Abstract
Yellow plumage is common in chickens, especially in breeds such as the Huiyang Bearded chicken, which is indigenous to China. We evaluated plumage colour distribution in F1, F2, and F3 populations of an Huiyang Bearded chicken × White Leghorn chicken cross, the heredity of the yellow plumage trait was distinguished from that of the gold plumage and other known plumage colours. Microscopic analysis of the feather follicles indicated that pheomelanin particles were formed in yellow but not in white feathers. To screen genes related to formation of the pheomelanin particles, we generated transcriptome data from yellow and white feather follicles from 7- and 11-week-old F3 chickens using RNA-seq. We identified 27 differentially expressed genes (DEGs) when comparing the yellow and white feather follicles. These DEGs were enriched in the Gene Ontology classes ‘melanosome’ and ‘melanosome organization’ related to the pigmentation process. Down-regulation of TYRP1, DCT, PMEL, MLANA, and HPGDS, verified using quantitative reverse transcription PCR, may lead to reduced eumelanin and increased pheomelanin synthesis in yellow plumage. Owing to the presence of the Dominant white locus, both white and yellow plumage lack eumelanin, and white feathers showed no pigments. Our results provide an understanding of yellow plumage formation in chickens.
Similar content being viewed by others
Introduction
Plumage colour in birds, coat colour in mammals, and skin colour in humans have long been the focus of pigment research. Among these, birds display the most fascinating and complex colouration, which has been shown to be caused by melanin (eumelanin and pheomelanin), carotenoids, porphyrins, polyenes and structural colours1,2. In general, the formation of different plumage colours in chickens is mainly attributed to variations in the quantity, proportion and location of eumelanin and pheomelanin in the feather3. Eumelanin makes plumage appear black and dark brown, whereas pheomelanin makes it appear red and yellow4. Both eumelanin and pheomelanin are indole-polymers with tyrosine as a precursor5. During eumelanin synthesis, tyrosinase (TYR) catalyses the hydroxylation of tyrosine to 3,4-dihydroxyphenylalanine (dopa) and the oxidation of dopa to dopaquinone, which is then oxidized to form eumelanin; this occurs via the TYR family, involving TYR, tyrosinase-related protein 1 (TYRP1), and dopachrome tautomerase (DCT). Dopaquinone is transformed into 5-S-cysteinyldopa when cysteine or glutathione provides sulfhydryl; it eventually forms pheomelanin5.
In recent decades, with the rapid development of sequencing technology, significant progress has been made in the study of functional genes related to plumage colour in chickens. Various plumage colour causal genes, such as the Dominant white gene (I), recessive white gene (c), Silver gene (S), molted gene (mo) and Sex-linked barring gene (B), have been cloned6,7,8,9,10,11. Especially for Dominant white allele (I), a strong dilutor of eumelanin owing to an insertion in the transmembrane region of the premelanosome protein (PMEL), it has no effect on the pigmentation of pheomelanin6,12,13. Therefore, it is widely used in the breeding of white-feathered chickens. In addition, many genes, such as those in the tyrosinase (TYR) gene family, as well as melanocortin 1 receptor (MC1R) and melanogenesis associated transcription factor (MITF), have been found to play important roles in melanin synthesis6,14,15. Mutations in the PMEL, solute carrier family 45 member 2 (SLC45A2), and cyclin-dependent kinase inhibitor 2A (CDKN2A) can alter melanin content, generating specific plumage colours6,9,11. The formation of plumage colour is also influenced by the expression of various genes related to pigment formation in feather follicles during the growth period16. As the pivotal position for feather formation and melanogenesis, transcriptome profiling of the feather follicle has been performed to elucidate the process of morphogenesis and to identify candidate genes of diverse feather shapes and colours17,18,19,20,21,22,23.
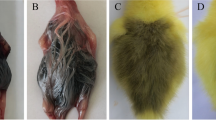
Yellow plumage is common in Chinese indigenous chicken populations. It is considered a marker of high-quality meat-type chickens, represented by Huiyang Bearded chickens (HB), which are very popular in the Chinese consumer market. The plumage colour (Fig. 1) is light yellow and is distinct from that of the commercial strain (Rhode Island Red chicken) with gold plumage by visual observation24,25. A causal mutation responsible for a similar yellow plumage phenotype (an 8.3 kb deletion upstream of the SOX10 gene) has been reported in chickens26. Polymorphisms in MC1R are related to plumage colour variations in Golden duckwing Araucana chickens27. A non-synonymous T-to-C substitution in TYRP1 is a causal mutation leading to a roux mutation in Japanese quail28. However, none of the above mutations were found in the HB population, which suggests that the formation mechanisms of yellow plumage of Chinese native chicken was different from the previously studies.
