Abstract
Cardiac tissue remodeling caused by hemodynamic overload is a major clinical outcome of heart failure. Uridine-responsive purinergic P2Y6 receptor (P2Y6R) contributes to the progression of cardiovascular remodeling in rodents, but it is not known whether inhibition of P2Y6R prevents or promotes heart failure. We demonstrate that inhibition of P2Y6R promotes pressure overload-induced sudden death and heart failure in mice. In neonatal cardiomyocytes, knockdown of P2Y6R significantly attenuated hypertrophic growth and cell death caused by hypotonic stimulation, indicating the involvement of P2Y6R in mechanical stress-induced myocardial dysfunction. Unexpectedly, compared with wild-type mice, deletion of P2Y6R promoted pressure overload-induced sudden death, as well as cardiac remodeling and dysfunction. Mice with cardiomyocyte-specific overexpression of P2Y6R also exhibited cardiac dysfunction and severe fibrosis. In contrast, P2Y6R deletion had little impact on oxidative stress-mediated cardiac dysfunction induced by doxorubicin treatment. These findings provide overwhelming evidence that systemic inhibition of P2Y6R exacerbates pressure overload-induced heart failure in mice, although P2Y6R in cardiomyocytes contributes to the progression of cardiac fibrosis.
Similar content being viewed by others
Introduction
Cardiac remodeling is characterized by structural and morphological changes of the heart, including hypertrophy and fibrosis, and is a major clinical outcome of heart failure after cardiac injury1,2. Structural remodeling is thought to be a plasticity process of the heart to overcome hemodynamic overload, but cardiac resistance (i.e., robustness) to mechanical stress may be reduced by additional environmental factors, such as physical and chemical stresses3.
Purinergic receptors are activated by extracellular nucleotides and play important roles in cardiovascular physiology and pathophysiology4. Purinergic receptors are divided into two main groups, P1 and P2. P1 receptors are activated by adenosine, and mediate cardiodepressant and cardioprotective effects4. P2 receptors are subdivided into P2X and P2Y subfamilies, which consist of ligand-gated ion channels and G protein coupled receptors (GPCRs), respectively4. The P2Y family has eight subtypes (P2Y1, P2Y2, P2Y4, P2Y6, P2Y11, P2Y12, P2Y13 and P2Y14) that differ in their coupling G protein and ligand selectivity5. Purinergic signaling must be important for cardiovascular homeostasis because many purinergic receptors are expressed in human and mouse hearts6,7. The nucleotide, uridine triphosphate (UTP), induces a profibrotic response via P2Y2R8, while adenosine triphosphate (ATP) induces contraction9 and negatively regulates hypertrophic growth of cardiomyocytes10,11.
We have previously focused on the role of the uridine-responsive P2Y receptors, P2Y2R and P2Y6R, because they are upregulated in the mouse heart when exposed to pressure overload7. We have reported that treatment of rat cardiac fibroblasts with ATP downregulates angiotensin type 1 receptor (AT1R) through induction of inducible nitric oxide (NO) synthase12. P2Y2R also mediates ATP-induced suppression of cardiomyocyte hypertrophy10 and nutritional deficiency-induced cardiomyocyte atrophy11. P2Y6R, activated mainly by uracil diphosphate (UDP), changes the contractility of mouse cardiomyocytes13. Contractility of the aorta in response to UDP is different in P2Y6R-deficient mice compared with wild-type mice14. Therefore, P2Y6R may have an important role in cardiovascular contractility. In mouse aorta, P2Y6R levels are increased in an age-dependent manner and P2Y6R contributes to hypertensive vascular remodeling via its heterodimerization with AT1R15. In addition, P2Y6R has a deleterious role in atherosclerosis, being abundant in sclerotic lesions and promoting inflammation16,17. P2Y6R is also upregulated in pressure overloaded mouse hearts, and pharmacological inhibition of P2Y6R by MRS2578 attenuates pressure overload-induced cardiac fibrosis7. These findings indicate that P2Y6R in cardiovascular systems is a promising therapeutic target for cardiovascular dysfunction. However, it is not clear whether pressure overload-induced heart failure can be attenuated in P2Y6R-deficient mice. Indeed, deletion of P2Y6R in mice enhances isoproterenol-induced pathological cardiac hypertrophy18.
Several GPCRs, especially Gq protein-coupled receptors, are responsive to mechanical stress19,20. For example, AT1R, which is activated by angiotensin II, is directly activated by mechanical stretch without angiotensin II stimulation21. One of the major physiological roles of P2Y6R is to act as a mechano-activating GPCR in cardiomyocytes through ligand-dependent and -independent (AT1R-P2Y6R heterodimer-dependent) pathways7,15. However, whether these two mechano-activation mechanisms of P2Y6R have the same role is unknown. Therefore, we tested whether deletion of P2Y6R attenuates mechanical stress-induced cardiomyocyte hypertrophy in vitro. We demonstrate that knockdown of P2Y6R suppresses hypotonic stress-induced cell damage and hypertrophy in neonatal rat cardiomyocytes (NRCMs). However, P2Y6R hetero- and homo-deficient [P2Y6R(+/−) and P2Y6R(−/−)] mice show vulnerability to pressure overload induced by transverse aortic constriction (TAC). In addition, cardiomyocyte-specific P2Y6R-expressing mice also show elevated pressure overload-induced cardiac fibrosis and contractile dysfunction. P2Y6R deficiency did not affect doxorubicin (DOX)-induced heart failure; therefore, systemic deletion of P2Y6R specifically augments cardiac vulnerability to mechanical stress.
