Abstract
Dwarf architecture is an important trait associated with plant yield, lodging resistance and labor cost. Here, we aimed to identify a gene causing dwarfism in watermelon. The ‘w106’ (dwarf) and ‘Charleston Gray’ (vine) were used as parents to construct F1 and F2 progeny. Dwarf architecture of ‘w106’ was mainly caused by longitudinal cell length reduction and was controlled by a single recessive gene. Whole-genome sequencing of two parents and two bulk DNAs of F2 population localized this gene to a 2.63-Mb region on chromosome 9; this was further narrowed to a 541-kb region. Within this region, Cla015407, encoding a gibberellin 3β-hydroxylase (GA3ox), was the candidate gene. Cla015407 had a SNP mutation (G → A) in the splice acceptor site of the intron, leading to altered splicing event and generating two splicing isoforms in dwarf plants. One splicing isoform retained the intron sequences, while the other had a 13-bp deletion in the second exon of GA3ox transcript, both resulting in truncated proteins and loss of the functional Fe2OG dioxygenase domain in dwarf plants. RNA-Seq analysis indicated that expression of Cla015407 and other GA biosynthetic and metabolic genes were mostly up-regulated in the shoots of dwarf plants compared with vine plants in F2 population. Measurement of endogenous GA levels indicated that bioactive GA4 was significantly decreased in the shoots of dwarf plants. Moreover, the dwarf phenotype can be rescued by exogenous applications of GA3 or GA4+7, with the latter having a more distinct effect than the former. Subcellular localization analyses of GA3ox proteins from two parents revealed their subcellular targeting in nucleus and cytosol. Here, a GA3ox gene controlling dwarf architecture was identified, and loss function of GA3ox leads to GA4 reduction and dwarfism phenotype in watermelon.
Similar content being viewed by others
Introduction
Plant height is an important agronomic trait that affects the quality and yield of plants1. The cultivation of dwarf crops has been widely applied in wheat1, rice2, maize3, pea4 and peach5. At present, most vine watermelon (Citrullus lanatus L.) varieties are planted for commercial production. With the rapid spread of watermelon protection areas, it is a good opportunity for the selection and cultivation of dwarf watermelon. Dwarf watermelon have short shoots, and are easily cultivated and managed, allowing for an increase in planting density, which can lead to increased yields and financial benefits.
There are various mechanisms leading to plant dwarfism. Changes in biosynthesis or perception of plant hormones, such as gibberellin (GA)6, brassinosteroid7, auxin8 and strigolactone9 can cause plant dwarfism. Additionally, abnormal expression of transcription factors, such as HD-Zip II10, WOX11, AP212 and GRAS13 can also lead to dwarf architecture in plants.
GAs is a kind of diterpenoid carboxylic acids that widely exist in plants, including the functional active molecules GA1, GA3, GA4 and GA7, as well as the non-active molecules GA9, GA19, GA20, GA29 and GA5114,15. GAs have a variety of biological functions, including promoting the stem elongation14,15. In plants, GA biosynthesis and metabolism are catalyzed by six key enzymes, ent-copalyl diphosphate synthase (CPS), ent-kaurene synthase (KS), ent-kaurene oxidase (KO), ent-kaurenoic acid oxidase (KAO), GA 20-oxidase (GA20ox) and GA 3-oxidase (GA3ox), and its deactivation is catalyzed by GA 2-oxidase (GA2ox)14,15. GAs could promote the stem elongation by stimulating the degradation of DELLA proteins16. Mutations in GA biosynthetic genes lead to dwarfism by reducing the endogenous GA levels, leading to DELLA protein accumulation, and ultimately limiting internode elongation in plants5.
Among the six enzymes, GA20ox and GA3ox catalyze the final two steps of GA biosynthetic pathway, and GA2ox catalyzes the GA metabolic pathway17,18,19. GA20ox, the “Green revolution gene” in rice, encodes a key enzyme that catalyzes the penultimate step of GA biosynthesis, converting GA12 to GA9, and GA53 to GA2017. Mutations in GA20ox genes conferring dwarf phenotypes have been reported in rice20,21, barley22,23 and Arabidopsis24,25. GA3ox catalyzes the final step of the GA biosynthetic pathway, converting GA20 to GA1, GA9 to GA4, and GA5 to GA3, which leads to the active molecules GAs18. Mutations of GA3ox genes have been identified in maize18, rice26, Arabidopsis27 and Medicago sativa28, and they are causing the dwarf phenotypes in these plants. GA2ox catalyzes the deactivation of bioactive GAs or its precursors to inactive forms through 2β-hydroxylation reaction; thus, plays a direct role in the determination of bioactive GAs content19. The GA2ox genes leading to dwarf phenotypes have been reported in wheat6, switchgrass29, rice30 and tomato31.
Watermelon belongs to the Cucurbitaceae and is a diploid species with a chromosomal number of 2n = 2 × 11 and a genome size of 425 Mb32. Approximately 97 million tons of watermelon are produced worldwide each year, with China being the largest producer. Watermelon dwarf mutants have been identified and analyzed in previous studies. Dwarf mutants in watermelon are controlled by dw-133, dw-1s34, dw-235, dw-336, dsh37, Cldf38, dw39 and Cldw-140, and the dwarf traits were controlled by respective single recessive genes37,38,39,40. At present, the dsh gene has been identified through whole-genome sequencing of two bulk DNAs, and Cla010726 (GA20ox) was predicted to be the candidate gene37. In addition, a SNP mutation of GA3ox38,39 and a SNP deletion in an ABC transporter gene40 also lead to dwarfism phenotypes in watermelon.
In recent years, the combination of a bulked segregant analysis (BSA) and next-generation sequencing technology (BSA-Seq) has been widely applied to identify candidate genes controlling important agronomic traits in watermelon, such as dwarf phenotype37,38,39,40, lobed leaves41, yellow skin42, fruit shape43, fruit pigment accumulation44 and anthracnose resistance45. In this study, we investigated the inheritance of watermelon dwarf genes in the F2 population of ‘w106’ (dwarf) × ‘Charleston Gray’ (CG; vine), which indicated that the dwarf phenotype was controlled by a single recessive gene. The candidate gene, Cla015407 (GA3ox), was obtained through BSA-Seq and mapping analysis. A single nucleotide polymorphism (SNP) mutation (G → A) occurred in the splice acceptor site of the intron in Cla015407, which lead to altered splicing, resulting in two splicing isoforms in dwarf plants. This point mutation leads to loss function of GA3ox and GA4 level reduction in dwarf plants. This study identify a GA3ox gene controlling dwarf architecture in watermelon and will aid in revealing the molecular mechanism of plant height in future.
Materials and methods
Plant materials
Two watermelon parental lines, ‘w106’ (dwarf) and ‘CG’ (vine), were used as the female and male parents, respectively. The F1 plants were generated by crossing ‘w106’ and ‘CG’, and self-pollinated to produce the F2 progeny. The 15 plants of each parental line, 15 F1 plants and 98 F2 plants were used for genetic analysis and BSA-Seq. For fine mapping, the dwarf individuals of a larger F2 population were used. The cross and self-pollination were carried out at the Haining Base of Zhejiang Academy of Agricultural Sciences. The plants used for the genetic analysis, BSA-Seq and fine mapping were grown and evaluated in a greenhouse at Zhejiang Academy of Agricultural Sciences.