The exterior and plumage colour of Huiyang Bearded chicken29. This image cited from the Animal Genetic Resources in China Indigenous Breeds provided by China National Commission of Animal Genetic Resources.
In this study, a hybrid population of HB and White Leghorn chickens (WL) was established to observe the inheritance of yellow plumage. In the offspring population, the Dominant white allele was found to have no epistatic effect on yellow pluamge, which is different from brown, red, and other known plumage colours. Therefore, we collected the feather follicles of yellow and white plumage chickens (both with the Dominant white allele), and performed dynamic transcriptome profiling to identify candidate genes for yellow plumage in chickens. This study provides a foundation for plumage colour selection in chicken breeding.
Results
Plumage colour distribution in experimental chickens
The Huiyang Bearded chicken, which is native to the Guangdong province, China, has yellow plumage in both sexes (Fig. 1)29. The White Leghorn chicken, which is homozygous for the Dominant white gene, has white plumage. In the F1 population (684 cocks and 685 hens) bred from crossing male HB with female WL chickens, all cocks had white plumage; among the hens, plumage colours were distributed as follows: 5% white, 5% grey, 45% yellow, and 45% grey plume yellow head. The F2 population had the following plumage colour distribution in both cocks and hens: white (49%), yellow (28%), grey (12%), black (1%) and black and white barred (10%) (Supplementary Figure S1, Supplementary Table S1). Only the yellow plumage breeders of F2 were selected to reproduce an F3 population, of which 37% had white plumage.
Genetic analysis of the yellow plumage trait
All F0 HB cocks had yellow plumage, with the Silver locus (ss) and Dominant white locus (ii) genotypes, whereas all F0 WL hens were white, because they had the Dominant white locus (II) and Silver locus (S/W) genotypes. In the F1 hens, the Dominant white locus had the Ii genotype, and the Silver locus had the s/W genotype. However, a high proportion of yellow plumage individuals was observed among the F1 hens, which indicates that the gene controlling yellow plumage had an epistatic effect on the Dominant white gene. The presence of multiple plumage colours in F1 hens also suggests that the yellow plumage gene may be influenced by other plumage colour genes. In F1 cocks, the Dominant white locus had the Ii genotype and the Silver locus had the Ss genotype; the fact that all of the F1 cocks had white plumage indicates that the Silver gene had epistatic effects on the yellow plumage gene (Supplementary Figure S2). In both F1 and F2 individuals with white plumage, the frequency of the recessive white locus (cc) was zero, indicating that their white plumage was not caused by the recessive white allele (c). The emergence of black and white barred individuals in the F2 population was verified to be caused by the Sex-linked barring (B) allele. Interestingly, we found white barring on a yellow background in a few light yellow plumage chickens, similar to the black and white barred pattern; the presence of the B allele was also identified in these individuals. This shows that a yellow and white barred pattern plumage can occur when both B and yellow plumage alleles exist. In summary, these results suggest that the yellow plumage gene had an epistatic effect on the Dominant white gene, whereas the yellow plumage gene was hypostatic to the Silver gene; further, they suggest that the effects of the yellow plumage gene and Sex-linked barring gene are independent of one another.
We then identified the Dominant white and Silver loci in yellow and white plumage individuals in the F3 population. Six yellow and six white plumage individuals were sampled for RNA-seq analysis. They were homozygous in the recessive white locus (CC) and Silver locus (s/W), and homozygous (II) or heterozygous (Ii) in the Dominant white locus (Supplementary Table S2). This indicates that the phenotypic differences between the yellow and white plumage chickens were not due to these gene loci.
Pigment deposition via histological analysis of feather follicles
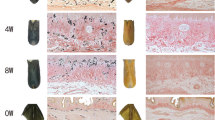
Histological sections of newly emerged yellow and white feather follicles revealed that they had similar cellular composition, with abundant keratinocytes and dermal cells. However, yellowish-brown particles were present in the yellow feather follicles, but not in white feather follicles (Fig. 2). As pheomelanin is the only type of melanin that can make a feather appear yellow, our preliminary conclusion was that yellow plumage colour was caused by increased pheomelanin production. As expected, eumelanin was not observed in the feather follicles of either plumage colour, because of the presence of the Dominant white locus (I).