Results
Knockdown of P2Y6R suppresses cell damage and hypertrophy induced by hypotonic stress in vitro
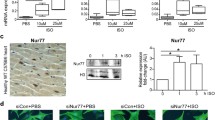
We previously reported that selective antagonist inhibition of P2Y6R suppressed cardiac remodeling and dysfunction after pressure overload7. However, effects of P2Y6R deficiency on pressure overload-induced cardiac remodeling have not been investigated. We therefore knocked down P2Y6R in NRCMs using two siRNAs (siP2Y6R #1 and #2), and examined cell damage and size after hypotonic stimulation, which is a model of in vitro pressure overload. Cytotoxicity was analyzed by measuring the activity of lactate dehydrogenase released by damaged cells, and the size of α-actinin-positive NRCMs was also determined. Cell damage and hypertrophy induced by hypotonic stress were significantly suppressed in P2Y6R knockdown NRCMs (Fig. 1A–C). These data indicate that P2Y6R deficiency can be protective against pressure overload-induced cardiac remodeling and dysfunction. These results are consistent with those of a previous study, which showed that pharmacological inhibition of P2Y6R improves cardiac dysfunction after pressure overload7.
Knockdown of P2Y6R in NRCMs suppresses hypotonic stress-induced cell damage and hypertrophy. (A) Activity of lactate dehydrogenase (LDH) released from damaged cells. (n = 3 independent experiments). (B) NRCMs transfected with negative control (siNC) or P2Y6R (siP2Y6R #1 and #2) siRNA were immunostained with an anti-actinin antibody. (n = 3 independent experiments). Scale bars, 50 µm. (C) Anti-actinin-immunostained NRCM surface area. (n = 3 independent experiments). Data are shown as means ± SEM. **P < 0.01, one-way ANOVA.
Mice lacking P2Y6R died suddenly after pressure overload from exacerbated heart failure with enhanced cardiac hypertrophy
To confirm the effect of P2Y6R deletion in vivo, wild-type [P2Y6R(+/+)], P2Y6R hetero-deficient [P2Y6R(+/−)] and P2Y6R homo-deficient [P2Y6R(−/−)] mice were subjected to pressure overload by transverse aortic constriction (TAC) surgery. qPCR confirmed constitutive and systemic P2Y6R deletion (Fig. 2A). As previously reported, deletion of P2Y6R in mice does not alter fertility or growth and does not induce any organ abnormalities14. Indeed, we observed no phenotypic differences between P2Y6R(+/+), P2Y6R(+/−), and P2Y6R(−/−) mice, including body weight, heart weight, and cardiac function, under homeostatic conditions. Surprisingly and in contrast to the expectation from the in vitro results, there was a rapid and high rate of death among P2Y6R(+/−) and P2Y6R(−/−) mice after TAC (Fig. 2B). The left ventricular contractile function was measured in surviving mice by echocardiography at 5 weeks after TAC (Table 1). Ejection fraction (EF) and fractional shortening (FS) was reduced in P2Y6R(+/−) and P2Y6R(−/−) TAC mice compared with that in P2Y6R(+/+) TAC mice, whereas P2Y6R deficiency did not affect basal cardiac contractility (Fig. 2C,D). Cardiac hypertrophy and fibrosis were evaluated by heart weight/body weight ratio and picrosirius red staining, respectively. Cardiac hypertrophy (Fig. 3A) but not fibrosis (Fig. 3B) was enhanced in P2Y6R(+/−) TAC and P2Y6R(−/−) TAC mice compared with P2Y6R(+/+) TAC mice. Consistent with echocardiography data (Fig. 2C,D), TAC-induced hypertrophy of P2Y6R(+/−) mice was the same extent with that of P2Y6R(−/−) mice (Fig. 3A), suggesting that heterozygous deletion of P2Y6R is critical and sufficient for TAC-induced cardiac dysfunction and remodeling. Fibroblast differentiation into myofibroblasts stimulates excessive ECM deposition, leading to cardiac fibrosis22. We therefore investigated whether P2Y6R deficiency affected TGF-β1-induced differentiation into myofibroblasts. TGF-β1 treatment of cardiac fibroblasts increased the expression of α-smooth muscle actin (α-SMA), a marker of differentiated myofibroblasts, and P2Y6R knockdown did not affect the expression of α-SMA after TGF-β1 treatment (Fig. 3C). Consistent with the in vivo result (Fig. 3B), P2Y6R deficiency did not affect the differentiation of fibroblasts into myofibroblasts. These data indicate that P2Y6R knockdown in vivo confers vulnerability to pressure overload, which is opposite to the in vitro results and to the findings of a previous inhibitor study7.
P2Y6R deficiency promotes pressure overload-induced heart failure. (A) The expression of P2Y6R in P2Y6R(+/+) (n = 6) and P2Y6R(−/−) (n = 4) mouse hearts was assessed by qPCR. Data are shown as means ± SEM. **P < 0.01, t-test. (B) Survival rate of P2Y6R(+/+), P2Y6R(+/−) and P2Y6R(−/−) mice after TAC. (n = 10 to 25 mice per treatment). *P < 0.05 compared to P2Y6R(+/+) TAC, Log-rank test. (C,D) Contractile function in P2Y6R(+/+), P2Y6R(+/−) and P2Y6R(−/−) mice, 5 weeks after TAC. Ejection fraction (C) and fractional shortening (D) (n = 7 to 10 mice per treatment). Data are shown as means ± SEM. *P < 0.05, **P < 0.01, one-way ANOVA.
