Abstract
The objective of this in vivo study was to compare bone-to-implant contact (BIC) and bone area fraction occupancy (BAFO) values of a new implant, designed to be inserted without bone preparation, using two different preparation protocols: no site preparation and prior limited cortical perforation, versus the values of a control implant using a conventional drilling protocol. Forty-one implants were inserted in 13 rabbits. Thirteen test implants with a new thread design were inserted using no bone preparation (NP), 14 test implants were inserted with limited cortical perforation (CP), and 14 conventional implants served as control. Five animals were sacrificed after 21 days and eight animals after 42 days. Histomorphometric analysis was performed and percentage of BIC and BAFO values were measured. ANOVA with Tukey post hoc and Mann–Whitney nonparametric tests were calculated to compare between the groups. Statistical analysis showed no significant difference in the measured values between any of the groups, neither compered by implant nor by compered day. The results demonstrated that biological osseointegration parameters of implant that was inserted without any bone preparation was non-inferior compared to conventional preparation. The clinical relevance is that novel implant designs may not require bone preparation prior to placement.
Similar content being viewed by others
Introduction
Various factors influence the long-term prognosis of dental implants and can affect osseointegration, such as surgical technique, host bed, implant surface, implant design, material biocompatibility, and loading conditions1. Osseointegration is defined as a direct contact between living bone and the implant on light microscopic level1.
A wider definition considers bone quality, as well as stable support of a prosthesis and lack of motion of the implant under functional loads, apposition of new bone, that is identified as normal bone and marrow at microscopic levels, and in direct contact with the implant, without interposed connective tissue2,3,4.
Bone-to-implant contact (BIC) percentages is considered essential requisite for implant stability and an indication of successful osseointegration5,6.
The most common implant insertion technique is based on a conventional drilling technique, in which gradual expansion of the osteotomy site by sequential enlargement of the drill diameter is performed8,9,10. Conversely, under-preparation of an implant site (also referred to as under-drilling) is defined as preparing the implant’s bed narrower than the implant’s inserted diameter11,12,13 while over-preparation osteotomy refers to preparing an implant’s bed wider than the implant's inserted diameter13. Other available techniques for implant insertion are bone compaction, osteodistraction and piezo surgery8.
Implant placement without any site preparation is rarely mentioned in published literature; the exception is with regard to orthodontic mini implants which are self-drilling i.e., the implant is inserted without need of predrilling, or "drill free" placement14. However, these orthodontic implants are not designated to achieve osseointegration, thus there are no available data regarding BIC and bone area fraction occupancy (BAFO) outcomes for implants placed without any site preparation.
There is disagreement within published literature regarding the influence of under-drilling on osseointegration, specifically on how this technique impacts the BIC and BAFO values. Reported BIC values when using standard drilling techniques as well as in the under-drilling approach range widely from 20.0% to 62.6%, respectively, in various studies13,15,16,17,18,19,20,21,22. Most studies reported no statistical difference between the two surgical approaches15,16,17,18,23, while others report significantly higher BIC values obtained with under-drilling14,15,16 and one study observed significantly lower BIC values in the undersized group22.
Implant insertion using the undersized drilling technique results in high insertion torque and consequently higher initial stability7. In this press-fit situation, the implant is situated in intimate contact with the bone walls15, hence it is more suitable for placement in Type 4 porous bone23. However, the undersized technique induces high pressure on the bone walls, mainly on high density bones, which may result in plastic deformation and microcracks.
Most implant's macrostructure is designed to be inserted following bone preparation. In this study, an implant with a unique macrostructure designed to be inserted without any implant osteotomy preparation is presented. The implant was placed following a superficial marking drill with or without preliminary cortical perforation.
The objective of this in vivo animal model study was to measure and compare BIC and BAFO values of a new implant using two different preparation protocols (no site preparation and prior limited cortical perforation), and the values of a conventional implant using a control protocol (conventional drilling technique).
Methods and materials
The study protocol was approved by the Research Ethics Committee of the University of Apollonia, Iasi, Romania. A test implant with a new thread design (Magix, Cortex, Israel) (Fig. 1) was inserted using no bone preparation (NP) and cortical perforation (CP) into 13 New Zealand rabbits. The features of the test implants macrostructure enabled insertion without bone preparation due to the single lead threaded tapered design with an expander-like internal core and flat neck, allowing ridge expansion (Fig. 1).