Characterisation of yellow and white phenotypes. (a) Feather follicles from yellow plumage types. (b) Feather follicles from white plumage types. (c–f) Haematoxylin-stained sections of newly emerged feather follicles from yellow (c, e) and white (d, f) chicken. Magnified area in (c, d) are shown in (e, f), respectively. Scale bar in (c, d) 300 μm. Scale bar in (e, f) 50 μm.
Summary of RNA sequencing data
Twelve libraries were sequenced from colourless RNA solutions obtained from samples of 12 individuals (white plumage from three 7-week-old chickens: 7W1, 7W2, and 7W3; yellow plumage from three 7-week-old chickens: 7Y1, 7Y3, and 7Y4; white plumage from three 11-week-old chickens: 1W1, 1W3, and 1W4; and yellow plumage from three 11-week-old chickens: 1Y1, 1Y3, and 1Y4); 140.7 Gb of data was obtained. The mapping rate of the filtered sequencing data to the reference genome exceeded 80%, and the GC content per library was more than 50% (Supplementary Table S3). For the uniquely aligned sequences in the gene annotation files, the numbers and proportions of three functional elements (mRNA, introns and intergenic regions) were obtained: 76.37% ± 2.46% reads per sample were aligned to the mRNA region (Supplementary Figure S3).
In total, 10,036–10,070 expressed genes were detected. On average, 52.68% of the expressed genes had low expression levels (fragments per kilobase million mapped (FPKM) < 1) in the feather follicles. A few genes had extremely high expression levels (FPKM ≥ 500); these accounted for 1.65% of the total number (Supplementary Figure S4).
Differentially expressed genes between the yellow and white feather follicles
Comparing the gene expression levels between yellow and white feather follicles, 177 differentially expressed genes (DEGs) were obtained in the 7-week age class and 163 DEGs in the 11-week age class (Fig. 3, Supplementary Table S4). Gene Ontology (GO) pathway enrichment analysis revealed the DEGs in the 7-week age class were mainly enriched in the GO classes ‘melanosome’, ‘lysosomal membrane’, and ‘early endosome’. Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway enrichment analysis for the 7-week age class found that ‘melanogenesis’ and ‘metabolic pathways’ were enriched. The DEGs in the 11-week age class were mainly enriched in the GO classes ‘melanosome’, ‘melanosome organization’, and ‘integral component of plasma membrane’(Table 1).
There were 27 overlapping DEGs that occurred in both age groups (Supplementary Figure S5, Supplementary Table S4). These were significantly enriched in the two pigment-related GO classes ‘melanosome’ and ‘melanosome organization’. The genes involved included PMEL, TYRP1 and MLANA (Table 1). Another overlapping DEG, HPGDS, which is involved in catalysing conjugation reactions with glutathione, might play an important role in pheomelanin synthesis.
Expression profiles of key pigment genes and verification of RNA-seq data
The expression levels of five key pigment genes (TYRP1, DCT, PMEL, MLANA and HPGDS) were measured using real-time quantitative reverse transcription PCR (qRT-PCR) of samples from feather follicles at various time points (3, 5, 7, and 11 weeks of age). The results showed that the expression of TYRP1 and DCT was lower in the yellow plumage group than in the white plumage group at the four ages, and the differences were significant (P < 0.05). Expression of PMEL, MLANA, and HPGDS genes was significantly lower in the yellow feather follicles than in the white feather follicles in chickens of 5, 7 and 11 weeks of age (P < 0.05); however, the differences were not significant in the 3-week age class (Fig. 4). This may be owing to the fact that at the 3 weeks of age, chicks are not yet at a critical time to moult. Among these five genes, TYRP1 and DCT expression levels were approximately tenfold lower in yellow than in white feather samples in 5-, 7- and 11-week old chickens, suggesting that these two genes might participate in the pigmentation and control of plumage colour in chickens.
Verification of gene expression level via quantitative real-time polymerase chain reaction (qRT-PCR). TYRP1, DCT, PMEL, MLANA, and HPGDS expression (a–e) comparisons between white and yellow feather follicles in 3- (3W and 3Y), 5- (5W and 5Y), 7- (7W and 7Y), and 11-week (11W and 11Y) ages. MFSD12, TYR, NR1H4, and THRSP expression (f–i) comparisons between white and yellow feather follicles in 7- (7W and 7Y) and 11-week (11W and 11Y) ages. Sample size in each group was five. **Indicates extremely significant difference (P < 0.01), *indicates significant difference (P < 0.05).
