Abstract
The mutational spectrum and prognostic factors of NRAS-mutated (NRASmut) acute myeloid leukemia (AML) are largely unknown. We performed next-generation sequencing (NGS) in 1,149 cases of de novo AML and discovered 152 NRASmut AML (13%). Of the 152 NRASmut AML, 89% had at least one companion mutated gene. DNA methylation-related genes confer up to 62% incidence. TET2 had the highest mutation frequency (51%), followed by ASXL1 (17%), NPM1 (14%), CEBPA (13%), DNMT3A (13%), FLT3-ITD (11%), KIT (11%), IDH2 (9%), RUNX1 (8%), U2AF1 (7%) and SF3B1(5%). Multivariate analysis suggested that age ≥ 60 years and mutations in U2AF1 were independent factors related to failure to achieve complete remission after induction therapy. Age ≥ 60 years, non-M3 types and U2AF1 mutations were independent prognostic factors for poor overall survival. Age ≥ 60 years, non-M3 types and higher risk group were independent prognostic factors for poor event-free survival (EFS) while allogenic hematopoietic stem cell transplantation was an independent prognostic factor for good EFS. Our study provided new insights into the mutational spectrum and prognostic factors of NRASmut AML.
Similar content being viewed by others
Introduction
Over the last two decades, our understanding of the molecular heterogeneity of acute myeloid leukemia (AML) has made significant advances through genomic discovery studies utilizing microarray and next-generation sequencing (NGS)- based “-omics ” technologies1. RAS oncogenes play important roles in diverse cellular events such as cell cycle, cell differentiation and survival2. RAS malfunction is strongly related to tumorigenesis and thus regarded as an important therapeutic target3. Mutations in the RAS genes (including KRAS, NRAS and HRAS) are discovered in 30% of all tumors4. KRAS is the most frequently mutated gene in cancers found in pancreatic (90%), colon (45%) and lung (35%), while NRAS mutations are more common in AML (10%)4,5.
Until now, the prognostic value of NRAS mutations in AML remains inconclusive. Recently, an integrated meta-analysis revealed that NRAS mutations did not influence the overall survival for adults with AML6. However, most of these reports evaluated NRAS in a binary fashion. The significance of variant allele frequency (VAF) of NRAS mutation at diagnosis, and the effect of companion gene mutations (co-mutations) in NRAS-mutated (NRASmut) AML are warranted for extensive evaluation. In this study, we examined patient outcomes in a series of NRASmut de novo AML cases in terms of co-mutations and the NRAS VAF at diagnosis.
Subjects and methods
Patients
NGS was performed in 1,149 cases of de novo AML at the First Affiliated Hospital of Zhengzhou University between June 2016 and September 2019. One hundred and fifty-two cases with NRASmut AML were screened out and enrolled in the study. The diagnosis and classification of AML were based on the WHO 2016 edition of classification of myeloid neoplasms and acute leukemia7. Patients were divided into good, intermediate and poor risk group according to “Chinese Guidelines for Diagnosis and Treatment of Adult Acute Myeloid Leukemia (Not APL) (2017)”8,9. The study was approved by the Ethics Committee of the First Affiliated Hospital of Zhengzhou University. Informed consent was obtained from all patients or their statutory guardian following the Declaration of Helsinki.
Treatment protocols
For M3 patients, all-trans retinoic acid and arsenic trioxide-based chemotherapy was given for the induction and consolidation therapy. Non-M3 patients received induction chemotherapy regimens include DA, IA, and MA regimens: a standard dose of cytarabine (Ara-C; 100 mg/m2/ day for 7 days) combined with daunorubicin (45 mg/m2/day for 3 days) or idarubicin (10 mg/m2/day for 3 days) or mitoxantrone (10 mg/m2/day for 3 days). After remission, young patients were consolidated with cytarabine (2–3 g/m2, once every 12 h for 3 days) based chemotherapy. For elderly patients, the chemotherapy consolidation was decided by the physicians in an individualized manner. A total of 24 patients underwent allogenic hematopoietic stem cell transplantation (allo-HSCT). Therapy recommendation was based on risk stratification and the results of minimal residual disease testing after 1–2 cycles of consolidation chemotherapy. The real treatment selection was based on both the physician’s recommendation and the patient’s preference. The last follow-up for surviving patients was conducted in December 2019.
