Abstract
Invasive fungal infections are a potentially life-threatening complication in immunocompromised patients. The aim of this study was to assess the efficacy and safety of posaconazole as compared with other antifungal agents for preventing invasive fungal infections in immunocompromised patients. Embase, CENTRAL, and MEDLINE were searched for randomized conweekmonthtrolled trials (RCTs) up to June 2020. A systematic review with meta-analysis of RCTs was performed using random-effects model. Trial sequential analysis (TSA) was conducted for the primary outcome to assess random errors. A total of five RCTs with 1,617 participants were included. Posaconazole prophylaxis was associated with a significantly lower risk of IFIs (RR, 0.43 [95% CI 0.28 to 0.66, p = 0.0001]) as compared to other antifungal agents. No heterogeneity was identified between studies (I2 = 0%). No significant associations were observed for the secondary outcomes measured, including risk reduction of invasive aspergillosis and candidiasis, clinical failure, all-cause mortality, and treatment-related adverse events, except for infection-related mortality (RR, 0.31 [95% CI 0.15 to 0.64, p = 0.0001]). Subgroup analysis favoured posaconazole over fluconazole for the prevention of IFIs (RR, 0.44 [95% CI 0.28 to 0.70, p = 0.0004]). TSA confirmed the prophylactic benefit of posaconazole against IFIs. Posaconazole is effective in preventing IFIs among immunocompromised patients, particularly those with hematologic malignancies and recipients of allogenic hematopoietic stem cell transplantation.
Similar content being viewed by others
Introduction
Invasive fungal infections (IFIs) remain a significant health threat in immunocompromised individuals, including blood cancer patients and transplant recipients1,2. In addition to prolonged hospital stays and increased healthcare costs, high mortality rates are reported in affected patients3. In view of the substantial disease burden associated with IFIs, primary antifungal prophylaxis is crucial in patients at high risk of prolonged neutropenia.
Triazoles are an important class of antifungals in clinical settings due to their effectiveness and availability for oral administration4,5. Structurally related to itraconazole, posaconazole is an extended-spectrum second-generation triazole with improved potency6. The azoles inhibit the cytochrome P450 lanosterol 14α-demethylase (CYP51) enzyme thereby blocking the synthesis of ergosterol and disrupting fungal cell membrane integrity4,5,6. The long side chain of posaconazole enables enhanced hydrophobic binding to CYP51, resulting in activity against many fluconazole- and voriconazole-resistant isolates6,7. Posaconazole demonstrates excellent antifungal activity against Candida and Aspergillus species7,8, which are known to be the predominant fungal pathogens of IFIs9,10. In contrast to older triazoles, posaconazole offers an additional coverage against Mucorales8,11.
To date, randomized controlled trials (RCTs) investigating the role of posaconazole in primary prophylaxis have demonstrated variable results12,13,14,15,16. However, current evidence on the efficacy and safety of posaconazole as compared to other antifungal agents has not been comprehensively evaluated. The objective of the current systematic review with meta-analysis and trial sequential analysis (TSA) was to provide reliable estimates on the efficacy and safety of posaconazole from RCTs to facilitate evidence-based decision-making on its prophylactic use in immunocompromised patients.
Methods
Study design
The protocol of this systematic review was registered in the International Prospective Register of Systematic Reviews (PROSPERO) under CRD42019148129. This study was performed based on the Cochrane Handbook for Systematic Reviews of Interventions17 and was reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA)18.
Data sources
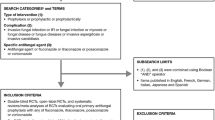
A systematic search for RCTs was performed in Embase, Cochrane Central Register of Controlled Trials (CENTRAL), and MEDLINE from inception to June 2020. Our search strategy included terms such as “immunocompromised host”, “leukemia”, “lymphoma”, “myelodysplastic syndromes”, “chemotherapy”, “transplants”, “graft vs host disease”, “invasive fungal infections”, “aspergillosis”, “candidiasis”, and “posaconazole”. The search was limited to human studies. A detailed description of the search strategy is provided in Supplementary material, Table 1.
Study selection
Studies included were RCTs that met the following eligibility criteria: study participants of any age who were at risk of prolonged neutropenia (patients with hematologic malignancies who received chemotherapy or transplant recipients under immunosuppressive treatment); intervention was posaconazole at any dose; comparators were any other antifungal agents; primary outcome was the incidence of proven/probable IFIs, categorized as per the revised criteria by the European Organization for the Research and Treatment of Cancer and the Mycoses Study Group (EORTC/MSG)19; secondary outcomes were the incidence of invasive aspergillosis, incidence of invasive candidiasis, clinical failure, all-cause mortality, infection-related mortality, and treatment-related adverse events.