Analysis of shoot sections
Shoots of dwarf and vine plants were fixed with 50% FAA for 24 h at 4 °C. Subsequently, these samples were dehydrated in a graded ethanol series, infiltrated with xylene and embedded in paraffin. Sections were sliced using an ultramicrotome and stained with safranin and fast green, and finally, observed under an optical microscope.
Bulk DNA construction and Illumina sequencing
DNAs were extracted using the CTAB method from leaves of both parental lines and F2 plants for BSA-Seq. Two bulk DNA samples, dwarf bulk (D-bulk) and vine bulk (V-bulk), were constructed by mixing equal amounts of DNAs from 25 dwarf plants and 25 vine plants from the F2 population, respectively. Paired-end DNA libraries were prepared according to the manufacturer’s instructions (Truseq Library Construction; Illumina, San Diego, CA, USA). First, genomic DNA was sheared into 350-bp fragments using a Covaris S220 sonicator (Woburn, MA, USA). Second, ends of the gDNA fragments were repaired, and 3′ ends were adenylated. Then, the size distributions and concentrations of the libraries were determined using an Agilent 2100 Bioanalyzer (Agilent Technologies, Waldbronn, Germany) and quantified by real-time PCR. Finally, DNA libraries were sequenced using an Illumina HiSeq X at Genepioneer (Nanjing, Jiangsu, China) according to the manufacturer’s instructions for paired-end 150-bp reads.
The short reads from D-bulk and V-bulk were aligned to the ‘97103’ reference genome32 using BWA software46. Alignment files were converted to SAM/BAM files using SAM tools47. SNPs and Insertion/deletion polymorphisms (InDels) were also assessed.
Gene location association analysis
All samples underwent variant calling using the Unified Genotyper function of the GATK program48. The SNPs and InDels were filtered using the Variant Filtration parameter of GATK. ANNOVAR, which is an efficient software tool, was used to annotate the SNPs or InDels based on the GFF3 files for the reference genome49. The homozygous SNPs/InDels between two parental lines were extracted from the vcf files.
A SNP index was used to indicate the proportion of reads harboring SNPs that differed from reference sequences50. An Euclidean distance (ED) value was calculated by comparing SNPs across the two bulk DNAs as follows: SNP-indexalt = Nalt/(Nalt + Nref), Δ(SNP-indexalt) = SNP-indexalt (V-bulk) −SNP-indexalt (D-bulk), SNP-indexref = Nref/(Nalt + Nref), Δ(SNP − indexref) = SNP − indexref (V-bulk) − SNP-indexref (D-bulk) and ED = [Δ(SNP-indexref)2 + Δ (SNP-indexalt)2]1/251. Using these formulae, we assessed whether the measured values fell within the following ranges, − 1 ≤ Δ(SNP-index) ≤ 1 and 0 ≤ ED ≤ 1.41451,52. The greater of the ED value, the closer of the object site52. The Δ(InDel-index) and EDs of InDel sites were calculated using the InDel-index53 as described above for calculating for SNP regions. Using a 1-kb sliding window, an average SNP/InDel-index was calculated over a 1-Mb interval.
Mapping of the candidate gene
To minimize the genetic interval and verify the accuracy of BSA-Seq, 161 simple sequence repeat (SSR) markers within the BSA-Seq region were developed based on the whole-genome sequencing of the two parental lines. These newly developed SSR markers were first screened for polymorphisms between the two bulk DNAs, then the polymorphic SSRs were used to screen for recombinants in the dwarf individuals of the F2 population.
The PCR was carried out in a total volume of 15 μL containing 7.5 μL 2 × TSINGKE Master Mix (Tsingke, Beijing, China), 0.5 μL of each primer (10 μM), 2 μL genomic DNA (~ 50 ng/μL) and 4.5 μL sterilized ddH2O. All the amplifications were performed on a Mastercycler nexus GSX1 (Eppendorf, Germany) under the following conditions: 95 °C for 5 min; 33 cycles of 30 s at 95 °C, 45 s at 55 °C and 45 s at 72 °C, followed by a final extension step at 72 °C for 10 min. The amplified products were separated on 8.0% non-denatured polyacrylamide gel with electrophoresis at 150 V constant power for 1 h. After fixation in 10% ethanol + 0.5% glacial acetic acid for 10 min, the silver staining in 0.2% AgNO3 for 12 min was performed. Samples were then rinsed in distilled water for 1 min and 1.5% NaOH + 0.4% formaldehyde for 6 min. The band pattern analysis was performed under a GL-800 Compact White Light Transmissometer (Kylin-Bell, Haimen, Jiangsu, China).
Cloning and sequence analysis of candidate gene
Total DNAs and RNAs were extracted from leaves of two parental plants using CTAB and TRIzol Reagent (Invitrogen, Carlsbad, CA, USA), respectively. First-strand cDNA was synthesized using a FastKing RT Kit (with gDNase) (Tiangen Biotech, Beijing, China). The PCR was carried out in a total volume of 25 μL containing 12.5 μL 2 × PCR buffer for KOD FX (Toyobo, Osaka, Japan), 0.5 μL KOD FX (1.0 U/μL) (Toyobo), 5.0 μL dNTPs (2 mM) (Toyobo), 0.5 μL of each primer (10 μM) (F: 5′-ATGGGAAGCATCAAAATAACCG-3′; R: 5′-TTAACCTACTTTAACCTGGCTG-3′), 2.0 μL cDNA (50 ng/μL) and 4.0 μL sterilized ddH2O. Amplifications of candidate genes were performed under the following conditions: 95 °C for 5 min; 33 cycles of 30 s at 95 °C, 45 s at 55 °C and 45 s at 72 °C, followed by a final extension step at 72 °C for 10 min. Amplification products were analyzed on 1.5% agarose gel and sent for sequencing.
Transcriptome sequencing of dwarf and vine plants
Transcriptome sequencing (RNA-Seq) was performed to analyze the expression of candidate genes and reveal the related pathways involved in dwarf architecture. Total RNAs were extracted from shoots of dwarf and vine plants in the F2 population using TRIzol Reagent (Invitrogen, Carlsbad, CA, USA). Approximately 10 µg total RNA was subjected to Poly(A) mRNA isolation using poly-T oligo-attached magnetic beads (Invitrogen, Carlsbad, CA, USA). Following purification, the mRNA was fragmented into small pieces and the cleaved RNA fragments were reverse-transcribed to create the final cDNA library. The average insert size for the paired-end libraries was 300 bp (± 50 bp). Then, the paired-end sequencing was performed on an Illumina HiSeq 4000 platform at Genepioneer (Nanjing, Jiangsu, China) following the vendor’s recommended protocol. Triplicates of each sample were carried out for Illumina sequencing.
Gene expression was assessed using the fragments per kilobase of transcript per million fragments mapped (FPKM) method54. The differentially expressed genes (DEGs) were determined using the criterion |log2(Fold Change)|≥ 1 and FDR < 0.05. The corresponding functions were revealed using the KEGG Automatic Annotation Server55.