Cytogenetics and fusion genes analysis
Cytogenetic analyses were performed by G-banding analysis according to the International System for Human Cytogenetic Nomenclature. Forty-three fusion genes including MLL-(AF4, AF6, AF9, AF10, AF17, AF1q, AF1p, AFX, ELL, SEPT6, ENL), NUP98-(HoxA9, HoxC11, HoxA11, HoxD13, HoxA13, PMX1), (NPM, FIP1L1, PML, PRKAR1A, STAT5b, NUMA1, PLZF)-RARα, (ETV6, FIP1L1)-PDGFRA, AML1-(ETO, MTG16, MDS1/EVI1), TEL-(JAK2, AML1, ABL), NPM-(ALK, MLF), (DEK, SET)-CAN, SIL-TAL1, E2A-HLF, TEL-PDGFRB, TLS-ERG, CBFβ-MYH11, E2A-PBX1 and BCR-ABL were detected with real-time PCR (RT-PCR) using Multiplex RT-PCR Fusion Gene Kits (Rightongene, Shanghai, China).
Next generation sequencing
We sequenced the mutational hotpots or whole coding regions of 22 genes (including FLT3, NPM1, KIT, CEBPA, DNMT3A, IDH1, IDH2, TET2, EZH2, RUNX1, ASXL1, PHF6, TP53, SF3B1, SRSF2, U2AF1, ZRSR2, NRAS, CBL, SETBP1, ETV6, and JAK2) with the standard NGS technology. The detection was based on a Illumina MiSeq System (Illumina, San Diego, CA) high-throughput sequencing platform by using a Rightongene AML/MDS/MPN Sequencing Panel (Rightongene, Shanghai, China). Details of the variant calling, filtering, and annotation are described in our recently published reports10.
Statistical analysis
Analyses were performed using SPSS software version 20.0 (Chicago, IL, USA) and Graphpad Prism™ 5.01 (San Diego, California, USA). Differences across groups were compared using the Pearson Chi-square analysis or Fisher exact test for categorical variables, and Mann–Whitney U test for continuous variables. Overall survival (OS) is defined as the time from diagnosis to death or the time of the last follow-up. Event-free survival (EFS) is defined as the time from diagnosis to relapse, death, or the time of the last follow-up. Survival analysis was estimated by Kaplan–Meier method and compared by the log-rank test. Multivariable analysis including variables with P < 0.10 in univariate analysis were performed for complete remission (CR), OS and EFS. P < 0.05 was considered to indicate statistical significance.
Results
Clinical and biological characteristics of NRAS mut AML
In the total cohort, NRAS mutations were found in 13% (152 of 1,149) cases. As shown in Table 1, median age was 44 (range 14–78) years, with 25 cases (16%) older than 60 years. Half of the cases were men. Twelve cases (8%) were M3 and 140 cases were non-M3 AML. The median white blood cell (WBC) count at diagnosis was 31 × 109/L, and in 27 cases (18%) it was ≥ 100 × 109/L. Forty-five cases (30%) had a bone marrow blast percentage of more than 80%. Forty-one cases (27%) was good-risk AML, 64 (42%) was intermediate-risk AML and 47 (31%) was poor-risk AML. Twenty-four cases (16%) received allogenic hematopoietic stem cell transplantation (allo-HSCT). Thirty-six cases failed to achieve CR after induction therapy and 61 cases died at the end of follow up. Thirty-five cases (16%) had more than two other recurrent genetic mutations. Forty-three fusion genes were detected in 135 cases and 16 cases were AML1-ETO positive; 15 cases were MYH11-CBFβ positive and 7 cases were MLL positive.
