the expression of AmCYP6AQ1 was slightly upregulated in insects treated with both pyrethroids, whereas the expression of AmCYP305a1 was downregulated in honeybees treated with deltamethrin. These data also showed that each breeding line of tested insects responded differently to pyrethroid treatment, taking into account changes in the expression level of the AmCYP450s genes tested (Fig. 6).
Finally, the expression levels of the AmCYP6AQ1 gene and the AmCYP305a1 gene were validated separately in insects under deltamethrin treatment and lambda-cyhalothrin treatment (Fig. 7, Table 6). The active substances used in our research model have little effect on changes in the expression level of the analysed AmCYP450s genes. In particular, when analysing the effect of deltamethrin on AmCYP450 expression, a minor increase in the expression level of the AmCYP6AQ1 gene 1 h post treatment and 24 h after pyrethroid exposure (1.40-fold change and 1.26-fold change, respectively, p < 0.01) was indicated. Accordingly, in lambda-cyhalothrin-treated insects, AmCYP6AQ1 showed a modest, statistically significant increase in expression level 24 h after pyrethroid treatment (1.34-fold change, with p < 0.01). Similarly, the expression level of the AmCYP305a1 gene was somewhat stable over time, reaching a 1.35-fold change (with p < 0.05) in bees 1 h after treatment with lambda-cyhalothrin (Fig. 7).
Expression of the two AmCYP450 genes AmCYP6AQ1 and AmCYP305a1 in Apis mellifera L. treated with either deltamethrin (A) or lambda-cyhalothrin (B), normalized against the indicated reference genes: (A) AmRPL32 and AmCHS6; (B) AmRPL32 and AmRPL13a. The effects of the two active compounds used to treat honeybees were considered separately. Blue bars: 1 h post treatment, orange bars: 24 h post treatment. Error bars represent the standard deviation. Error bars represent the standard deviation. The Mann–Whitney U-test was used. **p < 0.01, *p < 0.05.
Additionally, changes in AmCYP450s expression levels normalized against two unstable HKGs were also analysed (see Supplementary Figs. S2, S3 and S4). The use of inappropriate normalizers in differential gene expression analysis resulted in increased statistical significance at the expense of an increased error range and changes in the expression levels of target genes in individual research models (e.g., for the Nieska line, the AmCYP305a1 gene expression level 24 h after lambda-cyhalothrin treatment was almost 40 times higher than that obtained if the least stable genes were selected (see Supplementary Fig. S3). Moreover, the use of the highly unstable HKGs for validation gives different, highly discrepant results, as in the case of the Kortówka line, where after using the most stable genes, a decrease was observed in the level of AmCYP305a1 gene expression (1.19-fold change), while using the least stable genes resulted in a 3.11-fold increase in the expression of a given gene (see Supplementary Fig. S3).
Discussion
To minimize both biological and experimental errors in quantitative analyses performed by means of real-time qPCR, it is important to choose the most stable reference genes for normalization of RNA input. However, this requires an individualized research approach for each analysed parameter 9,17. One such parameter is the fitness and mortality of bees associated with commonly used insecticides, which has been extensively discussed18,19,20,21,22.
In this study, we investigated the expression stability of 14 candidate reference genes of A. mellifera L., belonging to Carnolian honeybees, exposed to pyrethroids. The selected subspecies of the honeybee was treated with two insecticides: deltamethrin23 and lambda-cyhalothrin24. It should be remembered that honeybees of various genetic background (like the three breeding lines described in the study: Alpejka, Nieska and Kortówka) might react differently at the level of insecticide sensitivity25,26 what can expressed at the molecular level.
Carnolian honeybees are highly adapted to nectar and climatic flow both in Poland and worldwide27. Analysis of all the obtained data described in this study on Carnolian honeybees under pyrethroid treatments indicated 5 stably expressed genes: AmHMBS (responsible for haem synthesis and porphyrin metabolism), AmCHS6 (responsible for synthesis of chitin), AmRPL32 (ribosomal protein gene), AmAct (encoding cytoskeletal structural proteins), and AmTub (encoding cytoskeletal structural proteins).