Measurement of endogenous GA levels using internal standards
At the fourth-true leave stage, the shoots of vine and dwarf plants in two parents and F2 population grown under the same condition were harvested. The endogenous levels of 18 kinds of GAs involved in GA biosynthetic and metabolic pathway (GA1, GA3, GA4, GA5, GA6, GA7, GA8, GA9, GA12, GA15, GA19, GA20, GA24, GA29, GA34, GA44, GA51 and GA53) were measured using 2H2-GA internal standards coupled with UPLC-MS/MS analyses at LC Sciences (Hangzhou, China). For the measurement, shoots from three seedlings of vine and dwarf plants were mixed and ground into fine powder in liquid nitrogen, respectively; weighed ~ 100 mg sample, added 1.0 ng/g corresponding 2H2-GA internal standards and 1 mL methanol/water (80/20, v/v) extracting solution at 4 °C for overnight, then centrifuged at 10,000×g for 20 min at 4 °C; the supernatant was taken and absorbed through the C18/SAX solid-phase extraction column; 2 mL methanol/water (20/80, v/v) was used to clean the SAX extraction column, and 3 mL ACN/FA (99/1, v/v) was used to desorb the target acid plant hormones retained on the SAX extraction column; the desorption solution was blow-dried with constant nitrogen flow at 40 °C and redissolved in 100 μL water, and 10 μL FA was added to the 100 μL solution and extracted twice with ether; the extracted organic phase was combined and blow-dried by nitrogen at room temperature, then dissolved in 100 μL ACN; adding 10 μL × 20 μmol/mL TEA and 10 μL × 20 μmol/mL BTA to the ACN solution, swirl for 35 min at room temperature and then blow-dried with nitrogen; redissolved the solution in 200 μL H2O/ACN (90/10, v/v) for subsequent UPLC-MS/MS analyses. The UPLC-MS/MS analyses were performed on Thermo Scientific Ultimate 3000-Thermo Scientific TSQ Quantiva. Three biological replicates were conducted for each measurement, and endogenous levels of GAs (ng/g fresh weight) were determined by means of three UPLC-MS/MS detection results. T-test was conducted for statistical analysis using SAS 8.0. The GAs with |log2Fold(FC)| ≥ 1 and statistical significance (p value < 0.05) were considered as significant difference.
Exogenous treatments with GA3 or GA4+7
In addition, the dwarf parental lines were employed to assess responses to exogenous application of GAs. The GA3 (Ryon, Shanghai, China) and GA4+7 (Ryon, Shanghai, China) are independently dissolved in a small amount of ethanol and then diluted with sterilized ddH20 to the final concentration of 500 μM. The seedlings were sprayed independently with 500 μM GA3 or GA4+7 for four times at 3-day intervals. The control seedlings were sprayed with sterilized ddH20. Three seedling were used for each treatment. The phenotypes of seedlings were analyzed and photographed 3-days after the last GA treatment.
Subcellular localization analyses of GA3ox protein
The CDS sequences of Cla015407 from ‘CG’ and ‘w106’ were amplified using gene-specific primer (F: 5′-CGGGATCCCGATGGGAAGCATCAAAATAAC-3′; R: 5′-GCTCTAGAGCTTAACCTACTTTAACCTGGCTG-3′) and cloned into the pFGC-eGFP plasmid via the BamH I and Xba I restriction sites. These recombinant plasmids were transformed into Agrobacteriumt tumefaciens GV3101 and transiently expressed in tobacco leaf cells. Images were acquired at 48 h using a Leica DMLE camera (Leica, Wetzlar, Germany).
Ethical standards
The authors declare that this study complies with the current laws of the countries in which the experiments were performed.
Results
Phenotypic and genetic analyses
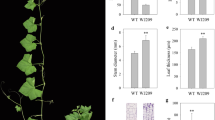
The height of ‘w106’ was significant shorter than that of ‘CG’ (Fig. 1a). Therefore, we conducted a microscopic observation of shoots of the ‘CG’ and ‘w106’ plants using paraffin sectioning. The cell sizes in transverse sections were not obviously different between the ‘CG’ and ‘w106’ plants (Fig. 1b). However, the cell lengths in longitudinal sections were obviously shorter in ‘w106’ than in ‘CG’ plants (Fig. 1c). Thus, the defective cell elongation appears to be the main cause for the reduced shoots and dwarf architecture in watermelon.
Phenotypic characterization and morphological analysis of two parental lines used in present study. (a) Phenotypic characterization of the two parental lines. Shoot length are reduced in ‘w106’ (right) as compared to ‘CG’ (left). (b) Transverse sections of shoots in two parental lines. (c) Longitudinal sections of shoots in two parental lines. The ‘w106’ (right) showed reduced longitudinal cell length compared with ‘CG’ (left).
To assess the inheritance of the dwarf trait in watermelon, crosses were made between ‘w106’ and ‘CG’. All the F1 plants showed the vine phenotype. Among the 98 F2 progeny, 72 individuals showed vine phenotype and 26 individuals showed dwarf phenotype, with the Chi-square test (χ2) confirming the segregation ratio to be 3:1 (Table 1). These results indicated that the dwarf trait in ‘w106’ was controlled by a single recessive gene, designated as the short internode (Si) locus.
Si gene located on chromosome 9 (0.80–3.43 Mb) using BSA-seq
We sequenced the genomes of the two parental lines and two bulk DNAs using the Illumina HiSeq™ PE150 platform. The high-throughput sequencing results obtained 50.30, 61.89, 65.48 and 70.68 Mb clean reads for female parent, male parent, D-bulk and V-bulk, respectively (Table 2). A total of 33.65 Gb clean data were generated for the two parental lines, and 40.84 Gb clean data were generated for the two DNA bulks, with approximately 42–57 × sequencing depth and more than 99.00% 5 × coverage per sample (Table 2). Data were aligned to the reference genome of watermelon ‘97103’ (https://cucurbitgenomics.org/organism/1), and 160,957 SNPs and 55,055 InDels, at a minimum of 5 reads, were identified between D-bulk and V-bulk. Each identified SNP or InDel was used to compute an SNP/InDel-index. The graph for Δ(SNP/InDel-index) was plotted and computed against the genome positions by combining SNP/InDel-index of D-bulk and V-bulk (Fig. 2a,b). At the 99% significance level, the Δ(SNP-index) and Δ(InDel-index) values located the region on chromosome 9 (0.72–3.93 Mb) and chromosome 9 (0.80–3.43 Mb), respectively (Fig. 2a,b; Supplementary Table S2). The results indicated that a candidate gene controlling the dwarf trait in ‘w106’ was located in the 0.80–3.43 Mb region of chromosome 9 (Fig. 2c).
Genetic mapping and prediction of the Si locus in watermelon by BSA-Seq and mapping analysis. (a) Δ(SNP-index) graph of BSA-Seq analysis. (b) Δ(InDel-index) graph of BSA-Seq analysis. (c) Locus at the interval of 0.80–3.43 Mb on chromosome 9 was identified to control watermelon dwarf trait by combining the Δ(SNP-index) and Δ(InDel-index) of BSA-Seq analysis. The x-axis represents the position of watermelon chromosomes. The y-axis represents the value of Δ-index for SNPs and InDels. The scatter in the figure indicates that the value of Δ-index is calculated. The black curve represents the fitting value of Δ-index and the pink dotted line represents a threshold line with an snpnum smooth fitting value of 99%. (d) Screening of recombinants in F2 progeny delimited the location of Si in an interval of 541-kb by two SSR markers dw37 and dw134. (e) Within the 541-kb interval, a SNP change occurring in the splice site acceptor for the intron of Cla015407, which encodes the GA3ox protein.
