Abstract
This study aimed to assess the benefit of postoperative adjuvant chemotherapy in stage II–III colorectal signet ring cell carcinoma (SRCC). Qualified postoperative patients were extracted from Surveillance, Epidemiology, and End Results (SEER) database from 2004 until 2015. We collected 1675 patients in the research, and 936 patients were subjected to adjuvant chemotherapy group. The proportions of married status, male, rectal cancer, grade III/IV, AJCC stage III and radiotherapy were higher; While, the rates of white race, ≥ 65 years old and located in cecum–transverse colon were lower in patients of chemotherapy group compared to no chemotherapy group (all P < 0.05). K-M plots revealed significantly better OS of adjuvant chemotherapy group than no chemotherapy group (P < 0.001). Meanwhile, there was no significantly different in CSS between the two groups (P = 0.93). However, after adjusting for confounding factors by multivariable Cox regression analysis, receipt of postoperative chemotherapy was still associated with better CSS and OS (CSS: hazard ratio [HR] = 0.719, 95% CI 0.612–0.844, P < 0.001) ; (OS: HR = 0.618, 95% CI 0.537–0.713, P < 0.001). Patients with stage II/III colorectal SRCC could receive survival benefit from postoperative adjuvant chemotherapy.
Similar content being viewed by others
Introduction
Colorectal cancer (CRC) ranks the third of cancer-associated death, causing great health burden globally1. The diverse pathological types of CRC have been uncovered to be having correlation with various clinical parameters and patient survival, with adenocarcinoma being most prevalent type2,3. Signet-ring cell carcinoma (SRCC) is a relatively rare histological subtype, consisting of 0.1–2.6% of CRC patients4,5, defined as the abundant presence of intracellular mucin in over 50% cells according to WHO6,7.
SRCC is considered as a distinct pathological subtype in CRCs. A series of differences among colorectal SRCC, mucinous adenocarcinoma (MC) and non-mucinous adenocarcinoma (NMC) have been consistently reported. To be specific, SRCC has been reported to be associated with younger age at diagnosis, more advanced stage and poorer clinical outcomes than MC and NMC7,8,9. In addition, massive lymphatic involvement, higher frequency of multiple metastatic organs and greater risks of peritoneal metastases are more commonly seen in SRCC9.
Because SRCC is relatively rare, there is a lack of consensus on therapeutic guidelines due to the difficulty in conducting large randomized controlled trials5. At present, surgical intervention is still the optimal option for colorectal SRCC patients. Moreover, the combined application of other therapeutics has been increasing, especially chemotherapy10. Hugen et al. have assessed the efficacy of adjuvant chemotherapy in colorectal SRCC, who further indicated the benefit of adjuvant chemotherapy in stage III SRCC patients11. Meanwhile, by analyzing the distinct metastatic patterns of colorectal SRCC toward different sites, Tao et al. have demonstrated better survival of received chemotherapy in metastatic colorectal SRCC patients12. However, there were still some studies showed that colorectal SRCC responded poorly to chemotherapy13,14. Thus, clear elucidation of the efficacy of postoperative chemotherapy in colorectal SRCC patients is of great significance.
The SEER database, the most authoritative and largest cancer dataset in North America15, records tumor data by covering almost 30% of population in the USA from diverse geographic regions, which could readily represent the population diversity16. Therefore, SEER is widely acknowledged as a valuable database for investigation into rare tumors17,18,19,20. Herein, in the present study, we collected eligible non-metastatic colorectal SRCC patients from SEER database to investigate the influence of adjuvant chemotherapy.
Materials and methods
Study population
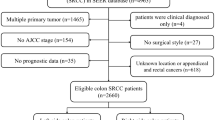
SEER*Stat v8.3.6 tool (released on August 8th, 2019) was adopted for selecting qualified subjects. Colorectal SRCC patients who were diagnosed from January 1, 2004 to December 31, 2015 were selected from the Incidence-SEER 18 Registries Custom Data (with additional treatment fields). Eligible patients were collected accordingly: (1) primary colorectal SRCC patients; (2) the diagnosis of SRCC was based on (ICD-O-3; coded as 8490/3). Patients were eliminated if they had: (1) more than one primary malignancies; (2) reported diagnosis source from autopsy or death certificate or no pathological diagnosis; (3) no AJCC stage; (4) no surgery; (5) AJCC stage I/IV; (6) no prognostic information. The remaining qualified populations were included, followed by assignment of patients into adjuvant chemotherapy group and no chemotherapy group according to whether they had chemotherapy or not.
