Abstract
Afghanistan has long history of ongoing conflicts, resulting in massive destruction of the country’s infrastructure. Illegal trade of livestock between Afghanistan and Pakistan boosted the spread of Foot & Mouth Disease (FMD). Current study was conducted to investigate outbreaks of FMD occurred between April-August 2014 in Nangarhar, Afghanistan. Descriptive data about suspected FMD cases were collected from the Civil Veterinary Hospital, Nangarhar to analyze spatio-temporal pattern of FMD. Case farms (n = 137) were selected from list of clinically confirmed FMD outbreaks available in the hospital. Control farms (n = 137) were enrolled from neighboring premises of case farms. The epidemic curve showed that the virus is continuously circulating among susceptible population. The mean age of the oldest lesion was 2.8 days. Foot & Mouth Disease was more likely to occur in female animals compared to male animals (p < 0.001). Farmers having no ability to clinically recognize FMD (OR 5.8, 95% CI 1.4–23.8); previously having any FMD case in herd (OR 11.8, 95% CI 3.0–45.8), farms where animals leave shed during day (OR 15.4, 95% CI 5.6–42.0), and farms, where neighboring farmers used to visit the premises (OR 3.5, 95% CI 1.2–9.9) were identified as risk factors. Current findings may be used to create awareness of concerned veterinary health authorities about FMD control.
Similar content being viewed by others
Introduction
Foot & Mouth Disease (FMD) is a highly contagious, viral disease of cloven-hoofed domesticated (including cattle, water buffalos, sheep, and goat)1 and wild animals2. Foot & Mouth Disease is an economically expensive disease due to heavy losses to livestock industry with high morbidity in adult animals (especially cattle and pigs), decreased production efficiency and also mortality in young stock3. The FMD virus (FMDV) belongs to the genus Aphthovirus and family Picornaviridae4. The virus has seven major serotypes: O, A, C, Asia 1 and SAT 1 (South African Territory), SAT 2, and SAT 35. These serotypes are immunologically unique and one serotype does not cross-protect against the others. However, continuous evolution of FMDV types have resulted into intra-serotypic subtypes that may cross-protect incompletely6. Continuous evolution of new isolates within a serotypes belonging to a particular geographic region make control of this disease difficult7.
Afghanistan was an agricultural country and exporter of livestock, but due to 40 years long ongoing conflicts and wars, its natural resources and trade has been damaged by military activities, refugees displacement, overexploitation of land and drought8,9. It is now an importer of meat and meat products and growing demand has increased profits for smuggling cattle to Afghanistan from Pakistan, with which it shares a long and porous border of 2,640 km10,11,12. Different type of agricultural and non-agricultural commodities are traded illegally at this border13. Nangarhar is considered as Afghanistan’s food basket due to its agriculture resources. About 70% of rural households, 64% of Kuchi nomads, and 18% of urban households in the province own livestock or poultry. The most commonly owned livestock are cattle (1–2 cows), donkeys, sheep, and goats. Different animal products like milk, meat, butter etc. is produced for household consumption while surplus is sold14. The buffalos are rarely present in herds15 and are usually imported from Pakistan for meat consumption16. The Nangarhar Province of Afghanistan is ecologically very similar to Pakistan and is connected through transhumance, trade and fattening enterprises12,17. Uncontrolled and Illegal movement of animals between two countries is linked to the enhanced transmission of trans-boundary animal diseases specifically FMD, which is endemic in both countries12,18,19. The seasonal movement of transhumant Kuchis tribes with their animals during winter also plays significant role in the spread of FMD regionally and globally20,21.
In Afghanistan and Pakistan serotypes O, A and Asia-1, are responsible for the outbreaks of FMD19,22,23,24. Continuous surveillance of virus transmission is essential to achieve better control of the disease in endemic countries. Worldwide strategy to respond in case of FMD is based on early detection and warning systems, prevention, and establishment of rapid response measures mechanism25.
Detailed epidemiological investigation of FMD outbreaks can give insight about disease patterns, which might be used for early warning and prospective control planning of the disease. Delayed case detection enhances disease transmission and epidemic risk, and subsequent economic losses in affected countries, which has been observed in previous FMD epidemics26,27. Comprehensive epidemiological knowledge of FMD is crucial for the development of efficacious surveillance and control programs12,28.
Foot & Mouth Disease is endemic in Afghanistan, however, epidemiological knowledge related to FMD is scarce in Afghanistan due to decades long war in the country, making many locations unsafe to visit, along with their geographical remoteness, which has hindered the delivery of effective veterinary services to the livestock sector by the Government and Non-Governmental Agencies (NGOs), and inefficient surveillance resulting in underreporting of FMD disease12,29.
Afghanistan was placed in stage 1 of OIE/FAO FMD-Progressive Control Pathway (FMD-PCP) by the GF-TADs FMD working group and regional advisory group for the West Eurasian region (7th Regional Progress Review Meeting, Bishkek, Kyrgyzstan, 2016, available at: https://www.fao.org/3/ca1257en/ca1257en.pdf). To reach at stage 2 of the FMD-PCP, it was advised to implement a risk-based strategic control plan and for that, investigation of outbreaks and identification of risk factors is very important.