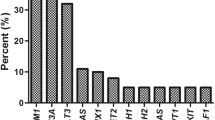
Most NRAS mutations (88 of 152; 57.9%) were found at codon 12. Mutations at codon 13 were found in 54 (35.5%) of 152 cases. The most frequent changes were from glycine to asparagine (codon 12: G12D, 59 of 152, 38.8%; codon 13: G13D, 44 of 152, 28.9%; Fig. 1). Mutations at codon 61 were detected in 31 (20.4%) of 152 cases, mostly from glycine to arginine (Q61R, 14 of 152, 9.2%; Fig. 1). It is worth noting that NRAS mutations at codons 146 which was a noncanonical N-RAS mutation were detected in one case. NRAS mutation types were divided into G12/13, Q61 and mix (G12/13 and Q61), with G12/13 accounting for 79% of the cases (Table 1). The median VAF of NRAS was 15% (range 1–59%).
NRAS mutations at codon 12, 13 and 61 of 152 de novo AML patients. Distribution and frequencies are given for NRAS mutations at codon 12, 13 and 61. The boxes in one column represent single patient cases. Mutations were color coded by mutation type. The histogram on the right showed the frequency distribution of all aberrations.
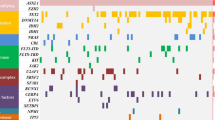
Companion gene mutations in NRAS mut AML
One hundred and thirty-five cases (89%) had at least one co-mutation besides NRAS. Fifty-four cases had one co-mutation, 46 cases with 2, 22 cases with 3, 10 cases with 4 and 3 cases with 5. As shown in Fig. 2, TET2 had the highest mutation frequency (51%), followed by ASXL1 (17%), NPM1 (14%), CEBPA (13%), DNMT3A (13%), FLT3-ITD (11%), KIT (11%), IDH2 (9%), RUNX1 (8%), U2AF1 (7%) and SF3B1(5%). Other mutated genes (including CBL, IDH2, EZH2, ETV6, SETBP1, FLT3-TKD, SRSF2, TP53, PHF6) are less than 5% in NRASmut AML; JAK2 and ZRSR2 mutations are absent in NRASmut AML. These gene mutations are further classified into functional groups as previously described: RAS pathway (100%)-NRAS and CBL; DNA methylation (62%)—TET2, DNMT3A and IDH1/2; chromatin modifying (18%)—ASXL1, EZH2; transcription factors (22%)-CEBPA, RUNX1, ETV6 and SETBP1; Tyrosine kinase (7%)—FLT3-ITD/TKD, KIT and JAK2; Spliceosome complex (12%)- U2AF1, SF3B1, SRSF2 and ZRSR2; Tumor suppressor (2%)-TP53 and PHF6; NPM1 gene (14%)-NPM1.
Response to induction therapy
One hundred and sixteen cases achieved CR after 1–3 cycles of induction therapy while 36 cases didn’t achieve CR. We validated the prognostic value of clinical variables and other genetic mutations in response to induction therapy. Comparison analysis was conducted considering variables such as gender (female vs. male), age (≥ 60 vs. < 60 years), AML types (Non-M3 vs. M3), NRAS type (mix vs. Q61 vs. G12/G13), NRAS VAF (≥ 15% vs. < 15%), WBC counts (≥ 100 vs. < 100 × 109/L), HGB counts (≥ 110 vs. < 110 g/L), PLT counts (≥ 100 vs. < 100 × 109/L), bone marrow blasts (≥ 80% vs. < 80%), peripheral blood blasts (≥ 20% vs. < 20%), number of co-mutations (≥ 3 vs. < 3), allo-HSCT (yes vs. no), risk group (high vs. inter vs. low -risk), ETO (positive vs. negative), MYH11-CBFβ (positive vs. negative), and the mutation status of other common AML co-mutations. In univariate analysis, it was shown that age ≥ 60 years, higher risk group, U2AF1 mutations and SF3B1 mutations were associated with lower CR rate (Table 2). While other factors were not associated with the induction outcome of the NRASmut AML patients (Table 2). In multivariate analysis, it was shown that age ≥ 60 years and U2AF1 mutations were independent prognostic factors for response to induction therapy (Table 2).