P2Y6R deficiency promotes hypertrophy of cardiomyocytes but not fibrosis after TAC. (A) Heart weight/body weight (HW/BW) ratio of P2Y6R(+/+), P2Y6R(+/−) and P2Y6R(−/−) mice, 6 weeks after TAC. (n = 7 to 14 mice per treatment). (B) Cardiac collagen volume fraction of P2Y6R(+/+) and P2Y6R(−/−) mice 6 weeks after TAC. The frozen section was stained with Picrosirius red. (n = 4 to 5 mice per treatment). Scale bars, 50 µm. C. Neonatal rat cardiac fibroblasts were transfected with negative control (siNC) or P2Y6R (siP2Y6R #1 and #2) siRNA and immunostained with an anti-α-SMA antibody. (n = 3 independent experiments). Scale bars, 50 µm. Data are shown as means ± SEM. **P < 0.01, one-way ANOVA.
Cardiomyocyte-specific overexpression of P2Y6R in mice also promoted cardiac dysfunction after pressure overload
From previously published findings and the data shown in Figs. 2 and 3, we hypothesized that an adequate level of P2Y6R expression is important for an appropriate response against pressure overload. Therefore, to assess the effects of P2Y6R overexpression in pressure overloaded-heart, we generated mice with cardiomyocyte-specific P2Y6R overexpression using an adeno-associated viral (AAV) vector. AcGFP and FLAG-P2Y6R were successfully expressed in cardiac tissue by the AAV vector (Fig. 4A,B). The survival rate of mice with cardiomyocyte-specific overexpression of FLAG-P2Y6R (FLAG-P2Y6R mice) after TAC was not significantly different compared to that of AcGFP mice (Fig. 4C). This indicates that cardiomyocyte-specific overexpression of P2Y6R does not induce sudden death, which is different from P2Y6R knockdown (Fig. 2B). Cardiomyocyte-specific overexpression of P2Y6R did not affect basal contractility in the heart, whereas EF and FS were significantly decreased in FLAG-P2Y6R mice compared with control (AcGFP) mice after TAC (Fig. 4D,E and Table 2). In contrast to P2Y6R(−/−) mice, morphological analysis showed more cardiac fibrosis but not hypertrophy in FLAG-P2Y6R mice (Fig. 5A–C). These data indicate that a suitable level of P2Y6R in cardiomyocytes may be important for mechanical sensation.
Cardiomyocyte-specific overexpression of P2Y6R promotes pressure overload-induced heart failure. (A,B) Overexpression of FLAG-P2Y6R proteins in cardiac tissue 13 weeks after adeno-associated viral (AAV) vector infection. Expression of FLAG-P2Y6R determined by western blotting of anti-FLAG immunoprecipitate from whole heart (A) and immunostaining of cardiac sections with anti-GFP or FLAG antibodies (B). (n = 3 mice per treatment). Scale bar, 100 µm. Asterisks show heavy and light chains of IgG. (C) Survival rate of control and P2Y6R-overexpression mice after transverse aortic constriction (TAC). (n = 5 to 9 mice per treatment). (D,E) Contractile function of control and P2Y6R-overexpression mice after TAC. Ejection fraction (D) and fractional shortening (E) (n = 5 to 9 mice per treatment). Data are shown as means ± SEM. #P < 0.05 versus AcGFP sham, *P < 0.05, **P < 0.01 versus AcGFP TAC, two-way ANOVA.
Cardiomyocyte-specific overexpression of P2Y6R promotes fibrosis but not hypertrophy of cardiomyocytes after transverse aortic constriction (TAC). (A) Heart weight/body weight (HW/BW) ratio of control and P2Y6R-overexpression mice after TAC. (n = 5 to 9 mice per treatment). (B) Cardiomyocyte cross sectional area of control and P2Y6R-overexpression mice 5 weeks after TAC was measured using hematoxylin–eosin staining. (n = 5 to 8 mice per treatment). Scale bar, 50 µm. (C) Cardiac collagen volume fraction of control and P2Y6R-overexpression mice 5 weeks after TAC. The paraffin section was stained with Picrosirius red. (n = 5 to 8 mice per treatment). Scale bar, 50 µm. Data are shown as means ± SEM. *P < 0.05, **P < 0.01, one-way ANOVA.
Non-mechano-induced heart failure was not affected by P2Y6R deficiency
Finally, we checked whether P2Y6R expression affects non-mechano-induced heart failure using a DOX-induced heart failure model23. DOX was administered to P2Y6R(+/+) and P2Y6R(−/−) mice and left ventricular contractile function was assessed by echocardiography. Left ventricular contractile function was similar among P2Y6R(+/+) and P2Y6R(−/−) mice after DOX infusion (Fig. 6A,B). Cardiomyocyte death is one of the major causes of DOX-induced cardiotoxicity, leading cardiac dysfunction24. P2Y6R knockdown did not affect DOX-induced death of NRCMs (Fig. 6C), indicating that P2Y6R deficiency does not affect cardiac remodeling induced by DOX. These data support P2Y6R expression affecting only mechano-induced-cardiac remodeling.