Risk factors associated with FMD outbreaks have been identified through questionnaire based studies previously in several developing countries like Bhutan1, Thailand30, Japan31, Ecuador32, Bangladesh33, Cameroon34, Ethiopia35 and Sri Lanka36. Several risk factors were identified through these studies and among them were; animal transaction (buying, selling, or animal exchange between farmers), contact through animal movement between villages, free ranging of cattle herd and farm management practices. Animal husbandry practices e.g. exchange/sharing of farm utensils and services, sharing of breeding animals and movement of farm workers/personnel. Extensive published data is not available about the risk factors of FMD outbreaks in Afghanistan and to achieve the goal of FMD-PCP control strategy, a well-established knowledge of disease determinants is required. The present study was conducted to investigate the 2014 outbreaks of FMD in the war-torn areas of Behsud and Surkhrod Districts of Nangarhar Province, Afghanistan and to identify potential risk factors associated with these outbreaks.
Results
Epidemic curve of the FMD outbreaks
Data were used from all infected premises from where animals were brought for clinical examination at CVH, Nangarhar (n = 177). The epidemic curve showed a pattern of propagated epidemic (CDC, 2012). It showed that the virus is spreading persistently from infected to susceptible population (Fig. 1). The mean age of the oldest lesion was 2.8 d [standard deviation (s) = 1.3], with a minimum age of 1 day and a maximum of 7 days on infected farm (Fig. 2). Proportion of premises with female FMD positive animals was higher (n = 132, 74.6%), as compared to premises with infected male animals (n = 45, 25.4%). Foot & Mouth Disease was more likely to occur in female animals as compared to male animals (χ2 = 42.8; p < 0.001). Majority of farmers (n = 78, 44%) reported that FMD was observed in their herds once in a year.
Spatial and temporal patterns of FMD outbreaks
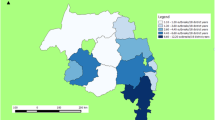
Outbreaks were spatially distributed in study area (Fig. 3). During temporal analysis of outbreak data, it was recorded that the maximum outbreaks (n = 63, 35.6%) occurred in July 2014 and in the Behsud District (n = 92). Maximum number of outbreaks (n = 17) occurred in Jamali village of Behsud District (Fig. 4) and the most affected species was sheep (n = 88, 49.7%), followed by cattle (n = 80, 45.2%) and goat (n = 9, 5.1%). The male:female ratio for ovine was 9 to 20.3, for bovine it was 3:17 and for caprine was 2:1. Average herd size was 10 (range between 1–82) animals.
Outbreak map of FMD cases in Nangarhar Province. Map was created using QGIS (https://qgis.org/en/site/) by the senior author (MC).
Case–control study
A total of 274 farmers (137 case farms, 137 control farms) were interviewed from the study area. Most of the farmers (n = 233, 85%) were rural smallholder farmers, 6.6% (n = 18) were rural commercial farmers and 8.4% (n = 23) were peri-urban commercial farmers. Average number of cattle in herd was 4 (range 0–20, there were 30 premises where no cattle was present), while average number of sheep was 2 (range 0–39, sheep were not present in the herd at 190 premises) and goats was 4 (range 0–60, no goat was present in herd at 106 premises) respectively. Most of the farmers (n = 74) possessed one acre of land. Out of 37 risk factors, 14 variables were selected for inclusion in multivariable analysis based on biological plausibility and selection criterion (Table 1). Nine factors having p > 0.25 were excluded from further analysis. Logistic regression analysis of 7 variables could not be conducted due to zero cell values in 2 × 2 contingency tables. Six variables were correlated [rho (ρ) ≥ 0.5] with others variables and from each pair of correlated variables only biologically plausible variables were retained in analysis. Two variables were excluded due to insufficient discordant pairs. In the final multivariable model, four variables were identified as significantly associated with the FMD outbreak (Table 2). Case farms were more likely to have farmers with no ability (unable to distinguish visible FMD lesion in their animals) to clinically diagnose FMD (OR: 5.8, 95% CI: 1.4–23.8, p < 0.05) compared to control farms. The odds of previously having any case of FMD in herd was 11.8 times more in case farms (95% CI 3.0–45.8, p < 0.001) when compared to exposure in control farms. Similarly, case farms where animals leave shed during day (usually for grazing outside the premises) were more likely to have FMD outbreaks (OR 15.4, 95% CI 5.6–42.0, p < 0.001) when compared to control farms. The case farms, where neighboring farmers used to visit the premises were 3.5 times more likely to have FMD (95% CI 1.2–9.9, p < 0.05).