Covariates and endpoint
The following clinicopathological parameters were analyzed: year of diagnosis (2004–2007, 2008–2011, 2012–2015); insured status (uninsured/unknown, any medicaid/insured); age (< 65, ≥ 65); marital status (unmarried, married); gender (female, male); race (black, white or others); primary site(cecum–transverse colon, descending colon–sigmoid, multiple, rectum and unknown); grade (grade I/II, grade III/IV, unknown); tumor size (≤ 5 cm, > 5 cm, unknown); AJCC stage ( stage II, stage III); lymph node dissection (none or biopsy, 1 to 3 regional lymph nodes removed, ≥ 4 regional lymph nodes removed, unknown); chemotherapy (no/unknown, yes) and radiotherapy (no/unknown, yes). The widowed or single (never married or having a domestic partner) or divorced or separated patients were classified as unmarried. The primary tumor site was classified as cecum–transverse colon (including the cecum, appendix, ascending colon, hepatic flexure and the transverse colon), descending colon–sigmoid (including the splenic flexure and descending and sigmoid colons), multiple, rectum and unknown. In addition, the staging of cancer is based on the 6th edition of AJCC stage system, which adapted to patients in the SEER database with a diagnosis time of 2004–2015.
The endpoint of this study was cancer‐specific survival (CSS) and overall survival (OS). CSS was defined as the period from diagnosis to death attributed to colorectal SRCC. OS was defined as the period from diagnosis to death from any cause.
Statistical analysis
Categorical data were compared by Chi‐square test between chemotherapy and no chemotherapy groups. Kaplan–Meier (K-M) method was adopted for univariate analysis to evaluate whether CSS and OS were different between two groups (log-rank test). Variables with P values lower than 0.1 in univariate analysis were incorporated into the multivariate Cox proportional hazard model. SPSS software (version 19.0) (SPSS Inc., Chicago, USA) was employed for statistical analysis, and Graph Pad Prism 5 was utilized for generating survival curve. A two-sided P < 0.05 indicated statistical significance.
Ethics statement
In order to obtain relevant data from the database, we signed the SEER Research Data Agreement (No.19817-Nov2018) and further searched for data according to the approved guidelines. The extracted data were publicly accessible and de-identified, and the data analysis was considered as non-human subjects by Office for Human Research Protection, therefore, no approval was demanded from institutional review board.
Results
Patient characteristics
In total, 1675 eligible patients were included in this research, and 936 patients received adjuvant chemotherapy while 739 patients did not. The process of patient selection was displayed in Fig. 1. The demographics, tumor characteristics and therapeutic features of both groups were summarized in Table 1. Except for year at diagnosis, insured status and lymph node dissection, multiple variables were significantly different between the two groups (all P < 0.05). There were more patients with married (57.59% vs. 48.44%), male (54.81% vs. 47.36%), located in rectum (29.38% vs. 9.47%), grade III/IV (84.08% vs. 82.41%), AJCC stage III (83.01% vs. 59.27%); less often white race (78.31% vs. 85.66%), ≥ 65 years old (36.65% vs. 73.88%) and located in cecum–transverse colon (52.56% vs. 76.45%) in chemotherapy group compared with no chemotherapy group. Furthermore, more subjects received radiotherapy in adjuvant chemotherapy group (28.31% vs. 2.71%).
Survival analysis of all patients
The median survival time of all included patients was 25.0 months (0–155 months). The 3-, 5- and 10-year CSS rate was 53.47%, 46.14% and 39.53%, respectively. In addition, the 3-, 5- and 10-year OS rate was 47.93%, 39.06% and 26.75%, respectively. K-M plots revealed significantly better OS of adjuvant chemotherapy group than no chemotherapy group (P < 0.001). Meanwhile, there was no significantly different in CSS between the two groups (P = 0.93).The survival curves of CSS as well as OS were displayed in Fig. 2.
In univariate analysis of CSS and OS, age, marital status, race, primary site, grade, tumor size, AJCC stage, lymph node dissection, chemotherapy and radiotherapy were risk factors for survival (P < 0.05), which were later incorporated into the multivariate Cox analysis. As a results, adjuvant chemotherapy was a significantly protective factor for survival (CSS: hazard ratio [HR] = 0.719, 95% CI 0.612–0.844, P < 0.001); (OS: HR = 0.618, 95% CI 0.537–0.713, P < 0.001). The concrete results of univariate analysis and multivariate analysis were listed in Tables 2 and 3 respectively.
Discussion
The multidisciplinary management of colorectal SRCC is required to select the optimal therapeutic strategies based on both natural history of tumor and tumor-associated prognostic factors. According to the present international guidelines, no specific therapy is recommended for SRCC histology in clinical practice21,22. Surgical intervention is vitally involved in treating localized tumors5. However, studies have shown the lower rate of curative resection as well as poorer outcome of colorectal SRCC14. Therefore, the application of other therapeutic approaches has been increasing, including chemotherapy10. However, relevant researches have shown that colorectal SRCC is relatively insensitive to the commonly applied chemotherapeutics, such as irinotecan, oxaliplatin as well as 5-fluorouracil13,14,23. Cabibi et al. have demonstrated that such drug resistance may be due to a low proliferative activity of tumor cells, as the analysis of 15 SRCC samples showed very low levels of Ki-67 expression (a proliferation marker) and weak positivity for thymidylate synthase (key enzyme for DNA synthesis pathways targeted by 5-FU)13,23,24.