P2Y6R deficiency fails to exacerbate doxorubicin-induced heart failure. (A,B) Contractile function of P2Y6R(+/+) and P2Y6R(−/−) mice, 3 weeks after DOX injection. Ejection fraction (A) and fractional shortening (B) were measured by echocardiography. (n = 6 mice per treatment). (C) Cell viability of NRCMs was assessed by MTT assays. NRCMs were transfected with negative control (siNC) or P2Y6R (siP2Y6R #1 and #2) siRNA and treated with vehicle or DOX (n = 4 independent experiments). Data are shown as means ± SEM. *P < 0.05, **P < 0.01, one-way ANOVA.
Discussion
We previously reported that activation of P2Y6R promotes cardiac remodeling in a pressure overload model7. Consistent with this, cardiomyocyte-specific overexpression of P2Y6R increased pressure overload-induced heart failure with severe fibrosis (Figs. 4, 5). Additionally, deletion of P2Y6R in vitro significantly suppressed cell damage and hypertrophy after hypotonic stimulation (Fig. 1). From these results, we hypothesize that deletion of P2Y6R in mice is protective against pressure overload-induced cardiac remodeling and heart failure. Surprisingly, there was a high death rate among P2Y6R-deficient mice within 5 days after TAC surgery (Fig. 2B). In addition, cardiac contractile function was reduced and cardiac hypertrophy was increased after TAC. Cardiac fibrosis did not change compared with that in wild-type mice (Figs. 2, 3). The results of P2Y6R knockout mice appear to conflict with those of P2Y6R overexpression mice. To explain this discrepancy, we need to take the difference between these two models into consideration. In the overexpression model, P2Y6R expression was driven by the cardiomyocyte-specific cTnT promoter, and P2Y6R signaling was directly altered in cardiomyocytes only. In P2Y6R(−/−) mice, P2Y6R is deleted in all cell types. The heart contains many cell types in addition to cardiomyocytes, including smooth muscle cells, endothelial cells, fibroblasts, connective tissue cells, mast cells, and immune system-related cells, and these cells cooperatively work together to maintain cardiac homeostasis25. Therefore, in vivo cardiac vulnerability caused by P2Y6R knockout may be caused by non-cardiomyocytes. Indeed, non-cardiomyocytes may contribute to hypertrophy and fibrosis26. For instance, inflammatory cells, such as macrophages and monocytes, infiltrate the heart in response to pressure overload and affect the hypertrophic response and pathogenesis of heart failure27. Deletion of activated cardiac fibroblasts suppresses fibrotic responses28,29,30. Moreover, purinergic signaling takes part in the inflammatory response31. Neutrophils, a type of inflammatory cell, release nucleotides during membrane deformation, which is important for neutrophil function32. Also in rat cardiac fibroblasts and neonatal rat cardiac myofibroblasts, many types of purinergic receptors are expressed33. Interestingly, the level of P2Y6R expression is selectively upregulated in endothelial cells after tumor necrosis factor α-stimulation34. Additionally, P2Y6R has critical roles in activating immune cells, such as macrophages and microglia in various tissues16,35,36,37. Based on these findings, our results concerning cardiac vulnerability in P2Y6R knockout and cardiomyocyte-specific P2Y6R overexpression mice after pressure-overload strongly indicate that P2Y6R signaling plays a pivotal role in a variety of cell types, including cardiomyocytes, fibroblasts and macrophages, and that this makes the relationship between P2Y6R and cardiovascular disease complex. Further study using cell type-specific P2Y6R knockout mice will be needed to elucidate the detailed mechanism.
The second possibility to explain this discrepancy is variety of P2Y6R activation modes. It has been recently reported about various modes of GPCR activation including biased activation and dimerization38. When GPCRs are activated, they can activate not only G protein-dependent but also β-arrestin-dependent signaling pathway. Moreover, in several situations, GPCR can selectively activate either G protein or β-arrestin signaling pathway, which is called biased activation. Interestingly, G protein and β-arrestin activate different downstream signaling molecules, leading different cardiac responses39. β1-adrenergic receptor-mediated G protein signaling pathway promotes harmful cardiac remodeling, whereas β-arrestin signaling pathway shows cardioprotective effect40. We previously identified that P2Y6R acts as G protein-biased modulator of angiotensin II type 1 receptor (AT1R)15. AT1R-P2Y6R heterodimerization converts AT1R signaling from β-arrestin to G protein pathway, leading vascular remodeling. P2Y6R is activated by extracellular UDP. We have previously reported that UDP-activated P2Y6R induces cardiac fibrosis through G12/13 pathway7. Interestingly, a non-nucleotide ligand for P2Y6R has recently been identified41. Moreover, several Gq-coupled GPCRs act as mechanosensors and are directly activated by mechanical stretch19. Therefore, these multiple modes of P2Y6R activation including biased activation and non-canonical ligands may result in complex phenotypes on cardiac remodeling.