In addition to the five key pigment genes described above, we selected one DEG (MFSD12) and three non-DEGs (TYR, NR1H4 and THRSP), and used qRT-PCR to measure gene expression in the feather follicles of 7- and 11-week old chickens, to verify the RNA-seq analysis results. MFSD12 showed significant differences in the expression levels (P < 0.05), whereas the three non-DEGs showed no significant differences (Fig. 4). This indicates that the RNA-seq analysis results are reliable.
Discussion
In birds, plumage colour variations are due to alterations in melanin production, distribution or deposition in feathers, and feather follicles are the only place where plumage melanin is produced30. In feather follicles, mature melanocytes produce melanosomes for melanogenesis; the newly synthesized melanin is then transferred to the keratinocytes of feathers, giving the feathers their particular colour31. There are two types of melanosomes, eumelanosomes and pheomelanosomes. Eumelanosomes are elliptical entity with a fibrillar matrix, and are responsible for eumelanin synthesis. Pheomelanosomes vary in shape, being predominantly rounded, and are responsible for the pheomelanin synthesis32,33. Our paraffin sections of feather follicles were not sufficiently magnified to observe melanosome structure and enable determination of the type. Nonetheless, we could identify the type of melanin produced by the colour displayed, and concluded that the pheomelanin, but not eumelanin, production was higher in the yellow than in the white feather follicles. Yellow or red coat colour has been shown to be associated with high levels of pheomelanin production: a 100-fold increase in the pheomelanin content was observed in the dorsal hairs of recessive yellow mice compared to black mice34. Higher levels of pheomelanin markers TTCA and 4-AHP were also observed in recessive red pigeons than in wild-type pigeons, based on the histology of regenerating feathers and chemical analysis of feather melanin17. Five down-regulated DEGs (TYRP1, PMEL, MLANA, HPGDS and TSPAN10) in our yellow plumage chickens were shared with the identified DEGs in recessive red pigeons17. As described in their hypothesis, pheomelanin-based phenotypes may result from changes in multiple pigmentation genes, reducing overall pigmentation and converting melanogenesis to pheomelanin production17. Our results are therefore consistent with previous studies, and confirmed that high levels of pheomelanin production contributed to the yellow plumage trait in our study.
In this study, we found that the Dominant white locus did not inhibit the pheomelanin synthesis and deposition. The Dominant white allele can alter the PMEL amyloid formation from functional to pathogenic, and inhibit eumelanin synthesis35,36. Similarly, previous studies have revealed that functional mutations in PMEL do not affect the production of pheomelanin in silver coat horses, silver mutation mouse, merle pattern domestic dogs, and yellowish plumage Japanese quail12,13,37,38. These results are understandable, given that the transmembrane glycoprotein encoded by PMEL is the main component of the fibrillar matrix of eumelanosomes rather than a component of pheomelanosomes33,39. A PMEL-silencing assay revealed that the mRNA expression of melanin biosynthesis genes, including TYR, did not differ from that in the control group40. This suggests that the Dominant white allele was functional at the protein level, but had no effect on the mRNA expression of melanin biosynthesis genes in our study. Some missense mutations of SLC45A2 resulted in a dominant Silver locus that made yellow plumage undetectable, confirming the previous reported dilution effect on pheomelanin by this locus9. Similarly, dilution of pheomelanin with no effect on eumelanin, or with only a slight effect, has been observed in SLC45A2 mutation horses41. It is interesting feature also an enigma concerning the specific inhibition of pheomelanin in Silver chickens. When the Sex-linked barring locus was present, the feathers of yellow plumage chickens showed a yellow and white barred pattern. This pattern can be caused by the B allele, the causative mutations are four single-nucleotide polymorphisms located in the CDKN2A gene11. These mutations cause high expression of CDKN2A and lead to the premature differentiation of melanocyte progenitor cells and premature depletion of melanocytes, this causes a white bar to appear where the melanocytes are absent11. Similarly, the diverse of feather patterns in pigeons are controlled by the distribution of melanocytes16. Lin et al.42 systematically explored the cellular and molecular basis of avian pigment pattern formation; they concluded that the diversity of avian pigment patterns is the result of modulating the presence, arrangement, or differentiation of melanocytes. Our findings elucidate the effect of several known pigment genes on yellow plumage; this may be helpful for the breeding of yellow plumage chickens.