Comparison of OS and EFS between different clinical and molecular characteristic groups
Comparison analysis of EFS and OS was conducted considering variables such as different gender (female vs. male), age (≥ 60 vs. < 60 years), AML types (Non-M3 vs. M3), NRAS type (mix vs. Q61 vs. G12/G13), NRAS VAF (≥ 15% vs. < 15%), WBC counts (≥ 100 vs. < 100 × 109/L), HGB counts (≥ 110 vs. < 110 g/L), PLT counts (≥ 100 vs. < 100 × 109/L), bone marrow blasts (≥ 80% vs. < 80%), peripheral blood blasts (≥ 20% vs. < 20%), number of co-mutations (≥ 3 vs. < 3), allo-HSCT (yes vs.no), risk group (high vs. inter vs. low -risk), ETO (positive vs. negative), MYH11-CBFβ (positive vs. negative), and the mutation status of other common AML co-mutations. The median follow-up time was 294 (5–1,219) days. As shown in Table 3, older cases (age ≥ 60 years) had shorter OS and EFS (P = 0.000, P = 0.000, respectively; Fig. 3a). M3 cases had longer OS and EFS (P = 0.030, P = 0.008, respectively). Higher risk group was associated worse OS and EFS (P = 0.002, P = 0.007, respectively; Fig. 3b). Cases who accepted allo-HSCT had longer OS and EFS (P = 0.016, P = 0.001, respectively; Fig. 3c). Presence of U2AF1 was associated with shorter OS and EFS (P = 0.000, P = 0.000, respectively; Fig. 3d). Presence of RUNX1 and SF3B1 was associated with shorter OS (P = 0.014, P = 0.032, respectively). Number of co-mutations ≥ 3 and presence of IDH2 was associated with shorter EFS (P = 0.025, P = 0.043, respectively). However, both NRAS type and NRAS VAF had no effect on EFS and OS.
Kaplan–Meier estimate of overall survival (OS) and event-free survival (EFS) in 152 NRASmut AML. OS and EFS were compared in (a) patients older than 60 years and patients younger than 60 years (b) different risk groups (c) patients who accepted allo-HSCT or not (d) patients with U2AF1 mutations or not.
Evaluation of possible prognostic factors
Multivariate analysis of factors related to OS included age (≥ 60 vs. < 60 years), AML types (non-M3 vs. M3), risk group (high vs. inter vs. low-risk), the time-dependent variable for allo-HSCT (yes vs.no), ASXL1 (mutated vs. wild type), RUNX1 (mutated vs. wild type), U2AF1 (mutated vs. wild type) and SF3B1 (mutated vs. wild type). As shown in Table 4, independent prognostic factors for poor OS included age ≥ 60 years, non-M3 types and U2AF1 mutations.
Multivariate analysis of factors related to EFS included age (≥ 60 vs. < 60 years), AML types (Non-M3 vs. M3), risk group (high vs. inter vs. low-risk), the time-dependent variable for allo-HSCT (yes vs.no), number of co-mutations (≥ 3 vs. < 3), IDH2 (mutated vs. wild type), RUNX1 (mutated vs. wild type) and U2AF1 (mutated vs. wild type). As shown in Table 4, age ≥ 60 years, non-M3 types and higher risk group were independent prognostic factors for poor EFS while allo-HSCT was an independent prognostic factor for good EFS.