Conversely, in several large sample-based retrospective studies, we have found that chemotherapy provides significant survival benefits for certain colorectal SRCC populations. Tao Shi et al. found that chemotherapy was related to better survival in metastatic colorectal SRCC12. Additionally, the clinical significance of chemotherapy on colorectal SRCC was evaluated in a population-based study involving 1972 patients from 1989 to 2010. The study found that patients with stage III colon SRCC receiving adjuvant chemotherapy had better survival compared to those without chemotherapy (5-year survival rate: 52% vs. 30%)11. In the present study, we analyzed 1675 stage II/III colorectal SRCC patients and found that chemotherapy could significantly prolong survival in CSS and OS. This is similar to the previous study. Unfortunately, we were not able to analyze the benefit of adjuvant chemotherapy in high-risk stage II patients specially, due to the low number of patients who received chemotherapy in this group and because the motivation for administration of chemotherapy was not registered.
As a large population based dataset, SEER could be used for cross-sectional assessment in a large number of tumor patients and simultaneously provide long-term follow-up data without inherent institutional bias. Nevertheless, several limitations are unavoidable in our research. First of all, as a retrospective study, the intrinsic selection bias exists in this study18,20. Furthermore, the effects of other adjuvant therapy are not assessed, and the specific type of chemotherapeutic regimen is unclear (single agent or doublet). Thus, we are unable to precisely elucidate whether differences exist in terms of adjuvant therapy throughout the study. Thirdly, therapeutic responses as well as recurrence rates are inaccessible from SEER database. Finally, several important prognostic information are unavailable from SEER database, such as: specific number of lymph node dissection ,extramural vascular invasion or obstruction/occlusion status, and Microsatellite stability/Microsatellite instability (MSS/MSI) status .Although it is better to obtain more details, we believed that the present available data from SEER database could fit our research objectives very well. The above concerns should be investigated in future studies.
Conclusion
In conclusion, our results have shown that stage II/III colorectal SRCC can gain survival benefit from postoperative adjuvant chemotherapy. This is a large population-based study to discuss adjuvant chemotherapy for patients with localized colorectal SRCC, and our present findings might be of significance for disease management and future prospective researches.
References
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 68, 394–424. https://doi.org/10.3322/caac.21492 (2018).
Fukui, Y. Mechanisms behind signet ring cell carcinoma formation. Biochem. Biophys. Res. Commun. 450, 1231–1233. https://doi.org/10.1016/j.bbrc.2014.07.025 (2014).
Hartman, D. J. et al. Signet ring cell colorectal carcinoma: A distinct subset of mucin-poor microsatellite-stable signet ring cell carcinoma associated with dismal prognosis. Am. J. Surg. Pathol. 37, 969–977. https://doi.org/10.1097/PAS.0b013e3182851e2b (2013).
Tung, S. Y., Wu, C. S. & Chen, P. C. Primary signet ring cell carcinoma of colorectum: An age- and sex-matched controlled study. Am. J. Gastroenterol. 91, 2195–2199 (1996).
Arifi, S., Elmesbahi, O. & Amarti Riffi, A. Primary signet ring cell carcinoma of the colon and rectum. Bull. Cancer 102, 880–888. https://doi.org/10.1016/j.bulcan.2015.07.005 (2015).
Zhang, M., Zhu, G., Zhang, H., Gao, H. & Xue, Y. Clinicopathologic features of gastric carcinoma with signet ring cell histology. J. Gastrointest. Surg. 14, 601–606. https://doi.org/10.1007/s11605-009-1127-9 (2010).
Vallam, K. C. et al. Adenocarcinoma of the rectum—a composite of three different subtypes with varying outcomes?. Clin. Colorectal Cancer 15, e47-52. https://doi.org/10.1016/j.clcc.2015.12.004 (2016).
Sung, C. O. et al. Clinical significance of signet-ring cells in colorectal mucinous adenocarcinoma. Mod. Pathol. 21, 1533–1541. https://doi.org/10.1038/modpathol.2008.170 (2008).
Hugen, N., van de Velde, C. J., de Wilt, J. H. & Nagtegaal, I. D. Metastatic pattern in colorectal cancer is strongly influenced by histological subtype. Ann. Oncol. 25, 651–657. https://doi.org/10.1093/annonc/mdt591 (2014).
Fakih, M. G. Metastatic colorectal cancer: Current state and future directions. J. Clin. Oncol. 33, 1809–1824. https://doi.org/10.1200/jco.2014.59.7633 (2015).