Deletion of P2Y6R has positive effects in atherosclerosis models16,17. However, as shown here, loss of P2Y6R increased pressure overload-induced heart failure. Moreover, isoproterenol-induced cardiac pathological hypertrophy is also augmented in P2Y6R-deficient mice18. This phenotypic difference between vascular and cardiac remodeling may depend on cell types. In an atherosclerosis model, agonist-induced P2Y6R activation causes leukocyte activation, such as rolling and adhesion16. In addition, P2Y6R signaling induces the release of inflammatory cytokines from macrophages17. These results indicate that P2Y6R-mediated pro-inflammatory responses in immune cells such as leukocytes and macrophages are primary contributors to atherosclerosis formation. However, in isoproterenol-induced hypertrophy, P2Y6R signaling suppresses isoproterenol-induced cardiomyocyte hypertrophy18 and this signaling may occur in cardiomyocytes.
DOX-induced heart failure is mainly caused by oxidative stress and not by mechanical stress23. The mechanism of cardiac remodeling induced by DOX is different from that induced by TAC. TAC induces myocardial hypertrophy and fibrosis, whereas DOX induces cardiac atrophy23 and cell death24. In this study, P2Y6R deficiency did not affect cardiac contractility after doxorubicin injection in vivo (Fig. 6A,B) or cell death of DOX-treated cardiomyocytes in vitro (Fig. 6C), indicating that P2Y6R does not participate in oxidative stress-induced cardiac injury, but specifically contributes to mechanical stress-induced heart failure.
In addition to TAC and DOX injection, administration of isoproterenol or angiotensin II is used in rodent heart failure models. In isoproterenol-induced heart failure models, deletion of P2Y6R exacerbates cardiac hypertrophy but not fibrosis18. These data are similar to our results in the pressure overload-induced heart failure model. There is no evidence that shows whether P2Y6R affects angiotensin II-induced heart failure. However, P2Y6R can interact with AT1R, and deletion of P2Y6R suppresses angiotensin II-induced hypertension and vascular remodeling15. P2Y6R may also participate in angiotensin II-induced cardiac remodeling.
The mortality of wild-type TAC mice presented in Figs. 2 and 4 was different. This difference may be caused by AAV infection because AAV infection induces an immune response42, which can alter cardiovascular homeostasis. In addition, the anesthetic inhalation protocol was different for mice with/without AAV infection because AAV infection altered the response to the anesthetic.
In conclusion, although P2Y6R in cardiomyocytes definitely plays a key role in mechanical stress-induced cardiac remodeling, our results provide solid evidence that systemic inhibition of P2Y6R fails to prevent heart failure after pressure overload in mice. Strategies targeting P2Y6R for the treatment of heart failure will need careful consideration.
A limitation of this study is that echocardiography was used to assess cardiac function. However, the detected contractile function in healthy mice was high (EF = 90–95%) compared with a report using MRI (EF = approximately 60%)43. This difference is caused by the resolution of our echocardiography device being lower than that of MRI and of echocardiography devices that are specialized for small animal experiments. In addition, M-mode echocardiography is two-dimensional and EF is a calculated parameter. In contrast, MRI can yield three-dimensional information and measure EF directly. Unexpectedly, many P2Y6R knockout mice died after pressure-overload, and we could only obtain a minimal number of samples. According to the principle of the 3Rs, we used the minimum number of samples for analysis. Therefore, several lines of evidence were obtained from in vitro experiments only (such as Fig. 3C).
Methods
Animals
All experiments using rodents were reviewed and approved by the ethics committees at the National Institutes of Natural Sciences and carried out in accordance with their guidelines (Inter-University Research Institute Corporation National Institutes of National Sciences Animal Experiment Regulations). C57BL/6J mice and Sprague–Dawley (SD) rats were purchased from SLC. Animals were maintained under a 12-h/12-h light/dark cycle. P2Y6R(−/−) mice (a kind gift from Professor Bernard Robaye, (Universite´ Libre de Bruxelles)) were backcrossed onto a C57BL/6J background to obtain P2Y6R(+/−) mice as described previously14. Eight-week-old male mice were used for experiments.
Isolation of neonatal rat cardiomyocytes and fibroblasts and transfection
Neonatal rat cardiomyocytes (NRCMs) and fibroblasts (NRCFs) were isolated from SD rat pups (1 to 2 days old) as previously described11,44. For knockdown, NRCMs and NRCFs were transfected with siRNA (20 nM) using Lipofectamine RNAiMAX Transfection Reagent (Invitrogen). siRNAs for rat P2Y6R (#1: P2ry6RSS300847, #2: P2ry6RSS300848) were purchased from Invitrogen.
Measurement of cell damage
Activity of lactate dehydrogenase (LDH) released from damaged cells was measured using the Cytotoxicity LDH assay kit WST (Dojindo). Absorbance at 490 nm was measured using a SpectraMax i3 plate reader (Molecular Devices). To induce hypotonic stimulation, NRCMs were incubated in control, 30%, or 50% hypotonic solution (70% DMEM and 30% distilled water, and 50% DMEM and 50% distilled water, respectively).
Measurement of cell surface area
NRCMs were seeded on 12φ glass base dishes (Iwaki) coated with Matrigel (Corning). After control or 30% hypotonic stimulation, NRCMs were fixed in 4% paraformaldehyde (FUJIFILM Wako) for 10 min, then permeabilized and blocked in 0.05% Triton X-100 with 1% bovine serum albumin (Nakalai Tesque) for 30 min. Anti-sarcomeric α-actinin (EA-53) (Abcam) was used as a primary antibody overnight at 4℃. Alexa-Fluor488-conjugated antibody (Life Technologies) was used as a secondary antibody. Stained NRCMs were mounted with ProLong Diamond Antifade Mountant containing DAPI (Invitrogen). Imaging was performed on a BZ-X700 microscope (KEYENCE). Cell surface area was measured using ImageJ. More than one hundred cells from five pictures were quantified for each specimen.