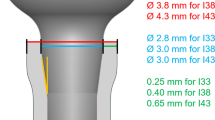
Additionally, a control implant designed for step osteotomy preparation (Dynamix, Cortex, Israel) was also inserted (Fig. 2). Both implants are self-tapping, self-drilling and self-condensing devices made of titanium alloy (Ti6Al4V) with sandblasted acid etched (SLA) surface and share the same dimensions of 10 mm length and 3.8 mm neck diameter.
Surgical procedure
The animals were anaesthetized using intramuscular injection of a combination of ketamine (Ketamidor 100 mg/ml, Richter Pharma ag, Wels, Bioveta, Czech Republic) (0.25 mg/kg of body weight) and Mepivastesin 3%( 3 M Espe, Germany). Incisions of 5 cm long were performed to expose the surface of the right and left tibial metaphysis. One or two of each type of implant were placed on each side.
Implant insertion
Three groups were tested and evaluated; all three groups were tested in each of the 13 animals. In group NP, a 1.5 mm bi-cortical marking drill was used followed by direct placement of the test implant (Fig. 3). In group CP, a bi-cortical 1.5 mm marking drill was used, then a 2 mm wide and 1 mm deep cortical bone perforation was performed followed by implant insertion (Fig. 4). In the control group, the implant was inserted according to the manufacturer’s protocol using a transosseous progressive sequence of drills: a marking drill, followed by a 2.0 mm pilot drill, a 2.8 mm drill and a final 3.2 mm drill. A 3.8 mm control implant was inserted under saline cooling.
The soft issues were sutured in separate layers and the animals received postoperatively subcutaneous enrofloxacin 20 mg/kg for 3 days and 0.6 mg/kg subcutaneous meloxicam for 2 days. Five animals were euthanized after 21 days, examining early healing stages of the bone, and 8 animals were euthanized after 42 days representing mature bone formation. Euthanasia was performed by an embutramide product T61 manufactured by MSD. Histology sections of bone samples were taken.
Histology and histomorphometry
The bone specimens were infiltrated with Remacryl resin starting with 50% ethanol/resin solution and subsequently 100% resin, with each step lasting 24 h. The photopolymerization was obtained using a 48-h exposure to blue-light. The implants were oriented in order to visualize the two different sides at the same time. After polymerization, the blocks were ground down in order to remove the excess of resin and expose the implant, which was glued on plastic slides using a methacrylate-based glue.
A Micromet high speed rotating blade microtome (Remet, Bologna, Italy) was used to separate the section from the block thus obtaining a 250 µm thick section. The section was then ground down to about 40 µm using a LS-2 grinding machine (Remet, Bologna, Italy), equipped with water-proof grinding paper. Grinding was followed by section polishing using a polishing paper and a 3-µm polishing cream.
The sections were then toluidine blue-stained and referred to optical microscopy at 10 × and 100 × magnification. The histomorphometric analysis was performed by digitizing the images from the microscope via a JVC TK-C1380 Color Video Camera (JVC Victor Company, Japan) and a frame grabber. The images were first acquired with a 10 × magnification, showing the entire implant surface (Figs. 5a–c and 6a–c). Large 100 × magnification images were taken for histological evaluation using the image analysis software IAS 2000 (Delta Sistemi, Italy), (Figs. 5d–f and 6d–f). The central section of each specimen was analyzed, and percentage of total implant-bone interface was calculated.
Micrographs of a 21-day site. Representative sections of the implant in × 10 magnification showing the entire implant section at its center, (a) NP group, (b) CP group and (c) control group. Cortical bone is adjacent to the implant neck in all groups, inter-thread space is occupied by bone marrow and newly formed bone. Representative sections of the implant in × 100 magnification showing bone formation at the thread pitch and valley in (d) NP group, (e) CP group and (f) control group. Trabecular bone fills the gaps, osteocytes are evident in all groups.