Further mapping analysis narrowed Si to a 541-kb interval and a candidate gene was predicted
To further narrow down the location of the Si locus detected by BSA-Seq, we selected 161 SSR markers from chromosome 9 (0.80–3.43 Mb) based on resequencing data of the two parental lines. All these SSR markers were first screened for polymorphisms between the two bulk DNA samples, and then, 16 polymorphic markers were applied to screen recombinants of the dwarf individuals in the F2 population. Finally, two flanking markers, dw37 (Chr9:1620039) and dw134 (Chr9:2161629), obtaining one and four recombinants, respectively, placed the Si locus in a 541-kb region (Fig. 2d). Additionally, no recombinant was obtained using the marker dw128 (Chr9:1835342), indicating that the target gene neighbored dw128. All the polymorphic SSR markers used in this study and the obtained recombinants are listed in Supplementary Table S3.
According to the watermelon genome annotation, 66 putative genes (Cla015361–Cla015427) were detected within the 541-kb interval (Supplementary Table S4). Within this region, three SNPs and one InDel were identified among the two parental lines and two bulk DNAs according to the whole-genome sequencing data (Table 3). Among these four variations, two SNP variations (Chr9:1620753 and Chr9:1621230) occurred in the intergenic region of the genome and an InDel (Chr9:1996536) occurred in the upstream of Cla015387 (WD43). One SNP occurred in Chr9:1857472 (from ‘G’ in ‘CG’ to ‘A’ in ‘w106’), locating at the splice-site acceptor in the intron of Cla015407 (Fig. 2e; Table 3). Cla015407 encodes GA3ox, which is involved in the GA biosynthetic pathway. Additionally, the genome location of Cla015407 (Chr9:1856847–1858103) neighbored the SSR marker dw128 (Chr9:1835342), which did not identify any recombinants and was near the target gene. Therefore, Cla015407 was predicted to be the candidate gene conferring the dwarf architecture of ‘w106’.
Sequences analyses of the candidate gene
To verify the sequences of Cla015407 at the DNA and mRNA levels, we cloned the DNA and coding sequence (CDS) of Cla015407 from both parental lines. Fragments of 1,257 bp were amplified at the DNA level from both parental lines (Fig. 3a), and the sequencing analysis further verified the G → A variation at the 626th nucleotide of Cla015407 (Fig. 3c).
Sequence analysis of Cla015407 from two parental lines. (a) Amplifications of Cla015407 in two parental lines on DNA level. (b) Amplifications of Cla015407 in two parental lines on cDNA level. (c) Sequencing results verified the SNP (G → A) in ‘CG’ and ‘w106’ on DNA level at 626th nucleotide. (d) Sequencing results of two splicing isoforms on cDNA level in ‘w106’ indicated that the full-length isoform retained the intron sequence and the truncated isoform had 13-bp deletion at the second exon compared with the CDS in ‘CG’. (e) Amino acids prediction of the two splicing isoforms indicted the loss of Fe2OG dioxygenase domain in ‘w106’ compared with ‘CG’.
At the cDNA level, a fragment was amplified from vine parent ‘CG’ and two fragments were amplified from dwarf parent ‘w106’, which indicated that this SNP mutation lead to altered splicing, generating two splicing isoforms in the dwarf plants (Fig. 3b). Sequence analyses of the splicing isoforms in ‘w106’ revealed that the full-length isoform (isoform 1) retained the intron sequences and contained the premature termination codon ‘TAG’ at the 505–507th nucleotides (Fig. 3d). Additionally, the truncated isoform (isoform 2) had a 13-bp deletion in the second exon compared with the CDS of ‘CG’ and contained the premature termination codon ‘TGA’ at the 520–522th nucleotides (Fig. 3d).
The proteins encoded by the transcripts of Cl015407 in vine and dwarf parents were also predicted. The transcript of Cl015407 in vine parent ‘CG’ encodes a protein with 377 aa (Fig. 3e). However, the full-length isoform (isoform 1) in the dwarf parent ‘w106’ contains an unspliced intron, introducing a stop codon (TAG) just after the splice donor site, thus translation of this full-length transcript is prematurely terminated and produces a protein with 168 aa (Fig. 3e). Moreover, the truncated isoform (isoform 2) has a 13-bp deletion in the second exon of Cla015407 in dwarf parent ‘w106’ and contains a premature termination codon ‘TAG’ at 520–522th nucleotides, leading to frame shift and premature termination, and resulted in a truncated protein with 173 aa residues (Fig. 3e). In summary, the two transcripts of Cl015407 from the dwarf plants resulted in truncated proteins and lost the functional Fe2OG dioxygenase domain.
DEGs identification between dwarf and vine plants and their KEGG pathway enrichment analyses
Transcriptome analyses of the shoots for dwarf and vine plants in the F2 population were carried out to reveal the expression patterns of the candidate gene and GA biosynthetic and metabolic genes. A total of 3,027 genes showed differential expression, with 1,144 up-regulated and 1,883 down-regulated in dwarf plants compared with vine plants (Fig. 4a). In addition, the heatmap generating by Cluster 3.0 clearly divided these 3,017 DEGs into two Clusters (I and II) (Fig. 4b).
Volcano plot and heatmap of DEGs and their functional analysis. (a) Volcano plot of DEGs for vine plants and dwarf plants in F2 population. (b) Heatmap of DEGs for vine plants and dwarf plants in F2 population. The expression of DEGs were indicated by log2(FPKM + 0.001). (c) The KEGG pathways enriched for the up-regulated genes in dwarf plants compared with vine plants. (d) The KEGG pathways enriched for the down-regulated genes in dwarf plants compared with vine plants. The size of each circle represents the number of significantly DEGs enriched in the corresponding pathway. The enrichment factor was calculated using the number of enriched genes divided by the total number of background genes in the corresponding pathway. The q value was calculated using the Benjamini–Hochberg correction. A pathway with q < 0.05 is considered significantly overrepresented.
The KEGG pathway enrichment analyses were carried out for these DEGs (Fig. 4c,d). The up-regulated genes in dwarf plants were significantly enriched in KEGG pathways of ‘plant hormone signal transduction’ and ‘photosynthesis’ (Fig. 4c). Moreover, the down-regulated genes in dwarf plants were significantly enriched in KEGG pathways of ‘pentose and glucuronate interconversions’, ‘biosynthesis of secondary metabolites’, ‘stilbenoid, diarylheptanoid and gingerol biosynthesis’, ‘starch and sucrose metabolism’, ‘metabolic pathways’, ‘phenylpropanoid biosynthesis’, ‘cyanoamino acid metabolism’, ‘linoleic acid metabolism’, ‘alanine, aspartate and glutamate metabolism’, ‘ascorbate and aldarate metabolism’, and ‘phenylalanine metabolism' (Fig. 4d).