Cardiac fibroblast differentiation
NRCFs were seeded on 12φ glasses. After treatment of 10 ng/mL TGF-β1, NRCFs were fixed in 4% paraformaldehyde for 10 min, then permeabilized and blocked in 0.05% Triton X-100 with 1% bovine serum albumin for 30 min. To check differentiation of NRCFs, anti-α-SMA antibody (Sigma) was used as a primary antibody overnight at 4˚C. Alexa-Fluor488-conjugated antibody was used as a secondary antibody. Stained NRCFs were mounted with ProLong Diamond Antifade Mountant containing DAPI. Imaging was performed on a Nikon A1Rsi microscope (Nikon). Expression level of α-SMA was measured using ImageJ. Five pictures were quantified for each specimen.
Transverse aortic constriction (TAC) surgery
To induce cardiac pressure overload, TAC surgery was performed. The procedure was optimized, based on a previous study45. Briefly, male mice were anesthetized with isoflurane (abbvie), then intubated and ventilated. The chest cavity was opened at the intercostal area. Then, the transverse aorta was constricted with a 7–0 nylon suture between the brachiocephalic artery and the left carotid artery to the width of a 27-gauge needle. After closing the chest cavity, buprenorphine was administrated intraperitoneally as an analgesic.
Measurement of cardiac functions
Echocardiography was performed as described previously45. Mice were anesthetized with isoflurane and cardiac function was measured by Nemio-XG echocardiography (Toshiba) with a 14 MHz imaging transducer.
Morphological analysis
Mouse hearts were harvested, washed in PBS, and fixed in 10% neutral-buffered formalin (Nacalai Tesque). Fixed cardiac tissues were embedded in paraffin and sectioned. For frozen sections, hearts were embedded in O.C.T. compound (Sakura Finetek) and snap-frozen with liquid nitrogen. For measurement of cross sectional cardiomyocyte areas, hematoxylin/eosin staining was performed. For collagen volume fraction, Picrosirius red staining was conducted. Quantification of cardiomyocyte cross sectional area and collagen volume fraction were performed using ImageJ software.
Cardiomyocyte-specific overexpression of P2Y6R in mice
All experiments using recombinant DNA were reviewed and approved by the safety committees for recombinant DNA of the National Institute for Physiological Sciences and were performed in accordance with their guidelines (National Institute for Physiological Sciences Recombinant DNA Experiment Rules). Adeno-associated viral (AAV) vectors encoding cardiomyocyte-specific expressed AcGFP and FLAG-tagged P2Y6R (FLAG-P2Y6R) under control of cardiac troponin T were generated as described previously46. AAV vectors were injected intraperitoneally into 7-day-old C57BL/6J male mice. Expression was examined by western blotting using an anti-FLAG M2 horseradish peroxidase-conjugated antibody (Sigma) following immunoprecipitation using anti-FLAG M2 beads (FUJIFILM Wako) and by immunofluorescence using an anti-FLAG antibody (Sigma). For western blotting, cardiac tissue was harvested and lysed in cell lysis buffer [20 mM HEPES (pH 7.4), 100 mM NaCl, 3 mM MgCl2, and 1% Triton X-100] with protease inhibitor cocktail (Nacalai Tesque) and Phosstop (Roche) to extract proteins. SDS-PAGE was performed in 12% polyacrylamide gels and proteins were then transferred to PVDF membranes. Proteins on membranes were reacted with antibodies and detected using the ImageQuant LAS4000 system (GE).
Administration of doxorubicin (DOX)
15 mg/kg DOX (Sand) in saline was administrated intravenously to P2Y6R(+/+) and P2Y6R(−/−) mice on the first day of the experiment. Cardiac function after DOX administration was monitored by echocardiography.
Statistical analysis
The results are presented as means ± SEM from at least three independent experiments. Statistical comparisons were performed by two-tailed Student’s t-test (for two groups) or one-way analysis of variance followed by a Newman-Keuls comparison procedure (for three and more groups). Significance was accepted for values of P < 0.05.
References
Cohn, J. N., Ferrari, R. & Sharpe, N. Cardiac remodeling-concepts and clinical implications: a consensus paper from an International Forum on Cardiac Remodeling. J. Am. Coll. Cardiol. 35, 569–582 (2000).
Azevedo, P. S. et al. Cardiac remodeling: concepts, clinical impact, pathophysiological mechanisms and pharmacologic treatment. Arq. Bras. Cardiol. 106, 62–69 (2016).
Nishimura, A. et al. Depolysulfidation of Drp1 induced by low-dose methylmercury exposure increases cardiac vulnerability to hemodynamic overload. Sci. Signal. https://doi.org/10.1126/scisignal.aaw1920 (2019).
Sunggip, C. et al. Purinergic P2Y6 receptors: a new therapeutic target of age-dependent hypertension. Pharmacol. Res. 120, 51–59 (2017).
Burnstock, G. Purine and pyrimidine receptors. Cell. Mol. Life Sci. https://doi.org/10.1007/s00018-007-6497-0 (2007).