Discussion
In Afghanistan and Pakistan, FMD is the main trans-boundary endemic disease and has the ability to spread very rapidly crossing national and international borders, causing serious economic losses and have affected food security and national economics18,19. Due to continuous war and ongoing conflict in the region, very limited data is available about epidemiology of FMD in Afghanistan12. The current study investigated outbreaks of FMD and potential risk factors associated with these outbreaks in Behsud and Surkhrod Districts, Nangarhar Province, Afghanistan, to present the glimpse of epidemiological situation of disease in the country. A total of 177 outbreaks were reported by civil veterinary hospitals in two districts of the province. The propagated pattern of epidemic curve of FMD outbreaks in study area could be attributed to high density of susceptible livestock population (Cattle, Sheep and Goats) and poor biosecurity in these rural smallholder herds and villages. Mixing of diseased and healthy animals while grazing at pastures escalate the probability of exposure to circulating viruses and enhance disease spread37. It showed that the virus is spreading persistently from infected to susceptible population.
The outbreak map identified several villages in Behsud District as clusters of FMD with maximum number (n = 17) of outbreak reported in Jamali village during the study period. Behsud District has high density of livestock compared to Surkhrod District38. Livestock density can impact transmission dynamics of FMD and would require additional strategies to control disease in highly dense areas compared to low dense areas37.
The number of outbreaks accelerated in May, June and July 2014, corresponding to the increased demand and transportation of animals to regional markets during Ramadan and at the eve of Eid-ul-Fitar (Muslim celebration event) (June–July, 2014). Gunasekera, et al.36 reported escalated number of outbreaks due to peak movement of animals for the festival celebrations for Muslim and Buddhist community. Osmani, et al.12 also reported that there is high probability of spread of FMD in the region through the continued movement of refugees across the border between Afghanistan and Pakistan and significant legal and illegal movement of animals especially at the religious occasion such as Eid-ul-Adha. Animal movements have been recognized as one of the most common method of transmission of FMD1,37,39,40. As Nangarhar province share border with Pakistan, seasonal migrations of nomadic populations through transhumance routes between Pakistan and Afghanistan along with their livestock also poses risk of trans-boundary diseases. The illegal movement of animals across the borders and two-way trade of livestock remained a major problem in terms of disease management including FMDV19,41,42,43.
Out of 177 positive cases 25.4% were male and 74.6% were female, which has been reported previously44. In current study maximum number of FMD cases were reported on the day when the lesion age was approximately 2 days (n = 77, 43.5%). Previously the mean age of oldest lesion was reported as 1.80 day27.
Small ruminants play a crucial role in the epidemiology of FMD transmission as they often show inapparent infection in these hosts and are largely ignored during national vaccination programs39,45,46. Furthermore, these species have ability to become carriers and can act as reservoir for further infection and spread of disease, hence trade of live sheep and goats present a major risk of entry of FMD to disease-free countries47. In current study, small ruminants, both sheep (n = 88) and goat (n = 9) were reported to be infected with FMD, along with cattle (n = 80). Sheep are highly susceptible to virus infection and can excrete virus through aerosol route and have been reported for transmission of FMDV within countries and across borders46. The FMDV can persist in sheep for up to 12 months and in goats for 2–3 months48. Nearly every herd in Afghanistan has a small ruminants animal (sheep or goat), which put them at higher risk of remained as carrier and being ignored due to subclinical nature of disease in small ruminants47. Most of the farmers (44%) reported that the FMD occurred on their premises once in a year. Similar results were documented previously49.
In current study four risk factors were identified. A farmer with no ability to clinically recognize FMD was 5.8 times more likely to have positive farm. Ability to recognize FMD clinically enable farmers to report disease promptly and early detection eventually lead to rapid response and better management of cases at premises27. Given the potential of FMD for rapid spread, it is essential that suspected cases are quickly reported and investigated by means of rapid and accurate tests, so that control measures can be speedily implemented43.
Previously having any case of FMD in herd increased the likelihood of being a case farm 11.8 times (95% CI 3.0–45.8, p < 0.001) compared to control farms with no previous FMD case in herd. Presences of any previous case in herd or any infected premises nearby keep farmers alert and more vigilant about clinical signs. They interact with veterinary staff more frequently than others to seek help for existing cases. These visits and interaction improve the farmer’s awareness about the disease and hence increase the likelihood of early detection of any new case in the herd, subsequently helping veterinary authorities to fully understand the extent of FMD infection in livestock and identifying any arising outbreak27,50.
In this study, case farms where animals leave shed during day (usually for grazing outside the premises) were more likely to have FMD outbreaks (OR 15.4, 95% CI 5.6–42.0). Susceptible animal are usually exposed to large quantity of viruses shed by infected animals at a grazing area, which can spread disease to non-infected animals35,36,43,51.
Farms having frequent visits by the neighboring farmers were more likely to have FMD than those farms where access to premises was restricted (OR 3.5, 95% CI 1.2–9.9). Lack of biosecurity has been recognized as a major factor in the spread of FMD1,36,50,52.
Conclusion
Our findings have provided an overview of the FMD outbreaks in Nangarhar Province of Afghanistan and have identified risk factors for the occurrence of FMD outbreaks. These finding suggests continuous circulation of FMD virus in livestock of Afghanistan. It may pose a serious threat to the livestock industry of the country and to the global food security. The current findings could be used to generate suitable recommendations for control of FMD in the country. To achieve the goals of Progressive Control Pathway for FMD control, risk factors identified from this study may be included in the FMD control plan for Afghanistan. For future research, longitudinal and surveillance studies are recommended to monitor the virus circulation and disease dynamics.