Expression of GA biosynthetic and metabolic genes and phylogenetic analysis
As shown in Table 4, a total of 20 genes involved in GA biosynthesis and metabolism were detected as expressed at least one library, including one gene for CPS, one gene for KS, one gene for KO, two genes for KAO, six genes for GA20ox, two genes for GA3ox and seven genes for GA2ox. Most of these GA biosynthetic and metabolic genes in dwarf plants showed higher expression levels than those of vine plants. The expression of our candidate gene, Cla015407 (GA3ox), was significantly increased in dwarf plants. Moreover, one KO gene (Cla020710) and three GA20ox genes (Cla002362, Cla006227 and Cla008413) involved in GA biosynthesis, and two GA2ox gene (Cla015162 and Cla019586) involved in GA metabolism, were also significantly up-regulated in dwarf plants compared with those in vine plants.
Additionally, phylogenetic analysis of these GA20ox, GA3ox and GA2ox proteins in watermelon were carried out with a few selected GA20ox, GA3ox and GA2ox families in Arabidopsis and divided them into three major subgroups (I, II and Ш) (Supplementary Fig. S1). Subgroup I contained five watermelon GA20ox proteins (Cla002362, Cla006227, Cla006941, Cla008413 and Cla013892) and two Arabidopsis GA20ox proteins (AT5G07200 and AT1G44090). Subgroup II contained five watermelon GA2ox proteins (Cla015162, Cla017338, Cla005397, Cla009774 and Cla019586) and two Arabidopsis GA2ox proteins (AT1G47990 and AT1G30040). Subgroup Ш contained two GA3ox proteins (Cla022285 and Cla015407), two GA2ox proteins (Cla005259 and Cla007482) from watermelon and two GA3ox proteins (AT4G21690 and AT1G15550) from Arabidopsis. However, Cla010726, encoding the gibberellin 20-oxidase-like protein, did not belong to any of these subgroups.
Endogenous levels of GAs were changed in the dwarf plants
Except for GA7, the remaining 17 kinds of GAs were detected from the shoots of vine and dwarf plants in two parents and F2 population using the 2H2-GA internal standards coupled with UPLC-MS/MS analyses. Among these GAs, endogenous level of GA3 was significantly increased and GA4 was significantly decreased in dwarf plants of two parents and F2 population (Table 5). Additionally, endogenous levels of GA9 and GA29 were significantly increased in dwarf plants of F2 population. The results indicated the reduced level of bioactive GA4 might be the main cause for the dwarf phenotype in watermelon (Table 5).
Moreover, we constructed the GA biosynthetic and metabolic pathway combining the endogenous levels of GAs and genes involved in this pathway (Fig. 5). Two major pathways, GA53-pathway (involving GA53, GA44, GA19, GA20, GA1, GA8, GA29, GA5, GA3 and GA6) and GA12-pathway (involving GA12, GA15, GA24, GA9, GA4, GA34, GA51 and GA7), were included. The GA9 and GA4 in the GA12-pathway showed different changes of endogenous levels between the vine and dwarf plants (Fig. 5; Table 5). Endogenous level of GA9 was increased in dwarf plants of F2 population, which might be due to the increased expression level of GA20ox genes (Cla002362, Cla006227 and Cla008413) in dwarf plants as indicated in Table 4. Endogenous level of bioactive GA4 was decreased in dwarf plants, suggesting the mutation of Cla015407 (GA3ox) impaired the biosynthesis of GA4 in GA12-pathway. Endogenous level of GA29 and GA3 in the GA53-pathway were increased in dwarf plants (Fig. 5; Table 5). The increased GA29 content might be attributed to the increased expression level of GA2ox genes (Cla015162 and Cla019586) in dwarf plants as indicated in Table 4. Endogenous level of bioactive GA3 was increased in dwarf plants, which might be due to the up-regulation of another GA3ox paralogue, Cla022285, as a result of feedback of reduced GA4 content caused by the mutation of Cla015407 (GA3ox) (Table 4).
The predicted pathways of GA biosynthesis and metabolism in watermelon. The pathways were constructed according to the previous reports of GA biosynthetic and metabolic pathways in higher plants. Two major pathways, GA53-pathway and GA12-pathway were present in watermelon. The levels of GA3 and GA29 in GA53-pathway were increased in dwarf plants and marked with red color. The level of GA9 in GA12-pathway was increased and marked with red color, and level of GA4 in GA12-pathway was decreased in dwarf plants and marked with green color.
Exogenous GA applications can rescue the dwarf phenotype
We investigated the responses of dwarf plants to 500 µM GA3 and 500 µM GA4+7 applications and found that the independent applications of GA3 or GA4+7 could both rescue the dwarf phenotype in watermelon (Fig. 6). Moreover, GA4+7 treatments had a more distinct effect than GA3 treatments, resulting in greater plant height (Fig. 6). These observations further verified that the Si gene is a GA biosynthetic gene.
Subcellular localization of GA3ox proteins
The subcellular localization of GA3ox proteins from ‘CG’ and ‘w106’, namely CG-Cla015407, w106-Cla015407-Iso1, and w106-Cla015407-Iso2, were analyzed by transient expression of the green fluorescent protein (GFP) fusion proteins in tobacco leaf epidermal cells. As shown in Fig. 7, all of the three GA3ox proteins were localized in the nucleus and cytosol.
Subcellular localization of GA3ox proteins from two parental lines. Green fluorescent protein (GFP)-fusion proteins were transiently expressed in tobacco leaf epidermal cells. After 48 h of incubation, GFP signal was detected with a fluorescence microscope. Bright-field, nucleus marker, fluorescence, and merged images of CG-Cla015407-GFP, w106-Cla015407-Iso1-GFP, and w106-Cla015407-Iso2-GFP were shown.
Discussion
Plant height, as the key component of plant architecture, has been associated with both natural beauty and yield performance. Total cell number and cell size, resulting from cell division and expansion, determine the size of plant organs56. For example, the average cell size of the cucumber dwarf mutant scp2 was significantly smaller than that of wild type plants57. The cucumber Csdw mutant showed dwarfing phenotype and decreased internode length due to the reduced cell division in main stem58. The rice stemless dwarf 1 (std1) mutant showed severe dwarfing phenotype, abnormal cell morphology and reduced cell division rate59. Further analyses revealed that a large number of cells were blocked in the S and G2/M phases, with the presence of many multinucleate cells59. In present study, microscopic observations of stem transverse and longitudinal sections revealed that the reduced plant height could be mainly attributed to the shorter longitudinal cell lengths (Fig. 1b,c).
Genetic mapping and identification of dwarfism genes have occurred in cucurbits, such as scp-257, Csdw58, cp60 and scp-161 in cucumber; mdw162 in melon; and qCmB263 in pumpkin. In watermelon, the genes of dsh37, Cldf38, dw39 and Cldw-140, conferring dwarf phenotypes, were studied and identified. NGS-assisted BSA is an effective method to identify target genes controlling the dwarf traits by genotyping the bulked DNA samples having distinct or opposite extreme phenotypes in plants58. In this study, we employed the BSA-Seq to identify the candidate gene controlling the dwarf trait in watermelon and delimited the region to 0.80–3.43 Mb on chromosome 9 (Fig. 2a–c). A further mapping analysis finally located this gene in a 541-kb interval (Fig. 2d), with Cla015407 (GA3ox) being the candidate gene.
