Abstract
This meta-analysis assessed the association between vitamin D supplementation and the outcomes of critically ill adult patients. A literature search was conducted using the PubMed, Web of Science, EBSCO, Cochrane Library, Ovid MEDLINE, and Embase databases until March 21, 2020. We only included randomized controlled trials (RCTs) comparing the efficacy of vitamin D supplementation with placebo in critically ill adult patients. The primary outcome was their 28-day mortality. Overall, 9 RCTs with 1867 patients were included. In the pooled analysis of the 9 RCTs, no significant difference was observed in 28-day mortality between the vitamin D supplementation and placebo groups (20.4% vs 21.7%, OR, 0.73; 95% CI, 0.46–1.15; I2 = 51%). This result did not change as per the method of vitamin D supplementation (enteral route only: 19.9% vs 18.2%, OR, 1.19; 95% CI, 0.88–1.57; I2 = 10%; intramuscular or intravenous injection route: 25.6% vs 40.8%, OR, 0.48; 95% CI, 0.21–1.06; I2 = 19%) or daily dose (high dose: 20.9% vs 19.8%, OR, 0.83; 95% CI, 0.51–1.36; I2 = 53%; low dose: 15.6% vs 21.3%, OR, 0.74; 95% CI, 0.32–1.68; I2 = 0%). No significant difference was observed between the vitamin D supplementation and placebo groups regarding the length of ICU stay (standard mean difference [SMD], − 0.30; 95% CI, − 0.61 to 0.01; I2 = 60%), length of hospital stay (SMD, − 0.17; 95% CI, − 041 to 0.08; I2 = 65%), and duration of mechanical ventilation (SMD, − 0.41; 95% CI, − 081 to 0.00; I2 = 72%). In conclusion, this meta-analysis suggested that the administration of vitamin D did not provide additional advantages over placebo for critically ill patients. However, additional studies are needed to confirm our findings.
Similar content being viewed by others
Introduction
Vitamin D, a fat-soluble vitamin, is an essential nutrient in bone metabolism and calcium and phosphorus homeostasis. However, the system of vitamin D is complex, in which some novel pathways have been found for host response to vitamin D treatment including non-canonical pathways of vitamin D activation1,2 leading to production of non- or low-calcemic analogs3 and of lumisterol activation4. In clinical practice, vitamin D is used for the treatment of hyperproliferative skin diseases, hyperparathyroidism, and osteoporosis. Vitamin D also exhibits other non-skeletal pleiotropic properties, such as immunomodulatory, antimicrobial, cardiovascular, and muscular effects. Therefore, vitamin D deficiency is associated with many diseases including tuberculosis, nonalcoholic fatty liver disease, cardiovascular disease, and metabolic syndrome5,6,7. In the United States, adults aged 20–39 years are at the highest risk of vitamin D deficiency (the prevalence: 7.6%; 95% CI: 6.0–9.6%)8. One study conducted in Europe showed that 13.0% of 55,844 European individuals showed average serum 25(OH)D concentrations of < 30 nmol/L9. In China, 30.6% of elderly people have vitamin D deficiency10.
In addition to its prevalence in the general population, vitamin D deficiency is common among critically ill patients. Lee et al. showed that 64.5% (n = 120) of critically ill surgical patients had serum 25(OH)D concentrations of < 20 nmol/L11, and Higgins et al. reported that 26% (50/196) of patients admitted to a medical/surgical intensive care unit (ICU) had vitamin D levels of ≤ 30 nmol/L12. A retrospective cohort study showed that 54% (65/121) of patients with severe sepsis or septic shock had vitamin D levels lower than 15 mg/mL13, and another prospective multicenter study demonstrated vitamin D deficiency in 78.8% (197/250) of patients14. Furthermore, several studies document that vitamin D deficiency could be associated with poor outcomes in critically ill patients12,13,15,16,17,18. To improve the outcomes of critically ill patients, vitamin D supplementation was proposed for ICU patients. Several randomized controlled trials (RCTs) were conducted to investigate the effects of vitamin D supplementation on the outcomes of critically ill patients. However, their results are conflicting19,20,21,22,23,24,25,26,27,28. Some studies showed that vitamin D supplementation demonstrated positive effects by decreasing the length of hospital stay23, duration of mechanical ventilation (MV)26,27, and mortality rate24,27. However, some studies11,20,21,25,29,30 reported no change in the outcomes of critically ill patients. Even 2 meta-analyses, the included studies of which were published before 201731,32, provided inconsistent findings. Since 2017, four more RCTs24,25,26,27 have reported their findings. Therefore, we conducted an updated meta-analysis of RCTs to assess the association between vitamin D supplementation and the outcomes of critically ill patients.