Our study has several limitations due to unavoidable circumstances. Scarcity of the data due to continuous war and conflict in the region, resulting in massive damage to the veterinary infrastructure, was a major hindrance in collection and collation of data. As very limited veterinary health facilities were available in the area, very few outbreaks were reported to the veterinary authorities, which might have introduced reporting bias in data. A clinical diagnostic criterion based of clinical sign was used by the veterinarian to diagnose case and control farms due to unavailability of confirmatory diagnostic tests. This selection criterion might have led to misclassification bias especially when selecting control farms from an FMD endemic area 36. As case–control study was based on questionnaire data, recall bias might have encountered in data. Furthermore, exact geographical coordinate where the outbreak occurred, were not available, thus only the available village locations were considered in each district to develop distribution map.
Methods
Study area
The outbreak investigation was carried out in two districts (Behsud and Surkhrod) of the Nangarhar Province of Afghanistan (Fig. 5). Located at 34°10′ N latitude and 70°37′ E longitude in east of Afghanistan, Nangarhar shares a border with Khyber Pakhtunkhawa province of Pakistan. Pashtun are the major ethnic group of population on both side of the boundary. Jalalabad, the capital city of Nangarhar, is situated on an ancient trade route connecting Kabul to Peshawar and the Indian sub-continent via Kyber Pass53.
Map showing selected districts of Nangarhar, Afghanistan. Map was created using QGIS (https://qgis.org/en/site/) by the senior author (MC).
Study design
In veterinary medicine, plenty of published information is available about FMD outbreak investigation35,39,54,55,56. For current study, outbreak investigation was conducted following ten steps of outbreak investigation57. All study protocols including humans were approved by Institutional Review Committee for Biomedical Research, University of Veterinary and Animal Sciences, Lahore, Pakistan. There was no experimentation on human and animal subjects and only data was collected from the farmers. Local veterinary authorities in Afghanistan were contacted to seek permission to contact owners of animals from selected premises. In person contact was made to reach owners and objective of the study were explained and they were then asked to participate in the study. After informed consent from owner, a face-to-face interview was conducted to collect information about risk factors using a pre-structured questionnaire. Most farmers were reluctant to provide any written consent due to ongoing war and privacy. Hence, only those farmers, who were willing to participate in the study, provided data about outbreaks of FMD on their premises.
The methods in current study were carried out in accordance with relevant guidelines and regulations to collect observational data as given in Declaration of Helsinki.
FMD outbreak analysis
Outbreak definition
The current study was conducted to investigate the outbreaks of FMD in study area. According to National Livestock Census report of Afghanistan, the total number of cattle in the country is 3.7 million, number of sheep is 8.8 million, and number of goat is 7.3 million38. Exact data about the livestock population and composition in Nangarhar Province was not available. It is estimated that almost 70% of the households in the rural areas of Nangarhar keep one or two cows53. For the current study, an outbreak of FMD was declared if one or more clinical cases of FMD occurred in a herd during the same time period (incubation period). If a case occurred in a herd or farm, which was separated from other herd or farm by physical barriers such as rivers, streams, hills or mountains or by time barrier i.e. occurring in same farm or herd but at different time period, it was considered a separate outbreak. For outbreak investigation, an animal was considered positive for FMD based on the clinical definition i.e. high body temperature, excessive salivation, vesicles formation on the tongue, nose, lips, oral mucosa, plus the inter-digital spaces and coronary bands on the feet, along with few or all signs of reduced appetite, depression, fever, hypersalivation, and lameness36,58,59,60.
Data source for outbreak analysis
An outbreak was confirmed by the veterinary officer of the reporting Civil Veterinary Hospitals (CVH) based on clinical diagnosis in suspected animals attending CVH from the farms in the selected districts. Serological or molecular diagnosis of the samples was not available due to poor diagnostic facilities resulting from decades long war and conflict in Nangarhar. In Kabul, Central Veterinary Diagnostic and Research Laboratory (CVDRL) has facilities to detect and genotype FMDV, however, continuous conflict and budget constraints caused shortage of supplies and reagents in the country and laboratory heavily relies upon the financial aid of international organizations, such as the Food and Agricultural Organization of the United Nations (UNFAO), to support the diagnosis of the disease in its livestock12. As a result, diagnostic testing remained infrequent in the country.
All reported outbreaks/diseased animals during the study period were included in the analysis.
Data about these outbreaks from April to August 2014 were retrieved from the CVH of Nangarhar, Afghanistan on a predesigned questionnaire. Data about number of diseased animals, age of lesions, infection date, date of reporting, geographical location (village, district), species of infected animal, herd size, sex and age of animal were collected and analyzed.