Andrejew, R. et al. Targeting purinergic signaling and cell therapy in cardiovascular and neurodegenerative diseases. Adv. Exp. Med. Biol. https://doi.org/10.1007/978-3-030-31206-0_14 (2019).
Nishida, M. et al. P2Y6 receptor-Gα12/13 signalling in cardiomyocytes triggers pressure overload-induced cardiac fibrosis. EMBO J. https://doi.org/10.1038/emboj.2008.237 (2008).
Braun, O. Ö, Lu, D., Aroonsakool, N. & Insel, P. A. Uridine triphosphate (UTP) induces profibrotic responses in cardiac fibroblasts by activation of P2Y2 receptors. J. Mol. Cell. Cardiol. https://doi.org/10.1016/j.yjmcc.2010.05.001 (2010).
Balogh, J. et al. Phospholipase C and cAMP-dependent positive inotropic effects of ATP in mouse cardiomyocytes via P2Y11-like receptors. J. Mol. Cell. Cardiol. https://doi.org/10.1016/j.yjmcc.2005.03.007 (2005).
Sunggip, C. et al. TRPC5-eNOS axis negatively regulates ATP-induced cardiomyocyte hypertrophy. Front. Pharmacol. https://doi.org/10.3389/fphar.2018.00523 (2018).
Sudi, S. B. et al. TRPC3-Nox2 axis mediates nutritional deficiency-induced cardiomyocyte atrophy. Sci. Rep. https://doi.org/10.1038/s41598-019-46252-2 (2019).
Nishida, M. et al. Heterologous down-regulation of angiotensin type 1 receptors by purinergic P2Y2 receptor stimulation through S-nitrosylation of NF-κB. Proc. Natl. Acad. Sci. U. S. A. https://doi.org/10.1073/pnas.1017640108 (2011).
Wihlborg, A. K. et al. Positive inotropic effects by uridine triphosphate (UTP) and uridine diphosphate (UDP) via P2Y2 and P2Y6 receptors on cardiomyocytes and release of UTP in man during myocardial infarction. Circ. Res. 98, 970–976 (2006).
Bar, I. et al. Knockout mice reveal a role for P2Y6 receptor in macrophages, endothelial cells, and vascular smooth muscle cells. Mol. Pharmacol. 74, 777–784 (2008).
Nishimura, A. et al. Purinergic P2Y6 receptors heterodimerize with angiotensin AT1 receptors to promote angiotensin II-induced hypertension. Sci. Signal. 9, 1–13 (2016).
Stachon, P. et al. P2Y6 deficiency limits vascular inflammation and atherosclerosis in mice. Arterioscler. Thromb. Vasc. Biol. https://doi.org/10.1161/ATVBAHA.114.303585 (2014).
Garcia, R. A. et al. P2Y6 receptor potentiates pro-inflammatory responses in macrophages and exhibits differential roles in atherosclerotic lesion development. PLoS ONE https://doi.org/10.1371/journal.pone.0111385 (2014).
Clouet, S. et al. Loss of mouse P2Y6 nucleotide receptor is associated with physiological macrocardia and amplified pathological cardiac hypertrophy. J. Biol. Chem. 291, 15841–15852 (2016).
Y Schnitzler, M. M. et al. Gq-coupled receptors as mechanosensors mediating myogenic vasoconstriction. EMBO J. https://doi.org/10.1038/emboj.2008.233 (2008).
Erdogmus, S. et al. Helix 8 is the essential structural motif of mechanosensitive GPCRs. Nat. Commun. https://doi.org/10.1038/s41467-019-13722-0 (2019).
Zou, Y. et al. Mechanical stress activates angiotensin II type 1 receptor without the involvement of angiotensin II. Nat. Cell Biol. https://doi.org/10.1038/ncb1137 (2004).
Fu, X., Liu, Q., Li, C., Li, Y. & Wang, L. Cardiac fibrosis and cardiac fibroblast lineage-tracing: recent advances. Front. Physiol. https://doi.org/10.3389/fphys.2020.00416 (2020).
Shimauchi, T. et al. TRPC3-Nox2 complex mediates doxorubicin-induced myocardial atrophy. JCI Insight https://doi.org/10.1172/jci.insight.93358 (2017).
Prathumsap, N., Shinlapawittayatorn, K., Chattipakorn, S. C. & Chattipakorn, N. Effects of doxorubicin on the heart: From molecular mechanisms to intervention strategies. Eur. J. Pharmacol. https://doi.org/10.1016/j.ejphar.2019.172818 (2020).
Tirziu, D., Giordano, F. J. & Simons, M. Cell communications in the heart. Circulation https://doi.org/10.1161/CIRCULATIONAHA.108.847731 (2010).
Frieler, R. A. & Mortensen, R. M. Immune cell and other noncardiomyocyte regulation of cardiac hypertrophy and remodeling. Circulation 131, 1019–1030 (2015).
Heymans, S. et al. Macrophage MicroRNA-155 promotes cardiac hypertrophy and failure. Circulation https://doi.org/10.1161/CIRCULATIONAHA.112.001357 (2013).
Kaur, H. et al. Targeted ablation of periostin-expressing activated fibroblasts prevents adverse cardiac remodeling in mice. Circ. Res. https://doi.org/10.1161/CIRCRESAHA.116.308643 (2016).