Micrographs of a 42-day site. Representative sections of the implant in × 10 magnification showing the entire implant section at its center, (a) NP group, (b) CP group and (c) control group. The implants are in close contact with the cortical portion of the tibial bone at the neck and apex. The median portion is adjacent to bone marrow. Representative sections of the implant in × 100 magnification showing bone formation at the thread pitch and valley in (d) NP group, (e) CP group and (f) control group. Mature bone formation is seen at the inter-threads space.
The calculated parameters were the percentage of BIC and BAFO. BIC indicates the percentage of implant surface in direct contact with the bone over the entire length of the implant. The remaining surface is in contact with the marrow tissue (Figs. 5 and 6). BAFO indicates the area occupied by the mineralized bone matrix between the threads over the entire microscopic field. This was measured by outlining the bone surface area from the total field area between the threads and was expressed as percentage.
Statistical methods
ANOVA with Tukey post hoc and Mann–Whitney nonparametric tests (also called Wilcoxon rank-sum test) were calculated to compare the control group with NP and CP groups. The Mann–Whitney test was run to confirm the significance of ANOVA results. Because the sample size did not reach the minimal statistical power of 0.80 for all comparisons, a linear regression test was used to get the target power of 0.80 and α = 0.05, and weight function was used. Analyses were conducted using SPSS.
Ethical approval
All applicable international, national, and/or institutional guidelines for the care and use of animals were followed. All procedures performed in studies involving animals were in accordance with the ethical standards of the institution or practice at which the studies were conducted. The study protocol was approved by the Research Ethics Committee of the University of Apollonia, Iasi, Romania.
Results
A total of 41 implants were inserted in 13 rabbits, each rabbit received 3 to 4 implants (1 to 2 from each test group, in both the right and left tibiae). Thirteen test implants were inserted in group NP without bone preparation, 14 test implants were inserted in group CP with prior cortical perforation, and 14 conventional implants served as a control group.
Statistical analysis and histological examinations were conducted on a total of 39 implants: 12 implants in group NP, 13 implants in group CP and 14 in the control group. Two implants were excluded due to bone fracture occurring during the surgical procedure, one in group NP, another in group CP. Bone fracture requiring immobilization was diagnosed on post-operative day 1 in 2 additional cases, both in the control group. These implants were included in the statistical analysis.
There was no statistically significant difference in BIC and BAFO values between group NP and group CP, as well as between the test groups and the control group either on day 21, or on day 42 (Table 1). Histological examination comparing BIC and BAFO by days and by implant type revealed that BIC values ranged from 46.5 to 51.2% on day 21, and from 49.1% to 54.4% on day 42 (p > 0.05). BAFO values ranged from 43.8% to 53.1% on day 21, and from 44.3% to 49.1% on day 42 (p > 0.05), (Figs. 7 and 8). Additionally, no difference was noted within the groups comparing day 21 values to day 42 values (Table 2).
Discussion
The focus of the current study was to evaluate osseointegration of a new implant designed to be inserted without any prior bone preparation (ie, drill-less) and to compare its biological parameters to a control group that used a conventional implant inserted using a progressive sequence of drills. The study was conducted in an experimental animal model. The results demonstrated that BIC and BAFO values of the test groups were not statistically different compared to the control group, or within the two test groups.
Successful osteointegration relies upon bone formation, which may originate from the implant surface properties: chemical and physical design, this is referred to as “contact osteogenesis”, as well as from the bony margin of the surgical defect i.e. “distance osteogenesis”2. In our study, whether the surgical defect was made following direct insertion or after prior preparation did not result in statistical difference in the tested parameters. As to “contact osteogenesis”, chemically wise, both implants have the same surface treatment, however, the physical implant design differs.
Implant design is a key element affecting initial stability and the implant’s ability to sustain load and achieve successful osseointegration24. The new implant that was tested has a macrostructure that was specifically designed to be inserted without any initial preparation. It was able to be inserted into the bone in a direct mode, following merely a marking spot or following limited cortical perforation prior to implant placement. Additionally, the features of the test implant’s macrostructure enabled prepless insertion due to the single-lead threaded tapered design with an expander-like internal core and flat neck, allowing for ridge expansion (Fig. 1). The threads of this implant are deep and progressive; thread depth is defined as the distance between the major and minor diameter of the thread, with deeper threads having a positive effect upon implant stabilization in poor bone quality situations. Progressive threads also mean higher depth in the apical portion that decreases gradually coronally. This design is hypothesized to increase the load transfer to the more flexible cancellous bone instead of crestal cortical bone24. The test implant features deep and gradual threads (0.3, 0.6, 1.0 mm deep coronally to apically) indicated for insertions in Type 4 bone.