Hugen, N. et al. Colorectal signet-ring cell carcinoma: Benefit from adjuvant chemotherapy but a poor prognostic factor. Int. J. Cancer 136, 333–339. https://doi.org/10.1002/ijc.28981 (2015).
Shi, T. et al. Chemotherapy is associated with increased survival from colorectal signet ring cell carcinoma with distant metastasis: A surveillance. Epidemiol. End Results Database Anal. 8, 1930–1940. https://doi.org/10.1002/cam4.2054 (2019).
Pande, R. et al. Significance of signet-ring cells in patients with colorectal cancer. Dis. Colon Rectum 51, 50–55. https://doi.org/10.1007/s10350-007-9073-7 (2008).
Lee, W. S. et al. Treatment outcomes in patients with signet ring cell carcinoma of the colorectum. Am. J. Surg. 194, 294–298. https://doi.org/10.1016/j.amjsurg.2006.12.041 (2007).
Yu, J. B., Gross, C. P., Wilson, L. D. & Smith, B. D. NCI SEER public-use data: Applications and limitations in oncology research. Oncology 23, 288–295 (2009).
Cronin, K. A., Ries, L. A. & Edwards, B. K. The surveillance, epidemiology, and end results (SEER) Program of the National Cancer Institute. Cancer 120(Suppl 23), 3755–3757. https://doi.org/10.1002/cncr.29049 (2014).
Cahill, K. S. & Claus, E. B. Treatment and survival of patients with nonmalignant intracranial meningioma: Results from the Surveillance, Epidemiology, and End Results Program of the National Cancer Institute. Clinical article. J. Neurosurg. 115, 259–267. https://doi.org/10.3171/2011.3.jns101748 (2011).
Dudley, R. W. et al. Pediatric choroid plexus tumors: Epidemiology, treatments, and outcome analysis on 202 children from the SEER database. J. Neurooncol. 121, 201–207. https://doi.org/10.1007/s11060-014-1628-6 (2015).
Dudley, R. W. et al. Pediatric low-grade ganglioglioma: Epidemiology, treatments, and outcome analysis on 348 children from the surveillance, epidemiology, and end results database. Neurosurgery 76, 313–319. https://doi.org/10.1227/neu.0000000000000619 (2015) ((discussion 319; quiz 319–320)).
Hankinson, T. C. et al. Short-term mortality following surgical procedures for the diagnosis of pediatric brain tumors: Outcome analysis in 5533 children from SEER, 2004–2011. J. Neurosurg. Pediatr. 17, 289–297. https://doi.org/10.3171/2015.7.peds15224 (2016).
Labianca, R. et al. Early colon cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 24(Suppl 6), vi64–vi72. https://doi.org/10.1093/annonc/mdt354 (2013).
Van Cutsem, E., Cervantes, A., Nordlinger, B. & Arnold, D. Metastatic colorectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncology 25(Suppl 3), iii1–iii9. https://doi.org/10.1093/annonc/mdu260 (2014).
Cabibi, D. et al. Clinical relevance of thymidylate synthase expression in the signet ring cell histotype component of colorectal carcinoma. Eur. J. Cancer 40, 2845–2850. https://doi.org/10.1016/j.ejca.2004.07.034 (2004).
Cabibi, D. et al. Differing expression of metalloprotease and of adhesion molecules in signet-ring cell and intestinal colorectal carcinoma. Anticancer Res. 29, 4417–4422 (2009).
Acknowledgements
We thank the staff members of the National Cancer Institute and their colleagues across the United States and at Information Management Services, Inc., who have been involved with the Surveillance, Epidemiology, and End Results (SEER) Program.
Funding
This work is supported by a grant (to Zhi Wen Li) from Jilin province finance department project (2018 sczwszx-041).
Author information
Authors and Affiliations
Contributions
Z.Z. and Z.L. conceived the study. N.Y. and S.P. searched the database and literature. D.W. discussed and analyzed the data. Z.Z. wrote the manuscript. D.W. revised the manuscript. All authors approved the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, Z., Yan, N., Pan, S. et al. The value of adjuvant chemotherapy in stage II/III colorectal signet ring cell carcinoma. Sci Rep 10, 14126 (2020). https://doi.org/10.1038/s41598-020-70985-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-70985-0
- Springer Nature Limited
This article is cited by
-
Clinicopathologic and prognostic factors of patients with T3/T4 colorectal signet ring cell carcinoma: a population-based study
Journal of Cancer Research and Clinical Oncology (2023)
-
Comparison of survival outcomes and survival prediction in patients with primary colorectal MANEC and primary colorectal SRCC: a population-based propensity-score matching study
Journal of Cancer Research and Clinical Oncology (2023)
-
Molecular profiling of signet-ring-cell carcinoma (SRCC) from the stomach and colon reveals potential new therapeutic targets
Oncogene (2022)