Analysis of the expression stability of selected candidate reference genes with respect to individual breeding lines distinguished a common high-scoring gene, AmRPL13a, in terms of stability for all the tested lines. On the other hand, AmDORS, AmAct and AmTub were selected as the most stable genes in the Alpejka, Kortówka and Nieska breeding lines, respectively. Similarly common most stable genes were also observed between the Kortówka and Nieska lines (the AmARGK gene) and between the Alpejka and Nieska lines (the AmRP18S gene). The expression level of the AmARGK gene does not change after carbon dioxide narcosis in honeybee workers28; however, it should be noted that the amount of ARGK protein in the antennae can vary between bee families29 . The ribosomal genes (from the functional rRNA-coding regions) are structurally conserved and homogeneous throughout the nuclear and mitochondrial genomes in honeybees30 and are often used as reference genes for differential expression studies31,32,33. In research on the effects of imidacloprid treatment on honeybees, ribosomal genes have been shown to be upregulated13, which means that they should be approached with caution as potential reference genes. The analyses also show the variable levels of expression of target genes relative to the AmDORS gene described in the literature34. Depending on the breeding line tested, the expression stability results for individual genes were classified slightly differently (Tables 3, 4, 5); therefore, in experiments, both the population and the breeding line should be determined with full accuracy to avoid statistical errors in research.
The stability ranking of HKGs in honeybees under pyrethroid treatment, when the active compounds (deltamethrin or lambda-cyhalothrin) were considered separately, showed three common most stable reference genes: AmRPL32, AmTub and AmHMBS. These results were confirmed with the data previously obtained when active substances were analysed together; however, it should again be noted that the genes were placed at different positions in the ranking order (after deltamethrin treatment: AmRPL32, AmCHS6, AmTub, AmARGK and AmHMBS; after lambda-cyhalothrin treatment: AmRPL32, AmAct, AmRPL13a, AmHMBS and AmTub). Such differences may occur due to differences in the sample sizes analysed individually. For the entire set of Carnolian honeybees, all data obtained in the experiments were taken into account. In turn, for the analysis of bees after treatment with deltamethrin or lambda-cyhalothrin, data obtained for a specific pyrethroid active substance treatment/exposure were taken (limiting the sample size from 108 bees up to 72 individuals). This is why the selection of the sample is such an important aspect of research related to differential gene expression9.
The validation of the indicated most stable reference genes showed that the selection of inappropriate normalizers, the expression of which is not stable under the conditions being tested, can significantly affect the final results of the analysis of the target gene of interest. The values may vary by up to 40 times, as was observed for the expression level of the AmCYP6AQ1 gene in the Nieska line exposed to lambda-cyhalothrin (24 h after treatment), when we compared the results obtained by using the most stable genes and least stable genes for normalization (see Supplementary Fig. S3). The statistical significance and direction of changes in the level of expression between two time points were also divergent after the selection of relatively less stable reference genes for analysis (as was the case for the Nieska breeding line, as stated earlier) (see Supplementary Figs. S2, S3, S4). Therefore, optimization of testing data by using various statistical programs is very important when studying changes in the expression of target genes relative to that of a reference gene12. It should also be noted that the selection of HKGs may differ if the research model assumes testing on populations, not on specific breeding lines, as presented in Tables 2, 3, 4, 5. Previous work also showed that the differences in the expression stability results for reference genes may be due to the season in which the study was conducted35 and the stage of maturation of the tested individuals36. The expression levels of the AmCYP450 genes validated against the selected HKGs confirmed some behavioural observations for the developmental lines tested. Namely, slight changes in the expression of the AmCYP6AQ1 and AmCYP305a1 genes were observed for the Alpejka line (Fig. 6), in which individuals showed the highest liveliness among the three breeding lines during the experiment. Accordingly, the most considerable increase in the expression of these genes was demonstrated for the Nieska line (Fig. 6), the individuals of which tended to gather in groups and exhibited low activity (data not shown).