Case control study to identify risk factors of FMD outbreaks
Case and control farm selection
The study was done in areas frequently experiencing FMD outbreaks in two districts (Behsud, & Surkhrod) of Nangarhar Province. The eligible population was all farm premises having cloven-footed animals in villages of both districts in Nangarhar, Afghanistan. The final study population was farm premises whose animals attended CVH, Nangarhar, for treatment purpose. A case farm was defined as a farm premises having animals (cattle/buffalo/sheep/goat) with clinical signs or lesions characteristics of FMD (clinically diagnosed cases) with or without laboratory confirmatory diagnosis58 reported in the last 4 months to the CVH, Nangarhar. Control farm was defined as a farm with animal negative for FMD infection based on herd history and lack of clinical signs of FMD in the last 4 months. Control farms were selected at random from the surrounding neighborhood of case farms in the same village. The owner of the control farms was requested to participate in the study and after formal consent; data were collected from that farm. Each case farm was matched with the control farm on geographical area i.e. same village. Due to unavailability of funds and diagnostic facilities to test the disease or health status of animals at each case and control farms, only the clinical records of the animals at farms were considered for selection. The FMD status of selected case farms was confirmed on the information available in the records of CVH of the area. The trained veterinarian diagnosed the animals, based on clinical signs and recorded the data in hospital registry36.
Sample size for case–control
The sample size was computed to detect an odds ratio of > 2.0 with 95% confidence interval with 80% power and assuming that 33% of controls are exposed to FMDV. The minimum sample size required to conduct study was 274 samples (137 case farms and 137 control farms) with a case–control ratio of 1:1. The sample size calculation was done using WINPEPI software (Version 11.17. 2012)61. The case farms were selected from list of farm premises (N = 177) with confirmed status of FMD (outbreak data) and farmers were invited to participate. If farm owner, refused to participate, next farmer on the list was approached. Control farms were selected from the neighboring farm premises of these case farms with no history of FMD in past 4 months.
Questionnaire for case–control study
A predesigned questionnaire tested with 10 farmers in a pilot study, was used to retrieve information about risk factors of case and control farms. The questionnaire contained 37 questions about biologically plausible risk factors. The questionnaire was prepared after literature review of various studies conducted to identify risk factors in various parts of the world31,35,54,62. Data were collected in a face-to face interview with farm owner about demographic characteristics of farmer, socioeconomic status, feeding practices, biosecurity measures and other relevant factors (Annexure I). The questionnaire was administered in local language in a face-to-face interview with the farm owner, who was willing to participate in the study and fulfilled the selection criteria.
Statistical analysis
Epidemiological and temporal analyses were performed in R software (version 3.2.3)63. The frequency distribution of diseased animals by age group of animals, sex of animal, area of residence (District & Village), age of lesions, frequency of occurrence of FMD in same herd/animal, species affected, season and reporting date was calculated by using epiDisplay package in R software64. Chi-Square test was used to estimate any association among various characteristics. The epidemic curve was drawn to observe the dynamics of epidemic. We performed a logistic regression analysis using presence of clinical signs as the outcome of interest. All biologically plausible risk factors were screened in univariable analysis by using survival package (version, 2.37.7.0) in R software65. Predefined criteria i.e. p < 0.25, was used to select variables for inclusion in a multivariable model66. Significant variables at p < 0.25 in the univariable analysis were included in multivariable analysis. Collinearity of variables was tested by using R package ellipse67. A multivariable model was derived by forward stepwise selection procedure, following selection criteria i.e. to remove the variable with p > 0.2568. Variables with p ≤ 0.05 based on Wald Statistic (or log likelihood ratio test for categorical variables with 3 or more levels) were retained in the model. To estimate the strength of association, Odds ratios (ORs) and corresponding 95% confidence intervals (CIs) were calculated66.
Spatial analysis of FMD outbreaks
QGIS-2.14.3 (available at https://www.qgis.org/en/site/forusers/download.html#) was used to visualize the spatial distributions of diseased animals in study area. The coordinates of villages with outbreak were spatially retrieved via MapCarta (available at https://mapcarta.com). The village location records were obtained from CVH Nangarhar as provided by the farmers. A dot map for FMD outbreaks was created using QGIS (available at https://qgis.org/en/site/).
Data availability
The datasets generated during and/or analyzed during the current study are available from the corresponding author on reasonable request.
References
Dukpa, K. et al. Risk factors for foot-and-mouth disease in sedentary livestock herds in selected villages in four regions of Bhutan. N. Z. Vet. J. 59, 51–58. https://doi.org/10.1080/00480169.2011.552852 (2011).
Arzt, J. et al. The pathogenesis of foot-and-mouth disease II: Viral pathways in swine, small ruminants, and wildlife; myotropism, chronic syndromes, and molecular virus-host interactions. Transbound. Emerg. Dis. 58, 305–326 (2011).
James, A. D. & Rushton, J. The economics of foot and mouth disease. Rev. Sci. Tech. 21, 637–644 (2002).
Belsham, G. J. Distinctive features of foot-and-mouth disease virus, a member of the picornavirus family; aspects of virus protein synthesis, protein processing and structure. Prog. Biophys. Mol. Biol. 60, 241–260 (1993).