GA3ox catalyzes the last step of bioactive GA synthesis, which converts GA20 to GA1, GA5 to GA3, and GA9 to GA418. Mutations in GA3ox genes, such as dwarf1 (d1) from maize18, dwarf18 (d18) from rice26, GA4 from Arabidopsis27, Msdwf1 from Medicago sativa28, and le from pea64, resulted in dwarfism. For example, the coding sequences of GA3ox2 isolated from d18 alleles were analyzed in rice. In the strong allele, d18-Id18h, the deletion of a guanine base at 750 altered the reading frame, and in the weak allele, d18-dy, the 9-bp deletion deleted three amino acids26. The dwarf mutant Msdwf1 had an amino acid change in a conserved position of the GA3ox gene compared with the wild type in Medicago sativa28. Sequence alignment revealed a G-to-A transition conferring an alanine-to-threonine substitution at residue 229 in the le gene product in pea64. The same locus of GA3ox, Cla015407, conferring the dwarf phenotypes in watermelon were concurrently identified in this study and those of Wei et al38 and Gebremeskel et al39. Additionally, the SNP mutation (G → A) at position 626 of DNA sequence, locating at the splice acceptor site of intron, was simultaneously detected in our study and those of Wei et al38 and Gebremeskel et al39. Similar with previous reports, the SNP mutation leading to 13-bp deletion in the second exon of GA3ox transcript, was also observed in our dwarf parent ‘w106’. Moreover, another GA3ox transcript caused by this point mutation, which retained the intron sequence, was identified in our dwarf parent ‘w106’. However, this full-length isoform was not present in the dwarf parents ‘N21’ and ‘Duan125’ of Wei et al38 and Gebremeskel et al39.
In the present study, the candidate gene, Cla015407 (GA3ox), significantly increased the expression level in dwarf plants compared with the vine plants (Table 4). Additionally, one KO gene (Cla020710), three GA20ox genes (Cla002362, Cla006227 and Cla008413) and two GA2ox genes (Cla015162 and Cla019586) were also significantly up-regulated in dwarf plants (Table 4). The increased expression of GA related genes in this study were partially consistent with Wei et al38, in which the GA3ox (Cla015407), CPS (Cla006048), KAO (Cla021351, Cla006992 and Cla016164), and GA20ox (Cla002362, Cla006227 and Cla010726) were significantly up-regulated in dwarf line ‘N21’. Different from our results, the expression of GA3ox (Cla015407) in the dwarf parent ‘Duan125’ was significantly reduced according to the report of Gebremeskel et al.39. The increase in expression of GA related genes in our study might be due to the feedback of low levels of bioactive GA4 in dwarf plants as showing in Table 5. This is a well-known phenomenon whereby mutations or chemical intervention in GA biosynthesis or GA perception result in increases in GA20ox and GA3ox in a homeostatic mechanism14. Moreover, two transcripts of GA3ox, the intron retention isoform and 13-bp deletion isoform, were generated in the dwarf parent ‘w106’ in our study. Introns are often added to increase expression, although the mechanism through introns stimulate gene expression in plants remains unclear65. For instance, inserting the first intron from the UBQ10 gene into intronless genes markedly increased the latter’s mRNA accumulation to over 150-fold in Arabidopsis65. Therefore, the up-regulated GA3ox gene might also be attributed to altered splicing event in dwarf plants.
Endogenous levels of GAs were measured using 2H2-GA internal standards and UPLC-MS/MS and we find that GA3, GA9 and GA29 were significantly increased and GA4 was significantly decreased in dwarf plants (Fig. 5; Table 5). The results were different from a previous study of Gebremeskel et al39, in which significantly lower GA3 content was obtained in the dwarf parent ‘Duan125’ of watermelon. The increased content of GA9 in GA12-pathway might be resulted from the up-regulation of GA20ox genes (Cla002362, Cla006227 and Cla010726) in dwarf plants, and the reduced content of bioactive GA4 indicated that loss function of GA3ox (Cla015407) impaired the GA4 biosynthesis in GA12-pathway. Furthermore, GA51 content in GA12-pathway was increased in dwarf plants, however, not to a significant level. The results indicate that mutation of GA3ox (Cla015407) promotes the biosynthesis of GA51 and inhibits the biosynthesis of GA4 from GA9 in GA12-pathway. Moreover, endogenous level of GA29 in GA53-pathway was increased, which might be attributed to the increased expression level of GA2ox genes (Cla015162 and Cla019586) in dwarf plants. Additionally, endogenous level of bioactive GA3 in GA53-pathway was increased in dwarf plants, which aroused our interest. We speculated that the reduction in GA4 level in GA12-pathway resulted in increased expression, through release of feedback, of another GA3ox paralogue, Cla022285, with the ability to produce GA3 in GA53-pathway (Table 4; Fig. 5). In rice, two GA3ox genes, OsGA3ox1 and OsGA3ox2, have the activity for converting GA20 to GA1, GA5 to GA3, GA44 to GA38, and GA9 to GA426. Additionally, the maize dwarf-1 gene (putative GA 3β-hydroxylase) controls the three biosynthetic steps: GA20 to GA1, GA20 to GA5, and GA5 to GA366. However, there is no evidence that Cucurbits contains such an GA3ox enzyme currently67. In cucumber, four GA 3-oxidases (CsGA3ox1, -2, -3, and -4) were identified and all these GA 3-oxidases oxidized the C19-GA GA9 to GA4 as the only product67. In this study, the presences of GA1, GA5, GA6, GA3 and GA4 indicate that the GA3ox proteins have the activity for catalyzing GA20 to GA1, GA20 to GA5, GA5 to GA6, GA5 to GA3, and GA9 to GA4, and the absence of GA7 suggests that the GA3ox proteins do not have 2,3-desaturation activity in watermelon.
The rescue of the dwarf phenotype has been reported in GA-deficient mutants in plants. For instance, the application of GA3 could partially rescue the dwarf phenotype of the cucumber mutant Csdw58. In watermelon, the dwarf phenotypes of Cldf and dw could be rescued by exogenous GA3 application38,39. In the present study, exogenous applications of GA3 or GA4+7 could rescue the height of dwarf plants, with the latter have a more distinct affect than the former (Fig. 6). The results further confirmed the Si gene is a GA biosynthetic gene and the dwarf phenotype might be attributed to the reduced GA4 level.
Accession of sequencing data
Whole-genome sequencing data from this study can be accessed at sequence read archive (SRA) database from NCBI (https://www.ncbi.nlm.nih.gov/sra) with accession number SRR8893166 (female parent ‘w106’), SRR8893167 (male parent ‘Charleston Gray’), SRR8893168 (dwarf bulk) and SRR8893169 (vine bulk).
References
Würschum, T., Langer, S. M., Longin, C. F. H., Tucker, M. R. & Leiser, W. L. A modern Green Revolution gene for reduced height in wheat. Plant J. 92, 892–903 (2017).
Wing, R. A., Purugganan, M. D. & Zhang, Q. The rice genome revolution: From an ancient grain to Green Super Rice. Nat. Rev. Genet. 19, 505–517 (2018).
Peng, J. et al. “Green revolution” genes encode mutant gibberellin response modulators. Nature 400, 256–261 (1999).
Nomura, T. et al. Brassinosteroid deficiency due to truncated steroid 5alpha-reductase causes dwarfism in the lk mutant of pea. Plant Physiol. 135, 2220–2229 (2004).