Data extraction and quality assessment
Data extraction was performed independently by two reviewers (TYW and YSL) and transferred into a standardized data collection form. The extracted data included the study design, characteristics of the study participants, interventions, outcome definitions, and outcome measures. Data for all outcomes were extracted following the intention-to-treat principle. The risk of bias for every study was assessed independently by two reviewers (TYW and YSL) using the revised Cochrane Risk of Bias tool (RoB 2.0)20. Any disagreements were resolved by consensus among the reviewers.
Data synthesis and statistical analysis
In the primary meta-analysis, we compared posaconazole with other antifungal agents in terms of efficacy in reducing the risk of proven/probable IFIs. Random-effects model was used to generate pooled relative risk (RR) and the corresponding 95% confidence interval (95% CI). Statistical significance was considered at a two-tailed p-value < 0.05. Comparison was made between posaconazole and fluconazole in the subgroup analysis. Multiple sensitivity analyses were carried out to assess the robustness of results from our primary meta-analysis, by employing fixed-effects model and excluding trials with high risk of bias. Heterogeneity between the trials was quantitatively assessed using I2 statistic, whereby I2 estimate ≥ 50% was indicative of substantial heterogeneity17. Funnel plot asymmetry testing and Egger’s regression test were performed to assess publication bias21. A p-value of < 0.05 for Egger’s test was considered as statistical evidence of significant small-study effects. All statistical analyses were performed using STATA software version 15.0 (StataCorp, College Station, TX, USA).
Meta-analyses that include a small number of studies or study participants, may produce false positive results (type I error) due to random errors22. TSA adjusts the threshold for statistical significance by considering the accrued sample size from all included trials in the cumulative meta-analysis and provides the required information size to determine the reliability of conclusions obtained from a meta-analysis22,23. Therefore, we conducted a TSA to assess the effect of random errors on our primary meta-analysis using an exclusive software developed by the Copenhagen Trial Unit (available at https://www.ctu.dk)24. Control event rate and anticipated relative risk reduction from our primary meta-analysis were used to perform TSA. For zero-event trials, constant continuity correction method was applied by adding a correction factor of 0.5 to the number of events and patients in both the treatment and control arms25.
The quality of evidence derived from meta-analytic estimates was rated (high, moderate, low or very low) based on the Grading of Recommendations Assessment, Development and Evaluation (GRADE) approach by using GRADEpro GDT software online (available at https://gradepro.org). The certainty of effect estimates was evaluated by considering the study design, risk of bias, inconsistency, indirectness, imprecision, and publication bias26,27.
Ethics approval and consent to participate
Not applicable.
Consent for publication
Not applicable.
Results
Included studies and main characteristics
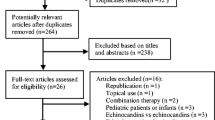
The process of identification, screening, and selection of studies is depicted in Fig. 1. The initial literature search identified 100 studies. After the removal of duplicates, 98 studies were screened for their eligibility. Of these, five RCTs12,13,14,15,16 met the eligibility criteria and were included in our primary meta-analysis. Table 1 describes the study characteristics of the included trials. A total of 1,617 participants aged ≥ 13-year-old with hematologic malignancies were included in this study. Three RCTs12,15,16 recruited patients who received chemotherapy and two RCTs13,14 enrolled patients who had undergone hematopoietic stem cell transplantation (HSCT) with/without the development of graft-versus-host disease (GVHD). Posaconazole oral suspension was used at a dose of 600 mg/day in most trials12,13,14,15, except one trial16 using 800 mg/day.
Risk of bias assessment
The risk of bias of all included trials is presented in Supplementary material, Fig. 1. Overall, one trial14 had low risk of bias, three trials12,13,16 had some concerns of bias, and one trial15 had high risk of bias. Majority of the trials12,13,15,16 did not provide information on randomization methods and allocation concealment. Although three trials14,15,16 followed an open-label design, outcome measurement was unlikely to be biased since the study endpoints were mostly binary outcomes that involved diagnostic procedures.