To summarize, regardless of the experimental conditions or tested breeding line of the examined insects, the above studies indicated the three following HKGs as reference genes to be considered, as they were classified by each analysis as being the most stable genes: AmRPL32, AmAct and AmRPL13a.
Methods
Insects used in the study
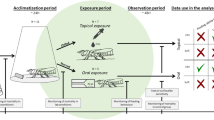
In this study, three breeding lines of A. mellifera L. were used, namely, Kortówka, Alpejka and Nieska, all belonging to Carniolan honeybees. The insects were taken from original hives by a beekeeper and were individually treated with a 1 µl dose of one of the following pyrethroids: deltamethrin (0.75 ml/L (4.8%)) or lambda-cyhalothrin (0.75 ml/L (4.81%)). Non-treated insects were used as a control. Then, the treated bees were gathered (6–15 insects, whereas for RNA isolation 6 insects were taken) in bee cages and collected 1 h and 24 h after treatment. During this time, the insects were kept at room temperature on a laboratory bench. Then, the insects were immediately frozen in liquid nitrogen and stored at -80 °C.
RNA isolation and cDNA synthesis
Single insects (from six biological replicates) were pulverized in liquid nitrogen using a mortar and pestle and were subsequently stored at − 80 °C for further analyses. Next, up to 100 mg of pulverized material was taken for total RNA extraction. RNA isolation was performed using 1 ml of TriReagent Solution (Invitrogen) followed by RNA precipitation with propanol. The resulting RNA pellet was washed with 70% ethanol, air-dried and resuspended in nuclease-free water. The concentration of the RNA, as well as its purity (the 260/230 and 260/280 values) were measured using a NanoDrop 2000 spectrophotometer (Thermo Scientific), whereas the quality of the RNA was assessed by means of gel electrophoresis.
Contaminant genomic DNA in the RNA samples was removed using dsDNase enzyme (Thermo Scientific). Next, cDNA synthesis was performed using the Maxima First Strand cDNA Synthesis Kit for RT-qPCR (Thermo Scientific) and 3 μg of total RNA. The resulting cDNA was finally diluted 3 times with water (50 ng/μl).
Primer selection and real-time quantitative PCR
The primers used in this study were designed using Primer3 online software37,38. The coding sequences of target transcripts were retrieved from GenBank and further analysed with Primer3 software to indicate the best pairs for RT-qPCR (Table 1). The selected primers were tested for their specificity: initially, all the tested sequences were verified by BLAST, and next, the primers were used in subsequent end-point RT-PCR to check the estimated size of the resulting amplicons. The RT-PCRs were performed in 20 μl reactions containing 1 × reaction master mix (DreamTaq PCR Master Mix, Thermo Scientific), 0.5 μM forward primer, 0.5 μM reverse primer and 1 μl of cDNA. The reaction was incubated for 3 min at 95 °C, followed by 35 cycles of 95 °C for 20 s, 60 °C for 30 s, and 72 °C for 30 s. After incubation at 72 °C for an additional 10 min, the reactions were resolved on a 1% agarose gel, and the PCR products were gel-purified and subsequently cloned into Escherichia coli DH10B using the CloneJET PCR Cloning Kit (Thermo Scientific). The recombinant plasmids were isolated from transformed bacteria, and the inserted cDNAs were sequenced by Genomed (Warsaw, Poland).