Discussion
High frequencies of NRAS mutations had been seen in AML patients11, indicating its important function in the pathogenesis and progression of AML. Although the prognostic value of NRAS mutations in AML patients remains inconclusive6,12, several large cohort studies indicated that NRAS mutations in AML did not influence the prognosis of patients11,13,14. A recently published meta-analysis also suggested that NRAS oncogene mutations were not correlated with the prognosis of patients with AML6. However, given the prevalence of NRAS mutations in AML, there is urgently need to explore the clinical characteristics, companion gene mutations and possible prognostic factors of NRASmut AML patients to provide evidence for clinical stratified diagnosis and treatment.
Our data showed that NRAS mutations were found in 13% of cases, which is consistent with findings in other literature that showed a range of 9.7% to 13.9% NRAS mutations11,14,15,16. The median age of NRASmut AML cases was 44 years and the median WBC counts was 31 × 109/L, which was consistent with a large cohort study in China in 201314. In our study, most NRAS mutations were found at codon 12 and the most frequent change were from glycine to asparagine, which was supported by previous reports11,17. Interestingly, we found that some samples have two NRAS mutations such as Q61K + Q61R, which have been confirmed by Sanger sequencing. This situation is very rare, it is not ruled out that two mutations have occurred in the same gene, but it may also be caused by mutations in the same allele. Nearly 90% of the cases had at least one companion gene mutation, which suggests that the molecular mechanism of patients with NRASmut AML is complicated, and multiple molecular interactions may exist. However, previous studies often focused on comparing the difference between NRASmut and NRAS wild-type patients11,14, with little attention on the molecular mutation spectrum. We observed that mutations of DNA methylation-related genes occurred in 62% NRASmut AML, the most common of which is TET2. This indicated that DNA methylation may play an important role in the pathogenesis in NRASmut AML and provided a basis for demethylation treatment of NRASmut AML patients.
AML in older patients generally had poorer prognosis due to poorer baseline performance status, and co-morbidities18. In our cohort of NRASmut AML, age ≥ 60 years also had a negative impact on both response to induction therapy and survival. Allogeneic HSCT which was usually considered the cure for AML, also showed survival benefit in our study. Traditional risk stratification schemes based on genetics and molecular biology were still applicable in patients with NRASmut AML and could well predict patients’ prognosis. Mutation Gene VAF of FLT3-ITD or NPM1 was reported to be significantly correlated with prognosis of AML19,20. However, we found that NRAS VAF had no correlation with either response to induction therapy or survival. FLT3-ITD was associated with increased risk of relapse while NPM1, AML1-ETO, MYH11-CBFβ were good prognostic factors18. In our study, however, recurrent genetic mutations including FLT3-ITD, NPM1, DNMT3A, TET2 and KIT and fusion genes including AML1-ETO and MYH11-CBFβ were not associated with survival. The discrepancy may be related to possible interplay of mutated genes.
U2AF1 belongs to mutations in splicing factor (SF) genes. Mutations in U2AF1 is a poor prognostic indicator in myelodysplastic syndrome21. Recently many studies proved that mutations in U2AF1 predict poor prognosis in patients with de novo AML22,23,24,25. Our study showed that U2AF1 was also an independent poor prognostic factor for survival in NRASmut AML patients. In a large study of 664 AML patients conducted by the German AML Cooperative Group, mutations in U2AF1 were one of the independent risk factors for achievement of CR126. Similar to this result, in our study, 90% U2AF1-mutated AML patients failed to achieve complete remission.
The limitations to our study need to be acknowledged. First, our study was retrospective and susceptible to selection biases. Second, some gene mutations may not be detected due to the limitation of technique. Prognostic effects of some important gene mutations may be ignored. Third, whether these findings are restricted to NRASmut AML need to be further explored by parallel comparison with non NRASmut AML. Fourth, the small sample sizes of some subgroups resulted in relatively low statistical power and the univariate analyses were not adjusted for multiple comparisons which may result in false positive results, especially in small subgroups. Because of these limitations, our conclusion needs validation in a larger and prospective cohort.