Primary efficacy outcome: incidence of proven/probable IFIs
Posaconazole demonstrated a statistically significant 57% reduction in risk of IFIs as compared to other antifungal agents (RR, 0.43 [95% CI 0.28 to 0.66], p = 0.0001, I2 = 0%) with no heterogeneity between studies (Fig. 2). Results from the subgroup analysis comparing posaconazole and fluconazole are depicted in Fig. 3.
Sensitivity analyses
Findings from sensitivity analyses were consistent with our primary meta-analysis. Posaconazole was significantly more effective than other antifungal agents in preventing IFIs when a fixed-effects model was applied (RR, 0.41 [95% CI 0.27 to 0.63], p = 0.00005, I2 = 0%) (Supplementary material, Fig. 2) and after excluding one trial15 with high risk of bias (RR, 0.44 [95% CI 0.28 to 0.71], p = 0.0007, I2 = 0%) (Supplementary material, Fig. 3). Per-protocol analysis was not feasible as some trials14,15 did not report the number of participants who had completed the treatment phase.
Trial sequential analysis
By using a median event proportion in the control group of 8.71% after excluding one trial15 with high risk of bias, an alpha of 5% (two-sided), and a power of 80%, the required information size to demonstrate or reject a 56% relative risk reduction of IFIs with posaconazole prophylaxis was 790 study participants (Fig. 4). The number of participants included in the primary meta-analysis surpassed the required information size. In addition, the cumulative Z-curve crossed both the conventional and trial sequential monitoring boundaries, suggesting that the evidence was significant and conclusive.
Publication bias
Funnel plot (Supplementary material, Fig. 4) showed weak asymmetry and Egger’s regression test (p = 0.442) (Supplementary material, Table 2) indicated no evident publication bias28, although the number of studies included in the primary meta-analysis was small.
Secondary efficacy outcomes
For invasive aspergillosis, posaconazole reduced the risk by 71% (RR, 0.29 [95% CI 0.08 to 1.09], p = 0.066) compared to control. However, statistical significance was not achieved and substantial heterogeneity was detected (I2 = 54.7%) (Fig. 5). In comparison with control, no significant associations were also observed for posaconazole in reducing the risk of invasive candidiasis (RR, 1.01 [95% CI 0.36 to 2.84], p = 0.982, I2 = 0%) (Fig. 6), clinical failure (RR, 0.82 [95% CI 0.58 to 1.15], p = 0.246, I2 = 70.6%) (Fig. 7), and all-cause mortality (RR, 0.77 [95% CI 0.59 to 1.01], p = 0.055, I2 = 22.2%) (Fig. 8). Meanwhile, posaconazole demonstrated a significant reduction in infection-related mortality (RR, 0.31 [95% CI 0.15 to 0.64], p = 0.001, I2 = 0%) (Fig. 9).
Safety outcome: treatment-related adverse events
Four RCTs12,13,14,15 provided information on the incidence of treatment-related adverse events. Shen et al15 did not report the overall number of patients who experienced adverse events; hence, this study was not included in our meta-analysis. Commonly documented antifungal-related adverse events were gastrointestinal disorders (nausea, vomiting, and diarrhea) and abnormalities in liver function. No significant difference was detected between posaconazole and other antifungal agents (RR, 1.09 [95% CI 0.71 to 1.66], p = 0.703, I2 = 73.3%) (Fig. 10).
GRADE: summary of evidence for posaconazole
Randomized trials are assigned with high rating provided there are no major study limitations. Most of the included trials12,13,16 in our primary meta-analysis had some concerns of bias due to the lack of blinding and allocation concealment. However, the quality of evidence remained unaffected as the overall limitations were not serious. No inconsistency was observed among trials included in the primary meta-analysis. In addition to different contributory factors to the risk of neutropenia, the use of interventions at different doses with variable duration of treatment across the studies suggested potential indirectness. The results of TSA indicated that the optimal information size was achieved and the 95% CI excluded the value of no effect.
We thereby concluded that the evidence for posaconazole in preventing IFIs among immunocompromised patients was of high quality. Table 2 shows the GRADE evidence and summary of findings for our primary outcome (refer to Supplementary material, Table 3 for other outcomes).