Methods
Study search and selection
We conducted a literature review using the databases of PubMed, Embase, Web of Science, EBSCO, Cochrane Library, Ovid Medline, Embase, and Proquest until March 21, 2020. The following search terms were used: “intensive care” “ICU,” “critically-ill,” “vitamin D,” “calcitriol,” “Cholecalciferol*,” “ergocalciferol*,” and “RCT.” Our meta-analysis only included RCTs that investigated the clinical efficacy of vitamin D supplementation compared with placebo for critically ill adult patients. The supplementation could be done in different ways, such as oral, enteral, or parenteral vitamin D administration as 1, 25-dihydroxyvitamin D (calcitriol) or 25-hydroxyvitamin D (cholecalciferol). Two authors (Lan SH and Chang SP) searched for related studies and examined the risk of bias in each study using the Cochrane Risk of Bias Assessment tool33. When they had different opinions, a third author (Lai CC) helped resolve the issue. Data, including the year of publication, study design, study location and duration, demographic characteristics of critically ill patients, regimen of vitamin D, patient outcomes, and adverse events, were extracted from each included study. This study followed the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) reporting guidelines.
Definitions and outcomes
Critically ill patients were defined as that the patients with acute respiratory failure required mechanical ventilation or the patients required ICU hospitalization. The primary outcome of the current study was the patients’ 28-day mortality. If data on 28-day mortality were not available, hospital mortality was used in the meta-analysis. Secondary outcomes included the length of ICU and hospital stay and the duration of MV. Doses of ≥ 300,000 and < 300,000 IU of vitamin D daily were defined as high and low doses, respectively, as per a previous study34.
Statistical analysis
We used Review Manager software (The Cochrane Collaboration 2008, Copenhagen) to develop a random-effects model and derive the pooled estimates and their associated 95% CIs. The odds ratio (OR) was used to evaluate the outcome of 28-day mortality. Standardized mean differences (SMDs) and 95% CIs were computed for continuous variables including length of ICU and hospital stay and the duration of MV.
Results
Study selection
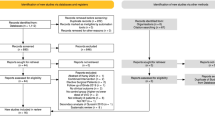
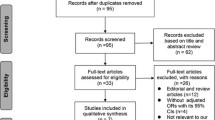
Our search yielded 444 studies in total from online databases from PubMed (n = 56), Web of Science (n = 71), EBSCO (n = 27), Cochrane library (n = 107), Ovid MEDLINE (n = 74), and Embase (n = 85); 272 duplicate studies were excluded. The remaining 172 articles were identified. Moreover, 142 studies were found to be irrelevant after the title and abstract were screened, and 19 studies were found to be irrelevant after the full text was screened. Eventually, 9 RCTs19,20,21,22,23,24,25,26,27 were included in this meta-analysis (Fig. 1, Appendix 1).
Study characteristics
Five RCTs19,21,22,26,27 were conducted in a single center, and three RCTs20,23,24 were conducted in two centers. Only one RCT25 was a multicenter study (Table 1). Four studies20,22,23,25 were conducted in the United States, three24,26,27 were in Iran, and two19,21 in Austria. Overall, these nine RCTs included a total of 1,640 critically ill patients. Three RCTs19,21,25 only enrolled patients with vitamin D levels ≤ 20 ng/mL, and two studies26,27 enrolled patients with vitamin D levels ≤ 20 ng/mL. Vitamin D was administered through the enteral route in five studies19,21,22,23,25, through intravenous or intramuscular injection in three20,24,27, and both routes in one26. Single-dose regimens of vitamin D were used in six studies19,20,22,24,25,27 and multidose regimens in three studies21,23,26. Almost all risks of bias were low in each study. The study by Miroliaee et al.24 and Hasanloei et al.26 had a high risk of allocation, and detection bias. The publication bias is shown in a funnel plot (Fig. 2).