Melanin production is determined by relevant enzymes and their metabolic capacity. For example, the presence of TYR allows the rapid oxidation of tyrosine to dopaquinone, thus initiating the eumelanin or pheomelanin synthesis pathways in melanocytes43. Mutations in the TYR gene can lead to severe inadequacy in melanin production. The recessive white feather colour in chickens is caused by the insertion of a retroviral sequence on intron 4 of the TYR gene, resulting in a substantial reduction in the expression of normal transcript that contains exon 58. The amount or activity of TYR, TYRP1, and DCT is also directly related to eumelanin content, because of their roles in the final step of eumelanin synthesis43. Further, the type of melanin produced is directly determined by the amounts of sulfhydryl compounds such as cysteine and glutathione that are present in the tissue (Fig. 5)43,44. Thus, the presence and activity of enzymes that catalyse glutathione reactions, such as glutathione peroxidase, glutathione reductase and glutathione S-transferase, will modify the progression of melanogenesis45. In previous studies, omics data were used to explore feather colour formation-related genes. The formation of Columbian plumage in chickens was related to changes in the expression of MED23, FZD10, WNT7B and WNT1121. DEGs between black and white feather colours in ducks were enriched in the pathways of melanogenesis (c-Kit/TYR/ TYRP1) and tyrosine metabolism (TYR/ TYRP1)23. In our study, DEGs were significantly enriched in multiple pathways related to melanin synthesis, and were concentrated in several genes. Down-regulation of five pigment-related genes (TYRP1, DCT, HPGDS, PMEL and MLANA) was confirmed by qRT-PCR in 5-, 7- and 11-week old chickens. The enzymes or proteins encoded by these five genes can participate in melanogenesis by acting as catalysts or providing sites for melanin synthesis46,47,48,49,50. Therefore, it is possible that functional changes in enzymes or precursors, caused by the down-regulation of these five pigment-related genes, lead to an increase in pheomelanin synthesis.
The synthetic process of eumelanin and pheomelanin. The blue arrow means that its corresponding coding gene is down-regulated in the yellow samples relative to the white samples. Dopa 3,4-dihydroxyphenylalanine, DHICA 5,6-dihydroxyindole-2-carboxylic acid, DHI 5,6-dihydroxyindole, GSH glutathione, TYR tyrosinase, TYRP1 tyrosinase-related protein 1, DCT dopachrome tautomerase, HPGDS haematopoietic prostaglandin D2 synthase.
TYRP1, the first pigment gene cloned in the tyrosinase gene family, encodes tyrosinase-related protein 1, a putative membrane-bound, copper-containing enzyme that plays a key role in the last step of eumelanin synthesis46,51. Previous studies have demonstrated strong correlations between changes in TYRP1 function and the pheomelanin-based coat colour of Japanese quail28, chickens52, cats46, rabbits53, and cattle54. Low expression levels of TYRP1 were associated with a pheomelanin-based coat colour. In French cattle breeds, the greatest expression of TYRP1 occurred in the black skin of Prim’Holstein cattle; in the pheomelanin-rich coats of the Blonde d’Aquitaine (blond), Limousin (red), and Salers (reddish brown) breeds, there were varying levels of complete repression of TYRP155. In catfish, lower expression levels of TYRP1 mRNA were detected in the albino yellow group than in the black wild-type group56. These results provide useful data for understanding the relationship between the lower expression of TYRP1 and the pheomelanin-based phenotype. DCT is an enzyme that metabolises dopachrome (DC) to 5, 6-dihydroxyindole-2-carboxylic acid (DHICA) during eumelanin synthesis47. The slaty mutation is known to reduce the activity of DCT. In cultured slaty melanocytes and slaty mutation mice, the content of eumelanin was reduced, whereas the content of pheomelanin was greatly increased57,58. DCT knockout mice showed a diluted coat colour relative to the non-knockout mice59. These studies indicate that DCT repression can affect both eumelanin and pheomelanin synthesis. Additionally, we confirmed that there was no significant difference in TYR expression between the yellow and white feather follicles. Thus, reduced TYRP1 and DCT levels caused a switch from eumelanin synthesis to pheomelanin synthesis (Fig. 5), which is a general trend under suppression of eumelanin synthesis34.