Type 4 bone, present in the human upper jaw, has a very light density, is porous, and presents difficulty when seeking to obtain rigid fixation; as a result, initial stability is often limited. The preparation of Type 4 bone necessitates narrower drills than the dimension of the final implant25. This type of preparation is referred to as under—drilling, also known as under preparation or undersized preparation11.
The advantages of under-drilling are high insertion torque and initial stability. Other advantages are ease of performance, shortened treatment time, and decreased surgical burden on the patient, as well as minimal postoperative morbidity9.
To our knowledge, there is no available data regarding osseointegration parameters in implants inserted without any prior preparation. The influence of under-drilling upon osseointegration is debatable. In a review article that examined the different aspects of undersized implant surgical preparation, the findings regarding BIC values were inconclusive15. An in-vivo experimental study evaluated the effect of drilling dimensions upon insertion torque and early implant osseointegration stages. Implants were placed in bone sites using undersized drilling protocol versus a conventional protocol. BIC and BAFO were examined, the results indicated there was no effect of the drilling dimension upon the tested parameters18. An additional in vivo experiment compared an undersized drilling protocol versus conventional methods and showed that the BIC percentage calculated was 60.6 ± 14.2% and 47.5 ± 11.7% respectively. The conclusion was that an undersized implant bed can improve BIC19.
A different study measured Crestal BIC and Total- BIC, of two implants inserted in an under-drilling or over-drilling protocol, at two time points: 21 or 42 days after insertion. Results demonstrated an increased short-term (21 days) C-BIC, in favor of the over-drilling protocol, however this difference became non-significant statistically at 42 days26.
Overall, there are discordant findings in the literature regarding the influence of under-drilling surgical techniques upon biological parameters, however most studies found no difference in BIC and BAFO between tested surgical approaches. The present study’s results demonstrated that BIC and BAFO values of the test groups were not statistically different compared to a control group or within the two test groups. Furthermore, there was no statistical difference within any group comparing BIC and BAFO after 21 days and 42 days. These findings are in agreement with most available data.
Under drilling complications are derived from high insertion torque which may cause bone microfractures and increased temperature generation27,28, resulting in compression necrosis of the bone, delayed healing process and increased bone resorption15,27. Microfractures occurrence depends on bone density and the discrepancy between the diameter of the implant and the implant’s bed29. In the present study two test insertion protocols were conducted. Implant insertion within the first test group was performed without any bone preparation (NP group). In the second test group (CP group), a cortical perforation was performed to avoid compact bone cracks as well as to examine the impact of this difference on the tested parameters. In our work, bone fracture that lead to implant removal occurred in two cases, one in the NP group, another in the CP group. Two other fracture occurrences were diagnosed on the second day in the control group and were followed by immobilization. Because this complication occurred in all three groups and in four different cases, one cannot conclude that using the drill-less implant insertion technique, with or without cortex perforation, affected the outcome.
Limitations
This study used two different implant types while testing different preparation techniques, which could have confounded our results as these implants differ in their macrostructure. However, the implants in this study have identical surface characteristics; both implants have the same surface treatment, and both are defined as self-tapping, self-drilling, and self-condensing. As mentioned above, it has been established that implant surface properties is an important factor affecting osteoblast differentiation and mineralization by influencing the level of bone-related genes and transcription factors2,26.
Another limitation is the sample size which, due to ethical reasons, is relatively small. For approximate calculation performed to be reliable, statistical weight function was used according to the received sample size, in order to achieve statistically validated conclusion.