Discussion
IFIs have long been recognized as a contributory factor to the significant increase in morbidity and mortality among immunocompromised patients, hence antifungal prophylaxis is of great importance in high-risk patients3. Posaconazole is a relatively potent triazole with promising antifungal activities against a wide array of fungal species6,7,8. Recent network meta-analyses ranked posaconazole highly for its efficacy in reducing the overall incidence of IFIs29,30,31,32. Similarly, the results of the present meta-analysis suggested that posaconazole significantly lowered the risk of IFIs as compared to control, with conclusive and high-quality evidence. This study also showed that posaconazole was beneficial in reducing infection-related mortality.
The findings from the subgroup analysis suggested that posaconazole was significantly superior to fluconazole in decreasing the risk of IFIs. Based on two of the studies12,13 included in the subgroup analysis, the incidence of invasive aspergillosis was significantly greater among patients who received fluconazole. This may be attributable to the selective antifungal activity of fluconazole against yeast pathogens, limiting its role in preventing invasive mold infections33, which translates into lower overall incidence of IFIs in patients on posaconazole prophylaxis. In clinical settings, fluconazole is one of the commonly used antifungal agents due to excellent tolerability and its high bioavailability34. However, the widespread use of fluconazole has driven the pathogenic shift to resistant strains of non-albicans Candida35,36. Moreover, the prophylactic role of fluconazole may be progressively diminishing owing to the rising incidence of invasive mold infections, notably invasive aspergillosis in HSCT recipients10,37. In light of the evolving epidemiological trends of IFIs, posaconazole stands out as a suitable antifungal agent for primary prophylaxis.
Other triazoles including itraconazole and voriconazole have also been recommended for the prevention of IFIs38,39,40,41. However, the use of both agents may be restricted due to poor tolerability and the associated higher incidence of adverse events33,39,42. The role of isavuconazole, a relatively new triazole in IFI prophylaxis is less well-studied. To our knowledge, trials are lacking to compare the efficacy and safety of posaconazole with voriconazole and isavuconazole. Echinocandins such as caspofungin, micafungin, and anidulafungin show antifungal properties against Candida and Aspergillus species with fewer adverse effects or drug interactions43,44. In addition to their favourable safety profiles, echinocandins have more predictable pharmacokinetics but they require daily intravenous administration43,44. In the published reviews, echinocandins were found to have significantly higher treatment success rates than triazoles for prophylaxis, with micafungin being the most studied agent45,46. Nevertheless, none of the included studies compared posaconazole to echinocandins. The present meta-analysis only included one comparative study between posaconazole and micafungin, underlining the need for more clinical trials.
Posaconazole administration may be associated with adverse effects such as nausea, vomiting, diarrhea, headache, and abnormalities in liver function47. In terms of safety profile, results from the present meta-analysis suggested that there was no significant difference observed between posaconazole and other antifungal agents. Nevertheless, posaconazole should be used with caution in patients with comorbidities due to possible drug–drug interactions47. Healthcare providers should also be wary of the potential development of resistance to posaconazole among fungal species48.
Currently, posaconazole is available as an oral suspension, delayed-release tablet, and intravenous formulation47. Posaconazole oral suspension was used in all of the trials included in this study. The effectiveness of this conventional formulation may be limited by its unpredictable and highly variable bioavailability; hence, it requires multiple daily dosing and concurrent administration with meals to maximize its systemic exposure49. In view of the greater bioavailability offered by the two newer formulations50,51,52,53, posaconazole delayed-release tablet and intravenous formulation are encouraged to be used in future clinical trials to compare their efficacy and safety with other antifungal agents.
There are several limitations to this systematic review. This review identified only five eligible RCTs and some trials involved small number of participants. Moreover, high-quality trials were lacking as those included in this study were mostly open-label trials. The duration of treatment and follow-up period were not identical across studies, which may have affected the summary effect estimates. In addition, the optimal duration of antifungal prophylaxis remained unknown. Posaconazole prophylaxis in other immunocompromised populations such as solid organ transplant recipients and autologous HSCT recipients were not discussed in the current review due to the absence of RCTs. The prophylactic role of posaconazole in allogenic HSCT recipients without GVHD and patients with hematologic malignancies other than acute myeloid leukemia and myelodysplastic syndrome were also less clear.
Conclusions
The present meta-analysis supports the use of posaconazole for IFI prophylaxis in patients with hematologic malignancies (particularly acute myeloid leukemia and myelodysplastic syndrome) and allogenic HSCT recipients with conclusive and high-quality evidence. However, additional well-designed trials are required to study the efficacy and safety of posaconazole delayed-release tablet and intravenous formulation in comparison with other antifungal agents. We also emphasize the need of future clinical trials in other patient settings to extensively study the role of posaconazole in the primary prevention of IFIs.