RT-qPCR was performed as follows: the 10 μl reaction mixture contained 1 × master mix (iTaq Universal SYBR Green Supermix, Bio-Rad), 0.5 μM forward primer, 0.5 μM reverse primer and 1 μl of cDNA. The reaction was incubated for 3 min at 95 °C, followed by 40 cycles of 20 s at 95 °C, 30 s at 60 °C, and 30 s at 72 °C. After the last cycle, a melting curve was generated by increasing the temperature from 60 °C to 95 °C. RT-qPCR was performed in three technical replicates using the real-time PCR system (QuantStudio5, Thermo Scientific).
Statistical analysis of HKGS and validation
Selection of the best reference genes was performed using previously described calculation algorithms, namely, geNorm17, BestKeeper39, NormFinder40 and the ΔCT method41. The detailed description of the methods was indicated in Supplementary File.
References
Rortais, A., Arnold, G., Halm, M.-P. & Touffet-Briens, F. Modes of honeybees exposure to systemic insecticides: estimated amounts of contaminated pollen and nectar consumed by different categories of bees. Apidologie 36, 71–83 (2005).
Gajendiran, A. & Abraham, J. An overview of pyrethroid insecticides. Front. Biol. 13, 79–90 (2018).
Johnson, R. M., Ellis, M. D., Mullin, C. A. & Frazier, M. Pesticides and honey bee toxicity–USA. Apidologie 41, 312–331 (2010).
Mullin, C. A. et al. High levels of miticides and agrochemicals in North American apiaries: implications for honey bee health. PLoS ONE 5, e9754. https://doi.org/10.1371/journal.pone.0009754 (2010).
Sanchez-Bayo, F. & Goka, K. Pesticide residues and bees–a risk assessment. PLoS ONE https://doi.org/10.1371/journal.pone.0094482 (2014).
Soderlund, D. M. et al. Mechanisms of pyrethroid neurotoxicity: implications for cumulative risk assessment. Toxicology 171, 3–59. https://doi.org/10.1016/s0300-483x(01)00569-8 (2002).
He, X. J. et al. Starving honey bee (Apis mellifera) larvae signal pheromonally to worker bees. Sci. Rep. 6, 1–9. https://doi.org/10.1038/srep22359 (2016).
Navajas, M. et al. Differential gene expression of the honey bee Apis mellifera associated with Varroa destructor infection. BMC Genom. 9, 301. https://doi.org/10.1186/1471-2164-9-301 (2008).
Bustin, S. A. et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin. Chem. 55, 611–622. https://doi.org/10.1373/clinchem.2008.112797 (2009).
Wieczorek, P., Wrzesińska, B. & Obrępalska-Stęplowska, A. Assessment of reference gene stability influenced by extremely divergent disease symptoms in Solanum lycopersicum L. J. Virol. Methods 194, 161–168. https://doi.org/10.1016/j.jviromet.2013.08.010 (2013).
Wrzesińska, B., Kierzek, R. & Obrępalska-Stęplowska, A. Evaluation of six commonly used reference genes for gene expression studies in herbicide-resistant Avena fatua biotypes. Weed Res. 56, 284–292. https://doi.org/10.1111/wre.12209 (2016).
Lü, J., Yang, C., Zhang, Y. & Pan, H. Selection of reference genes for the normalization of RT-qPCR data in gene expression studies in insects: a systematic review. Front. Physiol. 9, 1560 (2018).
Wu, M.-C., Chang, Y.-W., Lu, K.-H. & Yang, E.-C. Gene expression changes in honey bees induced by sublethal imidacloprid exposure during the larval stage. Insect Biochem. Mol. Biol. 88, 12–20. https://doi.org/10.1016/j.ibmb.2017.06.016 (2017).
Feyereisen, R. Evolution of insect P450. Biochem. Soc. Trans. 34, 1252–1255. https://doi.org/10.1042/bst0341252 (2006).
Gurkan, S. Insights into the defence of honey bees, Apis mellifera L., against insecticides (University of Liverpool, Liverpool, 2015).