Primary outcome
In the pooled analysis of nine RCTs, no significant difference was observed in 28-day mortality between the vitamin D supplementation and placebo groups (20.4% vs 21.7%, OR, 0.73; 95% CI, 0.46–1.15; I2 = 51%, Fig. 3). A sensitivity analysis performed after excluding individual studies did not change this result. Similarly, in the subgroup analysis of RCTs that enrolled only patients with vitamin D deficiency, no significant differences were observed in mortality (21.4% vs 19.7%, OR, 0.93; 95% CI, 0.57–1.52; I2 = 58%). This result did not change as per the method of vitamin D supplementation (enteral route only: 19.9% vs 18.2%, OR, 1.19; 95% CI, 0.88–1.57; I2 = 10%; intramuscular or intravenous injection route: 25.6% vs 40.8%, OR, 0.48; 95% CI, 0.21–1.06; I2 = 19%) or the daily dose (high dose: 20.9% vs 19.8%, OR, 0.83; 95% CI, 0.51–1.36; I2 = 53%; low dose: 15.6% vs 21.3%, OR, 0.74; 95% CI, 0.32–1.68; I2 = 0%). The similar trend was observed for subgroup with baseline vitamin D deficiency (19.3% vs 19.1%, OR, 0.80; 95% CI, 0.48–1.46; I2 = 63%). Finally, although the studies conducted from 2016 to 2019 seems to have a better outcome than those from 2011 to 2015, the pooled analysis of 5 studies conducted from 2016 to 2019 still did not show the significant difference between vit D and placebo group (OR, 0.50; 95% CI, 0.18–1.34, I2 = 0.70%).
Secondary outcome
The pooled analysis of seven studies19,20,21,22,23,26,27 reported no significant difference in the length of ICU stay between the vitamin D supplementation and placebo groups (SMD, − 0.30; 95% CI, − 0.61 to 0.01; I2 = 60%)(Fig. 4). Analysis of six studies19,20,21,22,23,25 reported no significant difference in the length of hospital stay between the vitamin D supplementation and placebo groups (SMD, − 0.17; 95% CI, − 041 to 0.08; I2 = 65%)(Fig. 4). Six studies19,20,21,23,26,27 reported no significant difference in the duration of MV between both groups (SMD, − 0.41; 95% CI, − 0.81 to 0; I2 = 72%)(Fig. 4). A subgroup analysis showed that high-dose vitamin D supplementation did not change length of ICU stay (SMD, − 1.82; 95% CI, − 5 to 1.35; I2 = 99%), length of hospital stay (SMD, − 0.09; 95% CI, − 0.25 to 0.06; I2 = 31%), and duration of MV (SMD, − 0.42; 95% CI, − 0.92 to 0.07; I2 = 70%). Similarly, low-dose vitamin D did not change length of ICU stay (SMD, 0.29; 95% CI, − 2.43 to 3; I2 = 97%), length of hospital stay (SMD, − 0.54; 95% CI, − 1.45 to 0.36; I2 = 78%), and duration of MV (SMD, − 0.65; 95% CI, − 1.66 to 0.37; I2 = 87%).
Discussion
This meta-analysis included nine RCTs with 1867 patients to compare the efficacy and safety of vitamin D supplementation with placebo in critically ill patients. The outcome was numerically better in the vitamin D supplementation group than control group, which may suggest biologically significant trends favoring vitamin D supplementation, however, these differences did not reach statistical significance. Overall, our results suggested that vitamin D supplementation did not significantly improve the outcomes of critically ill patients, which was supported by the following evidence. First, 28-day mortality did not change with vitamin D supplementation in the pooled analysis of 9 RCTs. Second, this difference remained unchanged in the sensitivity test. Third, we also found no significant improvement in the mortality of critically ill patients with vitamin D deficiency in the subgroup analysis. Fourth, compared with the placebo group, we found no significant difference in mortality in the vitamin D supplementation group with either enteral or injection administration of vitamin D and with administration of low- or high-dose vitamin D. Finally, we assessed the effect of vitamin D on the length of ICU and hospital stay and MV duration and found no significant difference between the vitamin D supplementation and placebo groups. Moreover, no difference was observed in the subgroup analysis of high and low doses of vitamin D. The aforementioned findings indicate that compared with placebo, the vitamin D supplementation is not associated with lower mortality in critically ill patients.
Our findings are consistent with those of a meta-analysis by Langlois et al.32, in which they included six RCTs of 695 patients, and they found that vitamin D did not reduce the mortality, length of ICU and hospital stay, and period on a ventilator. However, another meta-analysis by Putzu et al.31 including 7 studies of 716 patients between 2011 and 2016 showed that vitamin D supplementation was associated with lower mortality compared with placebo (OR, 0.70; 95% CI, 0.50–0.98, I2 = 0). This difference could be because we included a recent large-scale study of more than 1,000 patients by Ginde et al.25, in which the administration of high-dose vitamin D did not provide an additional benefit with respect to clinical outcomes, including mortality. Moreover, the data of clinical outcomes in the analysis by Putzu et al.31 had been reported by only 3 of 4 studies, which may limit the generalizability of their findings. Conversely, our study included more patients, more updated studies, and more subgroup analyses than previous studies31,32. In addition, all of our analyses showed consistent findings. Therefore, our findings provide stronger evidence regarding the effect of vitamin D supplementation on the outcomes of critically ill patients than previous studies.