In conclusion, this study examined and compared the biological parameters of a novel implant, inserted with two different drill-less surgical approaches. Within the limitations of the present study, we can conclude that this technique did not result in an inferior biological outcome as measured (BIC and BAFO) when compared to conventional preparation. Cortical bone perforation prior to implant placement did not affect these factors. Future studies are required to establish more information regarding biomechanical parameters and clinical outcomes when using no or limited bone preparation techniques for implant insertion.
References
Albrektsson, T., Branemark, P. I., Hansson, H. A. & Lindstrom, J. Osseointegrated titanium implants requirements for ensuring a long-lasting, direct bone-to-implant anchorage in man. Acta. Orthop Scand. 52(2), 155–170 (1981).
Shah, F. A., Thomsen, P. & Palmquist, A. Osseointegration and current interpretations of the bone-implant interface. Acta Biomater. 84, 1–15. https://doi.org/10.1016/j.actbio.2018.11.018 (2019).
Esposito, M., Hirsch, J.M., Lekholm, U.& Thomsen, P. Biological factors contributing to failures of osseointegrated oral implants. (I). Success criteria and epidemiology, Eur. J. Oral Sci. 106 (1), 527–551, https://doi-org.rproxy.tau.ac.il/10.1046/j.0909-8836..t01-2-.x (1998).
Brånemark, R.A. Biomechanical Study of Osseointegration. PhD Thesis, Göteborgs Universitet, Göteborg (1996).
Bernhardt, R., Kuhlisch, E., Matthias, C.S., Eckelt, U.& Stadlinger, B. Comparison of bone-implant contact and bone-implant volume between 2D-Histological sections and 3D-SRμCT slices. Eur. Cells Mater. 23, 237–248, https://doi.org/10.22203/eCM.v023a18 (2012).
Park, Y.S., Yi, K.Y., Lee, I.S.& Jung, Y.C. Correlation between microtomography and histomorphometry for assessment of implant osseointegration. Clin. Oral. Implants Res. 16, 156–160, https://doi-org.rproxy.tau.ac.il/10.1111/j.1600-0501.2004.01083.x (2005).
Alghamdi, H., Anand, P. S. & Anil, S. Undersized implant site preparation to enhance primary implant stability in poor bone density: A prospective clinical study. J. Oral Maxillofac. Surg. 69(12), e506–e512. https://doi.org/10.1016/j.joms.2011.08.007 (2011).
Falisi, G. et al. The effects of surgical preparation techniques and implant macro-geometry on primary stability: An in vitro study. Med. Oral Patol. Oral Cirugia Bucal. 22(2), e201–e206. https://doi.org/10.4317/medoral.21286 (2017).
Guazzi, P., Grandi, T. & Grandi, G. Implant site preparation using a single bur versus multiple drilling steps: 4-month post-loading results of a multicenter randomised controlled trial. Eur. J. Oral Implantol. 8(3), 283–290 (2015).
Stavropoulos, A., Cochran, D., Obrecht, M., Pippenger, B. E. & Dard, M. Effect of osteotomy preparation on osseointegration of immediately loaded, tapered dental implants. Adv. Dent. Res. 28(1), 34–41. https://doi.org/10.1177/0022034515624446 (2016).
Friberg, B. et al. On cutting torque measurements during implant placement: A 3-year clinical prospective study. Clin. Implant. Dent. Relat. Res. 1(2), 75–83 (1999).
Tabassum, A., Meijer, G. J., Walboomers, X. F. & Jansen, J. A. Evaluation of primary and secondary stability of titanium implants using different surgical techniques. Clin. Oral Implants Res. 25(4), 487–492. https://doi.org/10.1111/clr.12180 (2014).
Rea, M. et al. Healing of implants installed in over- or under-prepared sites—An experimental study in dogs. Clin. Oral Implants Res. 26(4), 442–446. https://doi.org/10.1111/clr.12390 (2015).
Smith, A., Hosein, Y. K., Dunning, C. E. & Tassi, A. Fracture resistance of commonly used self-drilling orthodontic mini-implants. Angle Orthod. 85(1), 26–32. https://doi.org/10.2319/112213-860.1 (2015).
Stocchero, M. et al. Biomechanical, biologic, and clinical outcomes of undersized implant surgical preparation: A systematic review. Int. J. Oral. Maxillofac. Implants 31(6), 1247–1263. https://doi.org/10.11607/jomi.5340 (2016).