Perkins, J. R. et al. ReadqPCR and NormqPCR: R packages for the reading, quality checking and normalisation of RT-qPCR quantification cycle (Cq) data. BMC Genom. 13, 296. https://doi.org/10.1186/1471-2164-13-296 (2012).
Vandesompele, J. et al. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 3, 0031. https://doi.org/10.1186/gb-2002-3-7-research0034 (2002).
Abramson, C. I., Aquino, I. S., Ramalho, F. S. & Price, J. M. The effect of insecticides on learning in the Africanized honey bee (Apis mellifera L.). Arch. Environ. Contam. Toxicol. 37, 529–535. https://doi.org/10.1007/s002449900548 (1999).
Alaux, C. et al. Interactions between Nosema microspores and a neonicotinoid weaken honeybees (Apis mellifera). Environ. Microbiol. 12, 774–782 (2010).
Botías, C. et al. Neonicotinoid residues in wildflowers, a potential route of chronic exposure for bees. Environ. Sci. Technol. 49, 12731–12740. https://doi.org/10.1021/acs.est.5b03459 (2015).
Brandt, A., Gorenflo, A., Siede, R., Meixner, M. & Büchler, R. The neonicotinoids thiacloprid, imidacloprid, and clothianidin affect the immunocompetence of honey bees (Apis mellifera L.). J. Insect Physiol. 86, 40–47. https://doi.org/10.1016/j.jinsphys.2016.01.001 (2016).
Manjon, C. et al. Unravelling the molecular determinants of bee sensitivity to neonicotinoid insecticides. Curr. Biol. 28, 1137–1143. https://doi.org/10.1016/j.cub.2018.02.045 (2018).
Christen, V., Joho, Y., Vogel, M. & Fent, K. Transcriptional and physiological effects of the pyrethroid deltamethrin and the organophosphate dimethoate in the brain of honey bees (Apis mellifera). Environ. Pollut. 244, 247–256. https://doi.org/10.1016/j.envpol.2018.10.030 (2019).
Liao, C.-H. et al. Short-term exposure to lambda-cyhalothrin negatively affects the survival and memory-related characteristics of worker bees Apis mellifera. Arch. Environ. Contam. Toxicol. 75, 59–65. https://doi.org/10.1007/s00244-018-0514-1 (2018).
Rinkevich, F. D. et al. Genetics, synergists, and age affect insecticide sensitivity of the honey bee, Apis mellifera. PLoS ONE 10, e0139841 (2015).
Friedli, A., Williams, G. R., Bruckner, S., Neumann, P. & Straub, L. The weakest link: Haploid honey bees are more susceptible to neonicotinoid insecticides. Chemosphere 242, 125145 (2020).
Roman, A., Popiela-Pleban, E. & Roman, K. Evaluation of the functional characteristics of selected breeding lines of Carniolan bees (Apis mellifera carnica). Sci. Ann. Pol. Soc. Anim. Prod. 10, 35–47 (2014).
Koywiwattrakul, P., Thompson, G. J., Sitthipraneed, S., Oldroyd, B. P. & Maleszka, R. Effects of carbon dioxide narcosis on ovary activation and gene expression in worker honeybees, Apis mellifera. J. Insect Sci. 5, 36. https://doi.org/10.1093/jis/5.1.36 (2005).
Woltedji, D. et al. Western honeybee drones and workers (Apis mellifera ligustica) have different olfactory mechanisms than eastern honeybees (Apis cerana cerana). J. Proteome Res. 11, 4526–4540. https://doi.org/10.1021/pr300298w (2012).
Gillespie, J., Johnston, J., Cannone, J. & Gutell, R. Characteristics of the nuclear (18S, 5.8 S, 28S and 5S) and mitochondrial (12S and 16S) rRNA genes of Apis mellifera (Insecta: Hymenoptera): structure, organization, and retrotransposable elements. Insect Mol. Biol. 15, 657–686. https://doi.org/10.1111/j.1365-2583.2006.00689.x (2006).