However, this study had several limitations. Although this study focused on critically ill patients, their clinical characteristics are heterogeneous. Some were admitted to the ICU for traumatic injury, and some had ventilator-associated pneumonia. The criteria of vitamin D deficiency varied across studies, and the disease severity of the study patients also differed. Therefore, potential positive effects of vitamin D supplementation on the patient outcomes could not be found in this pooled analysis. In addition, only limited studies reported the vit D3 level after treatment and their level increased after treatment. Thus, we cannot assess the association between the level of vitamin D after treatment and the clinical outcome. Further studies are warranted to discover specific populations who can benefit from vitamin D supplementation35.
Conclusion
This meta-analysis suggested that the administration of vitamin D did not provide additional advantages over placebo for critically ill patients. However, additional studies are needed to confirm our findings.
References
Slominski, A. T. et al. In vivo evidence for a novel pathway of vitamin D3 metabolism initiated by P450scc and modified by CYP27B1. FASEB. J. 26, 3901–3915 (2012).
Slominski, A. T. et al. Detection of novel CYP11A1-derived secosteroids in the human epidermis and serum and pig adrenal gland. Sci. Rep. 5, 14875 (2015).
Slominski, A. T. et al. Novel activities of CYP11A1 and their potential physiological significance. J. Steroid Biochem. Mol. Biol. 151, 25–37 (2015).
Slominski, A. T. et al. Characterization of a new pathway that activates lumisterol in vivo to biologically active hydroxylumisterols. Sci. Rep. 7, 11434 (2017).
Alagacone, S., Verga, E., Verdolini, R. & Saifullah, S. M. The association between vitamin D deficiency and the risk of resistant hypertension. Clin. Exp. Hypertens. 42, 177–180 (2020).
Wang, C. Y. et al. Association between vitamin D and latent tuberculosis infection in the United States: NHANES, 2011–2012. Infect. Drug Resist. 12, 2251–2257 (2019).
Kim, Y. S., Hwang, J. H. & Song, M. R. The association between vitamin D deficiency and metabolic syndrome in Korean adolescents. J. Pediatr. Nurs. 38, e7–e11 (2018).
Herrick, K. A. et al. Vitamin D status in the United States, 2011–2014. Am. J. Clin. Nutr. 110, 150–157 (2019).
Cashman, K. D. et al. Vitamin D deficiency in Europe: Pandemic?. Am. J. Clin. Nutr. 103, 1033–1044 (2016).
Wei, J., Zhu, A. & Ji, J. S. A comparison study of vitamin D deficiency among older adults in China and the United States. Sci. Rep. 9, 19713 (2019).
Lee, J. H., Doo, S. R., Kim, D., Park, Y. K., Park, E. J. & Lee, J. M. Vitamin D deficiency and mortality among critically ill surgical patients in an urban Korean hospital. Int. J. Vitam. Nutr. Res. 1–8 (2020).
Higgins, D. M. et al. Relationship of vitamin D deficiency to clinical outcomes in critically ill patients. JPEN. J. Parenter. Enteral. Nutr. 36, 713–720 (2012).
Rech, M. A., Hunsaker, T. & Rodriguez, J. Deficiency in 25-hydroxyvitamin D and 30-day mortality in patients with severe sepsis and septic shock. Am. J. Crit. Care. 23, e72-79 (2014).
Anwar, E. et al. Burden and outcome of vitamin D deficiency among critically ill patients: A prospective study. Nutr. Clin. Pract. 32, 378–384 (2017).
Zapatero, A. et al. Severe vitamin D deficiency upon admission in critically ill patients is related to acute kidney injury and a poor prognosis. Med. Intensiva. 42, 216–224 (2018).
Kvaran, R. B., Sigurdsson, M. I., Skarphedinsdottir, S. J. & Sigurdsson, G. H. Severe vitamin D deficiency is common in critically ill patients at a high northern latitude. Acta Anaesthesiol. Scand. 60, 1289–1296 (2016).
Guan, J. et al. A prospective analysis of hypovitaminosis D and mortality in 400 patients in the neurocritical care setting. J. Neurosurg. 127, 1–7 (2017).
Moraes, R. B. et al. Vitamin D deficiency is independently associated with mortality among critically ill patients. Clinics (Sao Paulo). 70, 326–332 (2015).
Amrein, K. et al. Short-term effects of high-dose oral vitamin D3 in critically ill vitamin D deficient patients: A randomized, double-blind, placebo-controlled pilot study. Crit. Care. 15, R104 (2011).