Data availability
All other data is available in the Supplementary Material and any further information is available upon request from the corresponding author.
References
Kriengkauykiat, J., Ito, J. I. & Dadwal, S. S. Epidemiology and treatment approaches in management of invasive fungal infections. Clin. Epidemiol. 3(1), 175–191 (2011).
Perfect, J. R., Hachem, R. & Wingard, J. R. Update on epidemiology of and preventive strategies for invasive fungal infections in cancer patients. Clin. Infect. Dis. 59(Suppl 5), S352–S355 (2014).
Menzin, J. et al. Mortality, length of hospitalization, and costs associated with invasive fungal infections in high-risk patients. Am. J.. Health Syst. Pharm. 66(19), 1711–1717 (2009).
Lass-Flörl, C. Triazole antifungal agents in invasive fungal infections. Drugs. 71(18), 2405–2419 (2011).
Nagappan, V. & Deresinski, S. Posaconazole: A broad-spectrum triazole antifungal agent. Clin. Infect. Dis. 45(12), 1610–1617 (2007).
Morris, M. I. Posaconazole: A new oral antifungal agent with an expanded spectrum of activity. Am. J. Health Syst. Pharm. 66(3), 225–236 (2009).
Diekema, D. J., Messer, S. A., Hollis, R. J., Jones, R. N. & Pfaller, M. A. Activities of caspofungin, itraconazole, posaconazole, ravuconazole, voriconazole, and amphotericin B against 448 recent clinical isolates of filamentous fungi. J. Clin. Microbiol. 41(8), 3623–3626 (2003).
Sabatelli, F. et al. In vitro activities of posaconazole, fluconazole, itraconazole, voriconazole, and amphotericin B against a large collection of clinically important molds and yeasts. Antimicrob. Agents Chemother. 50(6), 2009–2015 (2006).
Pagano, L. et al. The epidemiology of fungal infections in patients with hematologic malignancies: The SEIFEM-2004 study. Haematologica 91(8), 1068–1075 (2006).
Kontoyiannis, D. P. et al. Prospective surveillance for invasive fungal infections in hematopoietic stem cell transplant recipients, 2001–2006: Overview of the Transplant-Associated Infection Surveillance Network (TRANSNET) database. Clin. Infect. Dis. 50(8), 1091–1100 (2010).
Vehreschild, J. J. et al. Mucormycosis treated with posaconazole: Review of 96 case reports. Crit. Rev. Microbiol. 39(3), 310–324 (2013).
Cornely, O. A. et al. Posaconazole vs. fluconazole or itraconazole prophylaxis in patients with neutropenia. N. Engl. J. Med. 356(4), 348–359 (2007).
Ullmann, A. J. et al. Posaconazole or fluconazole for prophylaxis in severe graft-versus-host disease. N. Engl. J. Med. 356(4), 335–347 (2007).
Chaftari, A. M. et al. Comparison of posaconazole versus weekly amphotericin B lipid complex for the prevention of invasive fungal infections in hematopoietic stem-cell transplantation. Transplantation 94(3), 302–308 (2012).
Shen, Y. et al. Posaconazole vs. fluconazole as invasive fungal infection prophylaxis in China: A multicenter, randomized, open-label study. Int. J. Clin. Pharmacol. Ther. 51(9), 738–745 (2013).
Epstein, D. J. et al. Micafungin versus posaconazole prophylaxis in acute leukemia or myelodysplastic syndrome: A randomized study. J. Infect. 77(3), 227–234 (2018).
Higgins, J.P.T. et al. Cochrane Handbook for Systematic Reviews of Interventions version 6.0 (updated July 2019). Cochrane, 2019. hhtp://www.training.cochrane.org/handbook.
Moher, D., Liberati, A., Tetzlaff, J., Altman, D. G. & The PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. Ann. Intern. Med. 151(4), 264–269 (2009).
De Pauw, B. et al. Revised definitions of invasive fungal disease from the European Organization for Research and Treatment of Cancer/Invasive Fungal Infections Cooperative Group and the National Institute of Allergy and Infectious Diseases Mycoses Study Group (EORTC/MSG) Consensus Group. Clin. Infect. Dis. 46(12), 1813–1821 (2008).
Sterne, J. A. C. et al. RoB 2: A revised tool for assessing risk of bias in randomised trials. BMJ 366, l4898 (2019).