Shalabi, M. M., Wolke, J. G., De Ruijter, A. J. & Jansen, J. A. Histological evaluation of oral implants inserted with different surgical techniques into the trabecular bone of goats. Clin. Oral Implants Res. 18(4), 489–495. https://doi.org/10.1111/j.1600-0501.2007.01362.x (2007).
Pantani, F. et al. Influence of lateral pressure to the implant bed on osseointegration: An experimental study in dogs. Clin. Oral Implants Res. 21(11), 1264–1270. https://doi.org/10.1111/j.1600-0501.2010.01941.x (2010).
Campos, F. E. et al. Effect of drilling dimension on implant placement torque and early osseointegration stages: An experimental study in dogs. J. Oral Maxillofac. Surg. 70(1), e43-50. https://doi.org/10.1016/j.joms.2011.08.006 (2012).
Al-Marshood, M. M. et al. Study of the osseointegration of dental implants placed with an adapted surgical technique. Clin. Oral Implants Res. 22(7), 753–759. https://doi.org/10.1111/j.1600-0501.2010.02055.x (2011).
Duyck, J. et al. Effect of insertion torque on titanium implant osseointegration: An animal experimental study. Clin. Oral Implants Res. 26(2), 191–196. https://doi.org/10.1111/clr.12316 (2015).
Trisi, P., Todisco, M., Consolo, U. & Travaglini, D. High versus low implant insertion torque: A histologic, histomorphometric, and biomechanical study in the sheep mandible. Int. J. Oral Maxillofac. Implants. 26(4), 837–849 (2011).
Tabassum, A., Meijer, G. J., Walboomers, X. F. & Jansen, J. A. Biological limits of the undersized surgical technique: A study in goats. Clin. Oral Implants Res. 22(2), 129–134. https://doi.org/10.1111/j.1600-0501.2010.02016.x (2011).
Trisi, P., Berardini, M., Falco, A. & Podaliri, V. M. New osseodensification implant site preparation method to increase bone density in low-density bone: in vivo evaluation in sheep. Implant Dent. 25(1), 24–31. https://doi.org/10.1097/ID.0000000000000358 (2016).
Abuhussein, H., Pagni, G., Rebaudi, A. & Wang, H. L. The effect of thread pattern upon implant osseointegration. Clin. Oral Implants Res. 21(2), 129–136. https://doi.org/10.1111/j.1600-0501.2009.01800.x (2010).
Misch, C. Dental Implant Prosthetics 2nd edn. (Elsevier Mosby, St Louis, 2015).
Cohen, O. et al. Differences in crestal bone-to-implant contact following an under-drilling compared to an over-drilling protocol: A study in the rabbit tibia. Clin. Oral Invest. 20(9), 2475–2480. https://doi.org/10.1007/s00784-016-1765-8 (2016).
Cha, D. et al. Multiscale analyses of the bone-implant interface. J. Dent. Res. 94(3), 482–490. https://doi.org/10.1177/0022034514566029 (2015).
Stocchero, M. et al. Interosseous temperature change during installation of dental implants with two different surfaces and different drilling protocols: An in vivo study in sheep. J. Clin. Med. 8(8), 1198. https://doi.org/10.3390/jcm8081198 (2019).
O’Sullivan, D., Sennerby, L. & Meredith, N. Influence of implant taper on the primary and secondary stability of osseointegrated titanium implants. Clin. Oral Implants Res. 15(4), 474–480. https://doi.org/10.1111/j.1600-0501.2004.01041.x (2004).
Acknowledgements
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Author information
Authors and Affiliations
Contributions
O.Z. conceived the idea, designed the experiments, carried out the experimental work and was involved in the composition of the manuscript. F.M., M.I., B.A. & and M.M. wrote the manuscript and contributed to data analysis. All authors involved in the discussion to finalize the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Folkman, M., Becker, A., Meinster, I. et al. Comparison of bone-to-implant contact and bone volume around implants placed with or without site preparation: a histomorphometric study in rabbits. Sci Rep 10, 12446 (2020). https://doi.org/10.1038/s41598-020-69455-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-69455-4
- Springer Nature Limited