Cheng, J. et al. A single nucleotide mutation in GID1c disrupts its interaction with DELLA1 and causes a GA-insensitive dwarf phenotype in peach. Plant Biotech. J. 17, 1723–1735 (2019).
Ford, B. A. et al. Rht18 semidwarfism in wheat is due to increased GA 2-oxidaseA9 expression and reduced GA content. Plant Physiol. 177, 168–180 (2018).
Castorina, G. et al. The maize lilliputian1 (lil1) gene, encoding a brassinosteroid cytochrome P450 C-6 oxidase, is involved in plant growth and drought response. Ann. Bot. 122, 227–238 (2018).
Li, H. et al. An auxin signaling gene BnaA3.IAA7 contributes to improved plant architecture and yield heterosis in rapeseed. New Phytol. 222, 837–851 (2019).
Jiang, L. et al. DWARF 53 acts as a repressor of strigolactone signalling in rice. Nature 504, 401–405 (2013).
Chen, W. et al. Small Grain and Dwarf 2, encoding an HD-Zip II family transcription factor, regulates plant development by modulating gibberellin biosynthesis in rice. Plant Sci. 288, 110208. https://doi.org/10.1016/j.plantsci.2019.110208 (2019).
Wang, W. et al. Dwarf tiller1, a wuschel-related homeobox transcription factor, is required for tiller growth in rice. PLoS Genet. 10, e1004154. https://doi.org/10.1371/journal.pgen.1004154 (2014).
Magome, H., Yamaguchi, S., Hanada, A., Kamiya, Y. & Oda, K. Dwarf and delayed-flowering 1, a novel Arabidopsis mutant deficient in gibberellin biosynthesis because of overexpression of a putative AP2 transcription factor. Plant J. 37, 720–729 (2004).
Zhou, S. et al. Manipulation of plant architecture and flowering time by down-regulation of the GRAS transcription factor SlGRAS26 in Solanum lycopersicum. Plant Sci. 271, 81–93 (2018).
Hedden, P. & Phillips, A. L. Gibberellin metabolism: New insights revealed by the genes. Trends Plant Sci. 5, 523–530 (2000).
Yamaguchi, S. Gibberellin metabolism and its regulation. Annu. Rev. Plant Biol. 59, 225–251 (2008).
Sun, T. P. Gibberellin-GID1-DELLA: A pivotal regulatory module for plant growth and development. Plant Physiol. 154, 567–570 (2010).
Qin, X. et al. Gibberellin 20-oxidase gene OsGA20ox3 regulates plant stature and disease development in rice. Mol. Plant Microbe Interact. 26, 227–239 (2013).
Chen, Y. et al. The maize DWARF1 encodes a gibberellin 3-oxidase and is dual localized to the nucleus and cytosol. Plant Physiol. 166, 2028–2039 (2014).
Thomas, S. G., Phillips, A. L. & Hedden, P. Molecular cloning and functional expression of gibberellin 2-oxidases, multifunctional enzymes involved in gibberellin deactivation. Proc. Natl. Acad. Sci. USA 96, 4698–4703 (1999).
Oikawa, T., Koshioka, M., Kojima, K., Yoshida, H. & Kawata, M. A role of OsGA20ox1, encoding an isoform of gibberellin 20-oxidase, for regulation of plant stature in rice. Plant Mol. Biol. 55, 687–700 (2004).
Spielmeyer, W., Ellis, M. H. & Chandler, P. M. Semidwarf (sd-1), “green revolution” rice, contains a defective gibberellin 20-oxidase gene. Proc. Natl. Acad. Sci. USA 99, 9043–9048 (2002).
Xu, Y. et al. Characterization of the sdw1 semi-dwarf gene in barley. BMC Plant Biol. 17, 11. https://doi.org/10.1186/s12870-016-0964-4 (2017).
Teplyakova, S. et al. Impact of the 7-bp deletion in HvGA20ox2 gene on agronomic important traits in barley (Hordeum vulgare L.). BMC Plant Biol. 17, 181. https://doi.org/10.1186/s12870-017-1121-4 (2017).
Xu, Y. L. et al. The GA5 locus of Arabidopsis thaliana encodes a multifunctional gibberellin 20-oxidase: Molecular cloning and functional expression. Proc. Natl. Acad. Sci. USA 92, 6640–6644 (1995).
Luo, Y. et al. A single nucleotide deletion in gibberellin 20-oxidase1 causes alpine dwarfism in Arabidopsis. Plant Physiol. 168, 930–937 (2015).
Itoh, H., Ueguchi-Tanaka, M., Sentoku, N., Kitano, H. & Matsuoka, M. M. K. Cloning and functional analysis of two gibberellin 3β-hydroxylase genes that are differently expressed during the growth of rice. Proc. Natl. Acad. Sci. USA 98, 8909–8914 (2001).
Chiang, H. H., Hwang, I. & Goodman, H. M. Isolation of the Arabidopsis GA4 locus. Plant Cell 7, 195–201 (1995).
Dalmadi, A. et al. Dwarf plants of diploid Medicago sativa carry a mutation in the gibberellin 3-beta-hydroxylase gene. Plant Cell Rep. 27, 1271–1279 (2008).
Wuddineh, W. A. et al. Identification and overexpression of gibberellin 2-oxidase (GA2ox) in switchgrass (Panicum virgatum L.) for improved plant architecture and reduced biomass recalcitrance. Plant Biotechnol. J. 13, 636–647 (2015).
Liu, C. et al. Shortened basal internodes encodes a gibberellin 2-oxidase and contributes to lodging resistance in rice. Mol. Plant 11, 288–299 (2018).
Schrager-Lavelle, A. et al. The role of a class III gibberellin 2-oxidase in tomato internode elongation. Plant J. 97, 603–615 (2019).
Guo, S. et al. The draft genome of watermelon (Citrullus lanatus) and resequencing of 20 diverse accessions. Nat. Genet. 45, 51–58 (2013).
Mohr, H. C. Mode of inheritance of the bushy growth characteristics in watermelon. Proc. Assoc. South Agric. Work 53, 174 (1956).
Dyutin, K. E. & Afanaseva, E. A. Inheritance of the short vine trait in watermelon. Cytol. Genet. 21, 71–73 (1987).
Liu, P. B. W. & Loy, J. B. Action of gibberellic acid on cell proliferation in the subapical shoot meristem of watermelon seedlings. Am. J. Bot. 63, 700–704 (1976).
Huang, H. X., Zhang, X. Q., Wei, Z. C., Li, Q. H. & Li, X. Inheritance of male-sterility and dwarfism in watermelon [Citrullus lanatus (Thunb.) Matsum and Nakai]. Sci. Hortic. 74, 175–181 (1998).
Dong, W., Wu, D., Li, G., Wu, D. & Wang, Z. Next-generation sequencing from bulked segregant analysis identifies a dwarfism gene in watermelon. Sci. Rep. 8, 2908. https://doi.org/10.1186/s12870-016-0964-4 (2018).
Wei, C. et al. A point mutation resulting in a 13 bp deletion in the coding sequence of Cldf leads to a GA-deficient dwarf phenotype in watermelon. Hortic. Res. 6, 132. https://doi.org/10.1038/s41438-019-0213-8 (2019).