Radostits, O. M., Gay, C. C., Blood, D. C. & Hinchcliff, K. W. Veterinary Medicine. A Text Book of the Diseases of Cattle, Horses, Sheep, Pigs and Goats. 10th edn, (W.B. Saunders Co. Ltd., 2007).
Brehm, K. E., Kumar, N., Thulke, H. H. & Haas, B. High potency vaccines induce protection against heterologous challenge with foot-and-mouth disease virus. Vaccine 26, 1681–1687. https://doi.org/10.1016/j.vaccine.2008.01.038 (2008).
Knowles, N. J. & Samuel, A. R. Molecular epidemiology of foot-and-mouth disease virus. Virus Res. 91, 65–80 (2003).
Saidajan, A. Effects of war on biodiversity and sustainable agricultural development in Afghanistan. J. Dev. Sustain. Agric. 7, 9–13 (2012).
Goepner, E. War State, Trauma State: Why Afghanistan Remains Stuck in Conflict. (Cato Institute, Washington DC, 2018).
Yusufzai, R. in Global Meat News (2013).
Formoli, T. A. Impacts of the Afghan-Soviet war on Afghanistan’s environment. Environ. Conserv. 22, 66–69. https://doi.org/10.1017/S0376892900034093 (1995).
Osmani, A., Robertson, I. D., Habib, I. & Aslami, A. A. History and epidemiology of foot-and-mouth disease in Afghanistan: A retrospective study. BMC Vet. Res. 15, 340. https://doi.org/10.1186/s12917-019-2119-y (2019).
Sharif, M., Farooq, U. & Bashir, A. Illegal trade of Pakistan with Afghanistan and Iran through Balochistan: Size, balance and loss to the public exchequer. J. Agric. Biol. 2, 199–203 (2000).
Ates, S., Hassan, S., Biradar, C., Esmati, H. & Louhaichi, M. Characteristics of Baghlan and Nangarhar Provinces. (Technical Report. International Center for Agricultural Research in the Dry Areas (ICARDA), Amman, 2018).
Zafar, M. First Draft Country Report on the Status and Perspectives of the Animal Genetic Resources Development and Conservation in Islamic Republic of Afghanistan (FAO, Kabul, 2005).
Kandeepan, G., Biswas, S. & Rajkumar, R. Buffalo as a potential food animal. Int. J. Livestock Product. 1, 001–005 (2009).
Jamal, S. M. et al. Detection and genetic characterization of foot-and-mouth disease viruses in samples from clinically healthy animals in endemic settings. Transbound. Emerg. Dis. 59, 429–440 (2012).
Jamal, S. M., Ferrari, G., Ahmed, S., Normann, P. & Belsham, G. J. Genetic diversity of foot-and-mouth disease virus serotype O in Pakistan and Afghanistan, 1997–2009. Infect. Genet. Evolut. 11, 1229–1238. https://doi.org/10.1016/j.meegid.2011.03.006 (2011).
Rweyemamu, M. et al. Epidemiological patterns of foot-and-mouth disease worldwide. Transbound. Emerg. Dis. 55, 57–72. https://doi.org/10.1111/j.1865-1682.2007.01013.x (2008).
Macpherson, C. N. L. The effect of transhumance on the epidemiology of animal diseases. Prevent. Vet. Med. 25, 213–224. https://doi.org/10.1016/0167-5877(95)00539-0 (1995).
Fèvre, E. M., Bronsvoort, B. M. d. C., Hamilton, K. A. & Cleaveland, S. Animal movements and the spread of infectious diseases. Trends Microbiol. 14, 125–131, https://doi.org/10.1016/j.tim.2006.01.004 (2006).
Jamal, S. M., Ahmed, S., Hussain, M. & Ali, Q. Status of foot-and-mouth disease in Pakistan. Adv. Virol. 155, 1487–1491. https://doi.org/10.1007/s00705-010-0732-y (2010).
Ostrowski, S., Noori, H. & Rajabi, A. M. Foot-and-Mouth Disease in Wakhan District, Afghanistan (trans: Afghanistan Ecosystem Health Project Team W) (Wildlife Conservation Society, Bronx, 2010).
Valarcher, J.-F. et al. Global Foot-and-Mouth Disease Situation 2003–2004. 15 (Chania, Crete, 2004).
OIE. Foot and Mouth Disease Portal. https://www.oie.int/animal-health-in-the-world/fmd-portal/prevention-and-control/ (2018).
Gibbens, J. C. et al. Descriptive epidemiology of the 2001 foot-and-mouth disease epidemic in Great Britain: The first five months. Vet. Rec. 149, 729–743. https://doi.org/10.1136/vr.149.24.729 (2001).
McLaws, M., Ribble, C., Martin, W. & Wilesmith, J. Factors associated with the early detection of foot-and-mouth disease during the 2001 epidemic in the United Kingdom. Can. Vet. J. 50, 53–60 (2009).