Scharlaken, B. et al. Reference gene selection for insect expression studies using quantitative real-time PCR: the head of the honeybee, Apis mellifera, after a bacterial challenge. J. Insect Sci. 8, 33 (2008).
Boncristiani, H., Underwood, R., Schwarz, R., Evans, J. D. & Pettis, J. Direct effect of acaricides on pathogen loads and gene expression levels in honey bees Apis mellifera. J. Insect Physiol. 58, 613–620. https://doi.org/10.1016/j.jinsphys.2011.12.011 (2012).
Chaimanee, V., Evans, J. D., Chen, Y., Jackson, C. & Pettis, J. S. Sperm viability and gene expression in honey bee queens (Apis mellifera) following exposure to the neonicotinoid insecticide imidacloprid and the organophosphate acaricide coumaphos. J. Insect Physiol. 89, 1–8. https://doi.org/10.1016/j.jinsphys.2016.03.004 (2016).
Di Prisco, G. et al. A mutualistic symbiosis between a parasitic mite and a pathogenic virus undermines honey bee immunity and health. Proc. Natl. Acad. Sci. USA 113, 3203–3208 (2016).
Moon, K., Lee, S. H. & Kim, Y. H. Validation of quantitative real-time PCR reference genes for the determination of seasonal and labor-specific gene expression profiles in the head of Western honey bee, Apis mellifera. PLoS ONE 13, e0200369 (2018).
Lourenço, A. P., Mackert, A., dos Santos Cristino, A. & Simões, Z. L. P. Validation of reference genes for gene expression studies in the honey bee, Apis mellifera, by quantitative real-time RT-PCR. Apidologie 39, 372–385 (2008).
Untergasser, A. et al. Primer3—new capabilities and interfaces. Nucleic Acids Res. 40, e115–e115. https://doi.org/10.1093/nar/gks596 (2012).
Koressaar, T. & Remm, M. Enhancements and modifications of primer design program Primer3. Bioinformatics 23, 1289–1291. https://doi.org/10.1093/bioinformatics/btm091 (2007).
Pfaffl, M. W., Tichopad, A., Prgomet, C. & Neuvians, T. P. Determination of stable housekeeping genes, differentially regulated target genes and sample integrity: BestKeeper–Excel-based tool using pair-wise correlations. Biotechnol. Lett. 26, 509–515. https://doi.org/10.1023/B:BILE.0000019559.84305.47 (2004).
Andersen, C. L., Jensen, J. L. & Orntoft, T. F. Normalization of real-time quantitative reverse transcription-PCR data: a model-based variance estimation approach to identify genes suited for normalization, applied to bladder and colon cancer data sets. Cancer Res 64, 5245–5250. https://doi.org/10.1158/0008-5472.CAN-04-0496 (2004).
Silver, N., Best, S., Jiang, J. & Thein, S. L. Selection of housekeeping genes for gene expression studies in human reticulocytes using real-time PCR. BMC Mol. Biol. 7, 33. https://doi.org/10.1186/1471-2199-7-33 (2006).
Acknowledgements
We would like to thank Mrs. Daria Dworzańska and Dr. Joanna Zamojska for their support in preparing the insects and treating them with the pyrethroids. We also would like to thank Mr. Maciej Spychalski for his help in extracting RNA from the tested insects.
Author information
Authors and Affiliations
Contributions
A.O.S. and P.W. designed the experiment; P.W. carried out all molecular biological procedures; PF performed all statistical analyses; PW, PF, and AOS analysed and interpreted the obtained results; and AOS, PF and PW wrote the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wieczorek, P., Frąckowiak, P. & Obrępalska-Stęplowska, A. Evaluation of the expression stability of reference genes in Apis mellifera under pyrethroid treatment. Sci Rep 10, 16140 (2020). https://doi.org/10.1038/s41598-020-73125-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-73125-w
- Springer Nature Limited