Gebremeskel, H. et al. Molecular mapping and candidate gene analysis for GA3 responsive short internode in watermelon (Citrullus lanatus). Int. J. Mol. Sci. 21, 290. https://doi.org/10.3390/ijms21010290 (2019).
Zhu, H. et al. A single nucleotide deletion in an ABC transporter gene leads to a dwarf phenotype in watermelon. Front Plant Sci. 10, 1399. https://doi.org/10.3389/fpls.2019.01399 (2019).
Wei, C. et al. Genetic mapping of the LOBED LEAF 1 (ClLL1) gene to a 127.6-kb region in watermelon (Citrullus lanatus L.). PLoS ONE 12, e0180741. https://doi.org/10.1371/journal.pone.0180741 (2017).
Dou, J. et al. Genetic mapping reveals a marker for yellow skin in watermelon (Citrullus lanatus L.). PLoS One 13, e0200617. https://doi.org/10.1371/journal.pone.0200617 (2018).
Dou, J. et al. Genetic mapping reveals a candidate gene (ClFS1) for fruit shape in watermelon (Citrullus lanatus L.). Theor. Appl. Genet. 131, 947–958 (2018).
Oren, E. et al. The multi-allelic APRR2 gene is associated with fruit pigment accumulation in melon and watermelon. J. Exp. Bot. 70, 3781–3794 (2019).
Jang, Y. J. et al. An evolutionarily conserved non-synonymous SNP in a leucine-rich repeat domain determines anthracnose resistance in watermelon. Theor. Appl. Genet. 132, 473–488 (2019).
Li, H. & Durbin, R. Fast and accurate short read alignment with Burrows–Wheeler transform. Bioinformatics 25, 1754–1760 (2009).
Li, H. et al. The sequence alignment/map format and SAM tools. Bioinformatics 25, 2078–2079 (2009).
do Valle, I. F. et al. Optimized pipeline of MuTect and GATK tools to improve the detection of somatic single nucleotide polymorphisms in whole-exome sequencing data. BMC Bioinf. 17, 341. https://doi.org/10.1186/s12859-016-1190-7 (2016).
Wang, K., Li, M. & Hakonarson, H. ANNOVAR: Functional annotation of genetic variants from high-throughput sequencing data. Nucleic Acids Res. 38, e164. https://doi.org/10.1093/nar/gkq603 (2010).
Abe, A. et al. Genome sequencing reveals agronomically important loci in rice using MutMap. Nat. Biotechnol. 30, 174–178 (2012).
She, H. et al. Fine mapping and candidate gene screening of the downy mildew resistance gene RPF1 in Spinach. Theor. Appl. Genet. 131, 2529–2541 (2018).
Geng, X. et al. Rapid identification of candidate genes for seed weight using the SLAF-Seq method in Brassica napus. PLoS One 11, e0147580. https://doi.org/10.1371/journal.pone.0147580 (2016).
Singh, V. K. et al. Indel-seq: A fast-forward genetics approach for identification of trait-associated putative candidate genomic regions and its application in pigeonpea (Cajanus cajan). Plant Biotechnol. J. 15, 906–914 (2017).
Trapnell, C. et al. Transcript assembly and quantification by RNA Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 28, 511–515 (2010).
Kanehisa, M. & Goto, S. KEGG: Kyoto encyclopedia of genes and genomes. Nucleic Acids Res. 28, 27–30 (2000).
Breuninger, H. & Lenhard, M. Control of tissue and organ growth in plants. Curr. Top. Dev. Biol. 91, 185–220 (2010).
Hou, S. et al. A mutant in the CsDET2 gene leads to a systemic brassinosteriod deficiency and super compact phenotype in cucumber (Cucumis sativus L.). Theor. Appl. Genet. 130, 1693–1703 (2017).
Xu, L., Wang, C., Cao, W., Zhou, S. & Wu, T. CLAVATA1-type receptor-like kinase CsCLAVATA1 is a putative candidate gene for dwarf mutation in cucumber. Mol. Genet. Genom. 293, 1393–1405 (2018).
Fang, J. et al. Reduction of ATPase activity in the rice kinesin protein Stemless Dwarf 1 inhibits cell division and organ development. Plant J. 96(3), 620–634 (2018).
Li, Y. et al. Fine genetic mapping of cp: A recessive gene for compact (dwarf) plant architecture in cucumber, Cucumis sativus L.. Theor. Appl. Genet. 123, 973–983 (2011).
Wang, H. et al. The cytochrome P450 gene CsCYP85A1 is a putative candidate for super compact-1 (Scp-1) plant architecture mutation in cucumber (Cucumis sativus L.). Front. Plant Sci. 8, 266. https://doi.org/10.3389/fpls.2017.00266 (2017).
Hwang, J. et al. Fine genetic mapping of a locus controlling short internode length in melon (Cucumis melo L.). Mol. Breed. 34, 949–961 (2014).
Zhang, G. et al. A high-density genetic map for anchoring genome sequences and identifying QTLs associated with dwarf vine in pumpkin (Cucurbita maxima Duch.). BMC Genom. 16, 1101. https://doi.org/10.1186/s12864-015-2312-8 (2015).
Lester, D. R., Ross, J. J., Davies, P. J. & Reid, J. B. Mendel’s stem length gene (Le) encodes a gibberellin 3β-hydroxylase. Plant Cell 9, 1435–1443 (1997).
Emami, S., Arumainayagam, D., Korf, I. & Rose, A. B. The effects of a stimulating intron on the expression of heterologous genes in Arabidopsis thaliana. Plant Biotechnol. J. 11, 555–563 (2013).
Spray, C. R. et al. The dwarf-1 (dt) mutant of Zea mays blocks three steps in the gibberellin-biosynthetic pathway. Proc. Natl. Acad. Sci. USA 93, 10515–10518 (1996).
Pimenta Lange, M. J. et al. Functional characterization of gibberellin oxidases from cucumber, Cucumis sativus L.. Phytochemistry 90, 62–69 (2013).
Acknowledgements
This work was funded by the Youth Talents Training Program of Zhejiang Academy of Agricultural Sciences (2019R23R08E01). We thank Lesley Benyon, PhD, from Liwen Bianji, Edanz Group China (www.liwenbianji.cn/ac), for editing the English text of a draft of this manuscript.
Author information
Authors and Affiliations
Contributions
Y.Y.S. and M.F. conceived and designed the experiment. M.F. constructed the F1 and F2 progeny. Y.Y.S. carried out the bioinformatics analysis. Y.Y.S. and H.Q.Z. carried out the experimental analysis. Y.Y.S. wrote the manuscript, and M.F., Y.J.H., and P.A.G. gave insightful suggestions.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Sun, Y., Zhang, H., Fan, M. et al. A mutation in the intron splice acceptor site of a GA3ox gene confers dwarf architecture in watermelon (Citrullus lanatus L.). Sci Rep 10, 14915 (2020). https://doi.org/10.1038/s41598-020-71861-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-71861-7
- Springer Nature Limited
This article is cited by
-
Dwarfism of Ficus microcarpa in the Ryukyu islands, Okinawa, Japan
Plant Systematics and Evolution (2024)
-
Mutation in the GA3ox gene governs short-internode characteristic in a korean cucumber inbred line
Horticulture, Environment, and Biotechnology (2023)