In conclusion, our study provided new insights into the mutational spectrum and prognostic factors of NRASmut AML. These individuals companied with U2AF1 mutations experienced poor responses to chemotherapy and the mechanisms need to further evaluate. More detailed mutational spectrum information and large prospective studies are needed in the future for better prognostication of NRASmut AML.
References
Bullinger, L., Döhner, K. & Döhner, H. Genomics of acute myeloid leukemia diagnosis and pathways. J. Clin. Oncol. 35, 934–946. https://doi.org/10.1200/JCO.2016.71.2208 (2017).
Renneville, A. et al. Cooperating gene mutations in acute myeloid leukemia: a review of the literature. Leukemia 22, 915–931. https://doi.org/10.1038/leu.2008.19 (2008).
Cho, H., Shin, I., Ju, E., Choi, S. & Hur, W. First SAR study for overriding NRAS mutant driven acute myeloid. Leukemia 61, 8353–8373. https://doi.org/10.1021/acs.jmedchem.8b00882 (2018).
Wilson, C. Y. & Tolias, P. Recent advances in cancer drug discovery targeting RAS. Drug Discov. Today 21, 1915–1919. https://doi.org/10.1016/j.drudis.2016.08.002 (2016).
Nonami, A. et al. Identification of novel therapeutic targets in acute leukemias with NRAS mutations using a pharmacologic approach. Blood 125, 3133–3143. https://doi.org/10.1182/blood-2014-12-615906 (2015).
6Liu, X. et al. RAS mutations in acute myeloid leukaemia patients: a review and meta-analysis. Clin. Chim. Acta Int .J. Clin. Chem. 489, 254–260, https://doi.org/10.1016/j.cca.2018.08.040 (2019).
Arber, D. A. et al. The 2016 revision to the World Health Organization classification of myeloid neoplasms and acute leukemia. Blood 127, 2391–2405. https://doi.org/10.1182/blood-2016-03-643544 (2016).
[Chinese guidelines for diagnosis and treatment of adult acute myeloid leukemia (not APL) (2017)]. Zhonghua Xue Ye Xue Za Zhi 38, 177–182, https://doi.org/10.3760/cma.j.issn.0253-2727.2017.03.001 (2017).
Papaemmanuil, E. et al. Genomic classification and prognosis in acute myeloid leukemia. N. Engl. J. Med. 374, 2209–2221. https://doi.org/10.1056/NEJMoa1516192 (2016).
Yu, J. et al. Gene mutational analysis by NGS and its clinical significance in patients with myelodysplastic syndrome and acute myeloid leukemia. Exp. Hematol. Oncol. 9, 2. https://doi.org/10.1186/s40164-019-0158-5 (2020).
Bacher, U., Haferlach, T., Schoch, C., Kern, W. & Schnittger, S. Implications of NRAS mutations in AML: a study of 2502 patients. Blood 107, 3847–3853 (2006).
De Melo, M. B., Lorand-Metze, I., Lima, C. S., Saad, S. T. & Costa, F. F. N-ras gene point mutations in Brazilian acute myelogenous leukemia patients correlate with a poor prognosis. Leukemia Lymphoma 24, 309–317 (1997).
Bowen, D. T. et al. RAS mutation in acute myeloid leukemia is associated with distinct cytogenetic subgroups but does not influence outcome in patients younger than 60 years. Blood 106, 2113–2119 (2005).
Yang, X. et al. RAS mutation analysis in a large cohort of Chinese patients with acute myeloid leukemia. Clin. Biochem. 46, 579–583. https://doi.org/10.1016/j.clinbiochem.2012.12.022 (2013).
Neubauer, A. et al. Prognostic importance of mutations in the ras proto-oncogenes in de novo acute myeloid leukemia. Blood 83, 1603–1611 (1994).
Kiyoi, H. et al. Prognostic implication of FLT3 and N-RAS gene mutations in acute myeloid leukemia. Blood 93, 3074–3080 (1999).