Leaf, D. E., Raed, A., Donnino, M. W., Ginde, A. A. & Waikar, S. S. Randomized controlled trial of calcitriol in severe sepsis. Am. J. Respir. Crit. Care Med. 190, 533–541 (2014).
Amrein, K. et al. Effect of high-dose vitamin D3 on hospital length of stay in critically ill patients with vitamin D deficiency: the VITdAL-ICU randomized clinical trial. JAMA 312, 1520–1530 (2014).
Quraishi, S. A. et al. Effect of cholecalciferol supplementation on vitamin D status and cathelicidin levels in sepsis: A randomized, placebo-controlled trial. Crit. Care Med. 43, 928–937 (2015).
Han, J. E. et al. High dose vitamin D administration in ventilated Intensive Care Unit patients: A pilot double blind randomized controlled trial. J. Clin. Transl. Endocrinol. 4, 59–65 (2016).
Miroliaee, A. E. et al. Effect of vitamin D supplementation on procalcitonin as prognostic biomarker in patients with ventilator associated pneumonia complicated with vitamin D deficiency. Iran. J. Pharm. Res. 16, 1254–1263 (2017).
Ginde, A. A. et al. Early high-dose vitamin D3 for critically ill, vitamin D-deficient patients. N. Engl. J. Med. 381, 2529–2540 (2019).
Hasanloei, M. A. V. et al. Effect of oral versus intramuscular vitamin D replacement on oxidative stress and outcomes in traumatic mechanical ventilated patients admitted to intensive care unit. Nutr. Clin. Pract. 35, 545–558 (2020).
Miri, M., Kouchek, M., Rahat, Dahmardeh. A. & Sistanizad, M. Effect of high-dose vitamin D on duration of mechanical ventilation in ICU patients. Iran J. Pharm. Res. 18, 1067–1072 (2019).
Karsy, M., Guan, J., Eli, I., Brock, A. A., Menacho, S. T. & Park, M. S. The effect of supplementation of vitamin D in neurocritical care patients: RandomizEd Clinical TrIal oF hYpovitaminosis D (RECTIFY). J. Neurosurg. 1–10 (2019).
Leclair, T. R. et al. Vitamin D supplementation in mechanically ventilated patients in the medical intensive care unit. JPEN. J. Parenter. Enteral. Nutr. 43, 1037–1043 (2019).
Viglianti, E. M., Zajic, P., Iwashyna, T. J. & Amrein, K. Neither vitamin D levels nor supplementation are associated with the development of persistent critical illness: A retrospective cohort analysis. Crit. Care Resusc. 21, 39–44 (2019).
Putzu, A. et al. Vitamin D and outcomes in adult critically ill patients. A systematic review and meta-analysis of randomized trials. J Crit Care. 38, 109–114 (2017).
Langlois, P. L., Szwec, C., D’Aragon, F., Heyland, D. K. & Manzanares, W. Vitamin D supplementation in the critically ill: A systematic review and meta-analysis. Clin. Nutr. 37, 1238–1246 (2018).
Higgins, J. P. et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 343, d5928 (2011).
Kearns, M. D., Alvarez, J. A. & Tangpricha, V. Large, single-dose, oral vitamin D supplementation in adult populations: A systematic review. Endocr. Pract. 20, 341–351 (2014).
Martucci, G. et al. Trying to identify who may benefit most from future vitamin D intervention trials: A post hoc analysis from the VITDAL-ICU study excluding the early deaths. Crit. Care 23, 200 (2019).
Author information
Authors and Affiliations
Contributions
L.S.H. and L.C.C. wrote the main manuscript. C.S.P. and L.L.C. data collection and anlaysis. H.S.H. and L.W.T. critical review and supervision.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lan, SH., Lai, CC., Chang, SP. et al. Vitamin D supplementation and the outcomes of critically ill adult patients: a systematic review and meta-analysis of randomized controlled trials. Sci Rep 10, 14261 (2020). https://doi.org/10.1038/s41598-020-71271-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-71271-9
- Springer Nature Limited
This article is cited by
-
Update on vitamin D role in severe infections and sepsis
Journal of Anesthesia, Analgesia and Critical Care (2024)
-
Abnormal blood 25-hydroxyvitamin D in critically ill patients: prevalence, predictors, and its association with in-hospital mortality
European Journal of Medical Research (2022)
-
Administration of vitamin D and its metabolites in critically ill adult patients: an updated systematic review with meta-analysis of randomized controlled trials
Critical Care (2022)
-
Vitamin therapy in sepsis
Pediatric Research (2022)