Alkhamis, M. A., Perez, A. M., Yadin, H. & Knowles, N. J. Temporospatial clustering of foot-and-mouth disease outbreaks in Israel and Palestine, 2006–2007. Transbound. Emerg. Dis. 56, 99–107. https://doi.org/10.1111/j.1865-1682.2009.01066.x (2009).
Sumption, K. et al. Foot-and-mouth disease: situation worldwide and major epidemiological events in 2005–2006. FAO EMPRES (Emergency Prevention System) Focus-on 1, 1–11 (2007).
Cleland, P. C., Baldock, F. C., Chamnanpood, P. & Gleeson, L. J. Village level risk factors for foot-and-mouth disease in Northern Thailand. Prevent. Vet. Med. 26, 253–261. https://doi.org/10.1016/0167-5877(95)00552-8 (1996).
Muroga, N. et al. Risk factors for the transmission of foot-and-mouth disease during the 2010 outbreak in Japan: A case–control study. BMC Vet. Res. 9, 150. https://doi.org/10.1186/1746-6148-9-150 (2013).
Lindholm, A. et al. Epidemiologic aspects of a foot-and-mouth disease epidemic in cattle in Ecuador. J. Appl. Res. Vet. Med. 5, 17–24 (2007).
Sarker, S., Saranika, T., Haque, M. H., Islam, M. H. & Gupta, S. D. Epidemiological study on foot-and-mouth disease in cattle; prevalence and risk factor assessment in Rajshahi, Bangladesh. Wayamba J. Anim. Sci. 3, 71–73 (2011).
Bronsvoort, B. M. et al. Risk factors for herdsman-reported foot-and-mouth disease in the Adamawa Province of Cameroon. Prevent. Vet. Med. 66, 127–139. https://doi.org/10.1016/j.prevetmed.2004.09.010 (2004).
Negusssie, H., Kyule, M. N., Yami, M., Ayelet, G. & Jenberie, S. Outbreak investigations and genetic characterization of foot-and-mouth disease virus in Ethiopia in 2008/2009. Trop. Anim. Health Prod. 43, 235–243. https://doi.org/10.1007/s11250-010-9683-2 (2011).
Gunasekera, U. C. et al. Analyzing the foot and mouth disease outbreak as from 2008 to 2014 in cattle and buffaloes in Sri Lanka. Prevent. Vet. Med. 148, 78–88. https://doi.org/10.1016/j.prevetmed.2017.10.008 (2017).
Paton, D. J., Gubbins, S. & King, D. P. Understanding the transmission of foot-and-mouth disease virus at different scales. Curr. Opin. Virol. 28, 85–91. https://doi.org/10.1016/j.coviro.2017.11.013 (2018).
FAO. Afghanistan National Livestock Census, 2002–2003 Report 2006. (Food and Agriculture Organization of the United Nations, 2008).
Dukpa, K., Robertson, I. D. & Ellis, T. M. The epidemiological characteristics of the 2007 foot-and-mouth disease epidemic in Sarpang and Zhemgang districts of Bhutan. Transbound. Emerg. Dis. 58, 53–62. https://doi.org/10.1111/j.1865-1682.2010.01181.x (2011).
Shiilegdamba, E., Carpenter, T. E., Perez, A. M. & Thurmond, M. C. Temporal-spatial epidemiology of foot-and-mouth disease outbreaks in Mongolia, 2000–2002. Vet. Res. Commun. 32, 201–207. https://doi.org/10.1007/s11259-007-9018-6 (2008).
Kanwal, S., Saeed, A., Munir, M. & Arshad, M. Phylogenetics of foot and mouth disease virus in Punjab, Pakistan. Br. J. Virol. 1, 21–28 (2014).
Di Nardo, A., Knowles, N. J. & Paton, D. J. Combining livestock trade patterns with phylogenetics to help understand the spread of foot and mouth disease in sub-Saharan Africa, the Middle East and Southeast Asia. Rev. Sci. Tech. 30, 63–85 (2011).
Alexandersen, S., Zhang, Z., Donaldson, A. I. & Garland, A. J. M. The pathogenesis and diagnosis of foot-and-mouth disease. J. Comp. Pathol. 129, 1–36. https://doi.org/10.1016/S0021-9975(03)00041-0 (2003).
Farooq, U. et al. Sero-prevalence of foot and mouth disease in small ruminants of Pakistan. J. Anim. Plant Sci. 27, 1197–1201 (2017).
Rout, M. et al. Investigation of foot-and-mouth disease outbreaks in Chikkaballapur district of Karnataka. Indian J. Anim. Sci. 84, 231–235 (2014).
Kitching, R. P. & Hughes, G. J. Clinical variation in foot and mouth disease: Sheep and goats. Rev. Sci. Tech. 21, 505–512 (2002).
Barnett, P. V. & Cox, S. J. The role of small ruminants in the epidemiology and transmission of foot-and-mouth disease. Vet. J. 158, 6–13. https://doi.org/10.1053/tvjl.1998.0338 (1999).
Thomson, G. OIE Comprehensive Reports on Technical Items Presented to the International Committee or to Regional Commissions. 1996. The Role of Carrier Animals in the Transmission of Foot and Mouth Disease, 87–103.