Reuter, C. W. M. et al. Lack of noncanonical RAS mutations in cytogenetically normal acute myeloid leukemia. Ann. Hematol. 93, 977–982. https://doi.org/10.1007/s00277-014-2061-9 (2014).
Döhner, H., Weisdorf, D. J. & Bloomfield, C. D. Acute myeloid leukemia. N. Engl. J. Med. 373, 1136–1152. https://doi.org/10.1056/NEJMra1406184 (2015).
Patel, S. S. et al. High NPM1-mutant allele burden at diagnosis predicts unfavorable outcomes in de novo AML. Blood 131, 2816–2825. https://doi.org/10.1182/blood-2018-01-828467 (2018).
Schlenk, R. F. et al. Differential impact of allelic ratio and insertion site in FLT3-ITD-positive AML with respect to allogeneic transplantation. Blood 124, 3441–3449. https://doi.org/10.1182/blood-2014-05-578070 (2014).
Thol, F. et al. Frequency and prognostic impact of mutations in SRSF2, U2AF1, and ZRSR2 in patients with myelodysplastic syndromes. Blood 119, 3578–3584. https://doi.org/10.1182/blood-2011-12-399337 (2012).
Hou, H.-A. et al. Splicing factor mutations predict poor prognosis in patients with de novo acute myeloid leukemia. Oncotarget 7, 9084–9101. https://doi.org/10.18632/oncotarget.7000 (2016).
Hamilton, B. K. et al. Impact of allogeneic hematopoietic cell transplant in patients with myeloid neoplasms carrying spliceosomal mutations. Am. J. Hematol. 91, 406–409. https://doi.org/10.1002/ajh.24306 (2016).
Saygin, C. et al. Mutations in DNMT3A, U2AF1, and EZH2 identify intermediate-risk acute myeloid leukemia patients with poor outcome after CR1. Blood Cancer J. 8, 4. https://doi.org/10.1038/s41408-017-0040-9 (2018).
Ohgami, R. S. et al. Next-generation sequencing of acute myeloid leukemia identifies the significance of TP53, U2AF1, ASXL1, and TET2 mutations. Mod. Pathol. 28, 706–714. https://doi.org/10.1038/modpathol.2014.160 (2015).
Metzeler, K. H. et al. Spectrum and prognostic relevance of driver gene mutations in acute myeloid leukemia. Blood 128, 686–698. https://doi.org/10.1182/blood-2016-01-693879 (2016).
Acknowledgements
We thank all the treating physicians for allowing us to enroll their patients and the patients for allowing us to analyze their data. This work was supported by the National Natural Science Foundation of China [Grant Numbers 81800137 and U1804191].
Author information
Authors and Affiliations
Contributions
Y.F.L. and C.W. designed the project and prepared the typescript. S.J.W. and Z.Z.W. collected the data and performed statistical analyses. T.L. and Y.F.L. performed N.G.S. W.Q.W., Q.Q.H., X.S.X., D.M.W. and Z.X.J. provided clinical data. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wang, S., Wu, Z., Li, T. et al. Mutational spectrum and prognosis in NRAS-mutated acute myeloid leukemia. Sci Rep 10, 12152 (2020). https://doi.org/10.1038/s41598-020-69194-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-69194-6
- Springer Nature Limited
This article is cited by
-
Prioritizing cervical cancer candidate genes using chaos game and fractal-based time series approach
Theory in Biosciences (2024)
-
Anti-leukemia effects of omipalisib in acute myeloid leukemia: inhibition of PI3K/AKT/mTOR signaling and suppression of mitochondrial biogenesis
Cancer Gene Therapy (2023)
-
Immortalization-upregulated protein promotes pancreatic cancer progression by regulating NPM1/FHL1-mediated cell-cycle-checkpoint protein activity
Cell Biology and Toxicology (2023)
-
Droplet digital PCR for genetic mutations monitoring predicts relapse risk in pediatric acute myeloid leukemia
International Journal of Hematology (2022)