Egger, M., Davey Smith, G., Schneider, M. & Minder, C. Bias in meta-analysis detected by a simple, graphical test. BMJ 315(7109), 629–634 (1997).
Thorlund, K. et al. Can trial sequential monitoring boundaries reduce spurious inferences from meta-analyses?. Int. J. Epidemiol. 38(1), 276–286 (2009).
Brok, J., Thorlund, K., Gluud, C. & Wetterslev, J. Trial sequential analysis reveals insufficient information size and potentially false positive results in many meta-analyses. J. Clin. Epidemiol. 61(8), 763–769 (2008).
Thorlund, K. et al. Software for trial sequential analysis (TSA) ver. 0.9.5.5 Beta. Copenhagen Trial Unit, Centre for Clinical Intervention Research, Copenhagen, Denmark, free-ware. http://www.ctu.dk/tsa.
Thorlund, K. et al. User manual for trial sequential analysis (TSA). Copenhagen Trial Unit 1–115 (Centre for Clinical Intervention Research, Copenhagen, 2011).
Atkins, D. et al. Grading quality of evidence and strength of recommendations. BMJ 328(7454), 1490 (2004).
Guyatt, G. H. et al. GRADE: An emerging consensus on rating quality of evidence and strength of recommendations. BMJ 336(7650), 924–926 (2008).
Sterne, J. A. C. et al. Recommendations for examining and interpreting funnel plot asymmetry in meta-analyses of randomised controlled trials. BMJ 343, d4002 (2011).
Zhao, Y. J. et al. Network meta-analysis and pharmacoeconomic evaluation of fluconazole, itraconazole, posaconazole, and voriconazole in invasive fungal infection prophylaxis. Antimicrob. Agents Chemother. 60(1), 376–386 (2015).
Leonart, L. P. et al. A network meta-analysis of primary prophylaxis for invasive fungal infection in haematological patients. J. Clin. Pharm. Ther. 42(5), 530–538 (2017).
Lee, C. H., Lin, C., Ho, C. L. & Lin, J. C. Primary fungal prophylaxis in hematological malignancy: A network meta-analysis of randomized controlled trials. Antimicrob. Agents Chemother. 62(8), e00355-e418 (2018).
Su, H. C., Hua, Y. M., Feng, I. J. & Wu, H. C. Comparative effectiveness of antifungal agents in patients with hematopoietic stem cell transplantation: A systematic review and network meta-analysis. Infect. Drug Resist. 12, 1311–1324 (2019).
Nett, J. E. & Andes, D. R. Antifungal agents: Spectrum of activity, pharmacology, and clinical indications. Infect. Dis. Clin. N. Am. 30(1), 51–83 (2016).
Charlier, C. et al. Fluconazole for the management of invasive candidiasis: Where do we stand after 15 years?. J. Antimicrob. Chemother. 57(3), 384–410 (2006).
Marr, K. A., Seidel, K., White, T. C. & Bowden, R. A. Candidemia in allogeneic blood and marrow transplant recipients: Evolution of risk factors after the adoption of prophylactic fluconazole. J. Infect. Dis. 181(1), 309–316 (2000).
Lamoth, F., Lockhart, S. R., Berkow, E. L. & Calandra, T. Changes in the epidemiological landscape of invasive candidiasis. J. Antimicrob. Chemother. 73(Suppl 1), i4-13 (2018).
Valentine, J. C. et al. A population-based analysis of invasive fungal disease in haematology-oncology patients using data linkage of state-wide registries and administrative databases: 2005–2016. BMC Infect. Dis. 19(1), 274 (2019).
Fleming, S. et al. Consensus guidelines for antifungal prophylaxis in haematological malignancy and haemopoietic stem cell transplantation, 2014. Intern. Med. J. 44(12b), 1283–1297 (2014).
Baden, L. R. et al. Prevention and treatment of cancer-related infections, version 2.2016, NCCN Clinical Practice Guidelines in Oncology. J. Natl. Compr. Cancer Netw. 14(7), 882–913 (2016).
Patterson, T. F. et al. Practice guidelines for the diagnosis and management of aspergillosis: 2016 update by the Infectious Diseases Society of America. Clin. Infect. Dis. 63(4), e1-60 (2016).