Ostrowski, S., Noori, H. & Rajabi, A. M. Foot and Mouth Disease in Wakhan District, Afghanistan. (2010). https://www.wcsafghanistan.org/desktopModules/Brings2mind/DMX/download.aspx?Entryld=6277&Portalld=87&Downloadmethod=attachment.
Emami, J., Rasouli, N., McLaws, M. & Bartels, C. J. M. Risk factors for infection with foot-and-mouth disease virus in a cattle population vaccinated with a non-purified vaccine in Iran. Prevent. Vet. Med. 119, 114–122. https://doi.org/10.1016/j.prevetmed.2015.03.001 (2015).
Cleland, P. C., Chamnanpood, P., Baldock, F. C. & Gleesona, L. J. An investigation of 11 outbreaks of foot-and-mouth disease in villages in northern Thailand. Prevent. Vet. Med. 22, 293–302 (1995).
Ilbeigi, K., Bokaie, S., Aghasharif, S., Soares Magalhães, R. J. & Rashtibaf, M. Risk factors for recurrence of FMD outbreaks in Iran: A case-control study in a highly endemic area. BMC Vet. Res. 14, 253. https://doi.org/10.1186/s12917-018-1580-3 (2018).
RRERS. Regional Rural Economic Regeneration Assessment and Strategies (RRERS) Study/National Area Based Development Program, GRM International. (Ministry National Area Based Development Program, Ministry of Rural Rehabilitation and Development (MRRD), 2006).
Wee, S.-H. et al. Epidemiological characteristics of the 2002 outbreak of foot-and-mouth disease in the Republic of Korea. Transbound. Emerg. Dis. 55, 360–368. https://doi.org/10.1111/j.1865-1682.2008.01045.x (2008).
Gibbens, J. C. & Wilesmith, J. W. Temporal and geographical distribution of cases of foot-and-mouth disease during the early weeks of the 2001 epidemic in Great Britain. Vet. Rec. 151, 407–412 (2002).
Ayebazibwe, C. et al. Patterns, risk factors and characteristics of reported and perceived foot-and-mouth disease (FMD) in Uganda. Trop. Anim. Health Prod. 42, 1547–1559. https://doi.org/10.1007/s11250-010-9605-3 (2010).
Gordis, L. Epidemiology 5th edn. (Elsevier, Saunders, 2009).
OIE. in OIE Guidlines. (2013).
Jamal, S. M. & Belsham, G. J. Foot-and-mouth disease: Past, present and future. Vet. Res. 44, 116. https://doi.org/10.1186/1297-9716-44-116 (2013).
Hegde, R. et al. Epidemiology of foot and mouth disease in Karnataka state, India: A retrospective study. Virus Dis. 25, 504–509. https://doi.org/10.1007/s13337-014-0239-3 (2014).
Abramson, J. H. WINPEPI (PEPI-for-Windows): Computer programs for epidemiologists. Epidemiol. Perspect. Innov. 1, 6. https://doi.org/10.1186/1742-5573-1-6 (2004).
Hayama, Y., Muroga, N., Nishida, T., Kobayashi, S. & Tsutsui, T. Risk factors for local spread of foot-and-mouth disease, 2010 epidemic in Japan. Res. Vet. Sci. 93, 631–635. https://doi.org/10.1016/j.rvsc.2011.09.001 (2012).
R: A Language and Environment for Statistical Computing v. 3.2.3 (R Foundation for Statistical Computing, Vienna, 2015).
epiDisplay: Epidemiological Data Display Package v. 3.5.0.1 (2018).
Survey Package: Analysis of Complex Survey Samples. (2014).
Hosmer, D. W. & Lemeshow, S. Applied Logistic Regression. 2nd edn, (Wiley, New York, 2000).
ellipse Package: Functions for Drawing Ellipses and Ellipse-Like Confidence Regions v. 0.4.1 (2018).
Dohoo, I., Martin, W. & Stryhn, H. Veterinary Epidemiologic Research. (Transcontinental Prince Edward Island, 2003).
Acknowledgements
The authors are highly thankful to local veterinary officers (VOs) at the Afghanistan Livestock Department, Other government personnel, and farmers who cooperated with this investigation. The authors also thank Nangarhar University, Jalalabad, Nangarhar for facilitating first author during his research. This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
M.C. and A.W, designed the study. A.W. collected data. M.C. and A.W. analyzed data. S.R.H. provided support for field work and data collection. M.C., H.B.R., S.S.G. and A.W. drafted manuscript. M.C., A.W., H.B.R., and S.S.G. reviewed the paper.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wajid, A., Chaudhry, M., Rashid, H.B. et al. Outbreak investigation of foot and mouth disease in Nangarhar province of war-torn Afghanistan, 2014. Sci Rep 10, 13800 (2020). https://doi.org/10.1038/s41598-020-70489-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-70489-x
- Springer Nature Limited
This article is cited by
-
Foot-and-mouth disease virus dynamics in border areas of Pakistan with Afghanistan
Molecular Biology Reports (2024)