Kanisicak, O. et al. Genetic lineage tracing defines myofibroblast origin and function in the injured heart. Nat. Commun. https://doi.org/10.1038/ncomms12260 (2016).
Aghajanian, H. et al. Targeting cardiac fibrosis with engineered T cells. Nature https://doi.org/10.1038/s41586-019-1546-z (2019).
Jacobson, K. A. & Boeynaems, J. M. P2Y nucleotide receptors: Promise of therapeutic applications. Drug Discovery Today https://doi.org/10.1016/j.drudis.2010.05.011 (2010).
Chen, Y. et al. ATP release guides neutrophil chemotaxis via P2Y2 and A3 receptors. Science (80-) https://doi.org/10.1126/science.1132559 (2006).
Burnstock, G. Purinergic signaling in the cardiovascular system. Circ. Res. https://doi.org/10.1161/CIRCRESAHA.116.309726 (2017).
Riegel, A. K. et al. Selective induction of endothelial P2Y6 nucleotide receptor promotes vascular inflammation. Blood https://doi.org/10.1182/blood-2010-10-313957 (2011).
Nagai, J. et al. P2Y6 signaling in alveolar macrophages prevents leukotriene-dependent type 2 allergic lung inflammation. J. Clin. Invest. https://doi.org/10.1172/JCI129761 (2019).
Giannattasio, G. et al. The purinergic G protein-coupled receptor 6 inhibits effector T cell activation in allergic pulmonary inflammation. J. Immunol. https://doi.org/10.4049/jimmunol.1003669 (2011).
Koizumi, S. et al. UDP acting at P2Y6 receptors is a mediator of microglial phagocytosis. Nature https://doi.org/10.1038/nature05704 (2007).
Wang, W., Qiao, Y. & Li, Z. New insights into modes of GPCR activation. Trends Pharmacol. Sci. https://doi.org/10.1016/j.tips.2018.01.001 (2018).
Wisler, J. W., Rockman, H. A. & Lefkowitz, R. J. Biased G protein-coupled receptor signaling: changing the paradigm of drug discovery. Circulation 137, 2315–2317 (2018).
Patel, P. A., Tilley, D. G. & Rockman, H. A. Beta-arrestin-mediated signaling in the heart. Circ. J. https://doi.org/10.1253/circj.CJ-08-0734 (2008).
Brüser, A. et al. Prostaglandin E2 glyceryl ester is an endogenous agonist of the nucleotide receptor. Sci. Rep. https://doi.org/10.1038/s41598-017-02414-8 (2017).
Rabinowitz, J., Chan, Y. K. & Samulski, R. J. Adeno-associated Virus (AAV) versus immune response. Viruses https://doi.org/10.3390/v11020102 (2019).
Grune, J. et al. Evaluation of a commercial multi-dimensional echocardiography technique for ventricular volumetry in small animals. Cardiovasc. Ultrasound https://doi.org/10.1186/s12947-018-0128-9 (2018).
Numaga-Tomita, T. et al. TRPC3-GEF-H1 axis mediates pressure overload-induced cardiac fibrosis. Sci. Rep. https://doi.org/10.1038/srep39383 (2016).
Oda, S. et al. TRPC6 counteracts TRPC3-Nox2 protein complex leading to attenuation of hyperglycemia-induced heart failure in mice. Sci. Rep. https://doi.org/10.1038/s41598-017-07903-4 (2017).
Masuda, T. et al. Dorsal horn neurons release extracellular ATP in a VNUT-dependent manner that underlies neuropathic pain. Nat. Commun. https://doi.org/10.1038/ncomms12529 (2016).
Acknowledgements
We thank Professor Bernard Robaye (Universite´ Libre de Bruxelles) for the kind gift of P2Y6R knockout mice. We also thank The Research Support Center, Research Center for Human Disease Modeling, Kyushu University Graduate School of Medical Sciences for technical assistance. This work was supported by grants from JSPS KAKENHI to M.N. (19K22443, 19H03383), A.N. (19K07085), K.N. (18K14921), and Y.K. (20K15993), and JSPS Fellows to K.S. (19J20086) and by the Platform Project for Supporting Drug Discovery and Life Science Research (BINDS) from AMED (JP20am0101091, 20ak0101121h0001). This work was also supported by Joint Research of ExCELLS (No. 20-312) and NIPS (No. 20-224), and The Naito Foundation (to M.N.), the Takeda Science Foundation (to M.N. and A.N.), the Kato Memorial Bioscience Foundation (to A.N.) and the Suzuken Memorial Foundation (to M.N.). We thank Jeremy Allen, PhD, from Edanz Group (https://en-author-services.edanzgroup.com/ac) for editing a draft of this manuscript.
Author information
Authors and Affiliations
Contributions
A.N. and M.N. designed the project; K.S., A.N., C.S., T.I., K.N., Y.K., and T.T. performed experiments; H.S., and M.T. contributed reagents/analytical tools; K.S., A.N., and M.N. wrote the manuscript. M.N. edited the manuscript. All authors discussed the results and commented on the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shimoda, K., Nishimura, A., Sunggip, C. et al. Modulation of P2Y6R expression exacerbates pressure overload-induced cardiac remodeling in mice. Sci Rep 10, 13926 (2020). https://doi.org/10.1038/s41598-020-70956-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-70956-5
- Springer Nature Limited