Mellinghoff, S. C. et al. Primary prophylaxis of invasive fungal infections in patients with haematological malignancies: 2017 update of the recommendations of the Infectious Diseases Working Party (AGIHO) of the German Society for Haematology and Medical Oncology (DGHO). Ann. Hematol. 97(2), 197–207 (2018).
Enoch, D. A., Ludlam, H. A. & Brown, N. M. Invasive fungal infections: A review of epidemiology and management options. J. Med. Microbiol. 55(7), 809–818 (2006).
Aguilar-Zapata, D., Petraitiene, R. & Petraitis, V. Echinocandins: The expanding antifungal armamentarium. Clin. Infect. Dis. 61(Suppl 6), S604–S611 (2015).
Epstein, D. J., Seo, S. K., Brown, J. M. & Papanicolaou, G. A. Echinocandin prophylaxis in patients undergoing haematopoietic cell transplantation and other treatments for haematological malignancies. J. Antimicrob. Chemother. 73(Suppl 1), i60-72 (2018).
Wang, J. F., Xue, Y., Zhu, X. B. & Fan, H. Efficacy and safety of echinocandins versus triazoles for the prophylaxis and treatment of fungal infections: A meta-analysis of RCTs. Eur. J. Clin. Microbiol. Infect. Dis. 34(4), 651–659 (2015).
Lee, C. H. et al. Efficacy and safety of micafungin versus extensive azoles in the prevention and treatment of invasive fungal infections for neutropenia patients with hematological malignancies: A meta-analysis of randomized controlled trials. PLoS ONE 12(7), e0180050 (2017).
Merck & Co., Inc. Noxafil® (posaconazole) package insert (Merck & Co., Inc., Whitehouse Station).
Auberger, J. et al. Invasive fungal breakthrough infections, fungal colonization and emergence of resistant strains in high-risk patients receiving antifungal prophylaxis with posaconazole: Real-life data from a single-centre institutional retrospective observational study. J. Antimicrob. Chemother. 67(9), 2268–2273 (2012).
Krishna, G., Moton, A., Ma, L., Medlock, M. M. & McLeod, J. Pharmacokinetics and absorption of posaconazole oral suspension under various gastric conditions in healthy volunteers. Antimicrob. Agents Chemother. 53(3), 958–966 (2009).
Duarte, R. F. et al. Phase 1b study of new posaconazole tablet for prevention of invasive fungal infections in high-risk patients with neutropenia. Antimicrob. Agents Chemother. 58(10), 5758–5765 (2014).
Cornely, O. A. et al. Phase 3 pharmacokinetics and safety study of a posaconazole tablet formulation in patients at risk for invasive fungal disease. J. Antimicrob. Chemother. 71(3), 718–726 (2016).
Maertens, J. et al. Phase 1B study of the pharmacokinetics and safety of posaconazole intravenous solution in patients at risk for invasive fungal disease. Antimicrob. Agents Chemother. 58(7), 3610–3617 (2014).
Cornely, O. A. et al. Pharmacokinetics and safety results from the Phase 3 randomized, open-label, study of intravenous posaconazole in patients at risk of invasive fungal disease. J. Antimicrob. Chemother. 72(12), 3406–3413 (2017).
Funding
RKM acknowledges financial support from the Institute of Research, Development and Innovation, International Medical University, Kuala Lumpur, Malaysia.
Author information
Authors and Affiliations
Contributions
T.Y.W., Y.S.L., S.K.V., R.K.M. contributed to conception, design, data acquisition and interpretation, drafted the manuscript; D.V.G. contributed to data acquisition and analysis, drafted the manuscript; A.G., B.B., D.G., R.M.W. contributed to data interpretation, drafted the manuscript; P.S.W., S.M.C. contributed to data interpretation, critically revised the manuscript. All authors gave final approval and agreed to be accountable for all aspects of the work.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wong, T.Y., Loo, Y.S., Veettil, S.K. et al. Efficacy and safety of posaconazole for the prevention of invasive fungal infections in immunocompromised patients: a systematic review with meta-analysis and trial sequential analysis. Sci Rep 10, 14575 (2020). https://doi.org/10.1038/s41598-020-71571-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-71571-0
- Springer Nature Limited
This article is cited by
-
Communication between immune system and mycobiota impacts health and disease
Proceedings of the Indian National Science Academy (2022)
-
Isavuconazole and Liposomal Amphotericin B as Successful Combination Therapy of Refractory Invasive Candidiasis in a Liver Transplant Recipient: A Case Report and Literature Review
Mycopathologia (2022)