Haematopoietic prostaglandin D2 synthase (HPGDS) is a member of the sigma-class glutathione S-transferases (GSTs)60. HPGDS can be utilised as a cofactor in a variety of detoxification reactions, by catalysing the conjugation of mutagens and carcinogens with glutathione during mercapturic acid synthesis49,61. As a nucleophile, glutathione is the most abundant sulfhydryl compound in animal tissues and occurs mainly in the cytoplasm of aerobic organisms62. Glutathione levels are closely associated with the deposition of melanin in the coat or skin of vertebrates63. High concentrations of glutathione allow it to bind with dopaquinone to form 5-S-cysteinyldopa, thereby initiating the pheomelanin synthesis pathway. When the glutathione concentration decreases, dopaquinone is converted directly to dopachrome and ultimately forms eumelanin38. In melanoma cells, low levels of glutathione have been found to be associated with eumelanin-type pigmentation, while high levels were associated with pheomelanin-type pigmentation64,65. In mice, yellow hair follicles have high glutathione and total thiol concentrations, while black hair follicles have low glutathione concentrations66. Thus, we inferred that a reduction in HPGDS expression caused reduced mercapturic acid synthesis, which further increased pheomelanin synthesis in the yellow feather follicles. Our finding indicates that reduction in HPGDS might result in more glutathione providing sulfhydryl to participate in pheomelanin synthesis (Fig. 5).
PMEL is a melanosome matrix protein responsible for the formation of PMEL fibrils within eumelanosomes. PMEL fibrils are essential for the optimisation of normal eumelanosome morphogenesis and eumelanin deposition, as animals lacking PMEL expression exhibit varying degrees of eumelanin reduction6,12,39,48,67. Reduced expression of PMEL was found in Japanese quail with the yellowish phenotype13. Therefore, the loss of PMEL may also be one of the reasons for the formation of yellow plumage. MLANA, which encodes a melanoma antigen (melan-A) that is recognised by cytotoxic T lymphocytes, has been cloned while searching for melanoma antigens; it has been widely studied as a melanoma-specific antigen and melanosome-specific marker68. The expression of MLANA is observed only in melanocytes, melanomas, and retinal pigment epithelium, suggesting its association with melanogenesis68,69. Melan-A can form complexes with PMEL17, affecting both its stability and the processing required for melanosome structure and maturation, and participating in eumelanin synthesis50. In mice, knocking out the MLANA gene results in a reduction in melanin content. Likewise, the melanosomes of melanocytes in MLANA knockout mice showed morphological abnormalities, indicating that MLANA acts as a pigment gene for the biogenesis and maintenance of melanosomes70. Considering the above results, we believe that reduced expression of PMEL and MLANA also causes reduced eumelanin synthesis.
For yellow and white plumage formation, we inferred that the five pigment-related genes discussed cause a reduction in eumelanin and an increase in pheomelanin synthesis in yellow plumage. Further, due to the presence of Dominant white locus, both white plumage and yellow plumage lack the eumelanosomes required for normal eumelanin synthesis. Finally, only pheomelanin was detected in the yellow plumage samples, and none of two types of melanin was detected in the white plumage samples.
In conclusion, by analysing the plumage colour distribution among the offspring populations of HB × WL chickens, we revealed the heredity of the yellow plumage gene. The yellow plumage of HB was due to increased pheomelanin synthesis in the pheomelanosomes in the feather follicles. The transcriptome profiles and qRT-PCR validation revealed that down-regulation of TYRP1, DCT, PMEL, MLANA and HPGDS may alter eumelanin and pheomelanin content, leading to the formation of yellow plumage. We speculate that the phenotypic characteristics of the yellow plumage colour might be caused by the reduced expression of these genes. These findings provide new insights for future research in yellow plumage chickens.
Methods
Experimental animals and sampling
The F1 population was obtained by crossing HB cocks with WL hens. Yellow plumage females and white plumage males were selected from the F1 population to produce an F2 population. Yellow plumage females and males were then selected from the F2 population to produce an F3 population.
For the transcriptome sequencing and to verify gene expression, five white plumage and five yellow plumage chickens were randomly collected from the F3 population, and several newly emerged feathers were plucked from nape of the neck at four time points (3, 5, 7 and 11 weeks of age). The feather follicles were cut off 2 mm from the bottom end of the feather and then immediately put into RNA storage solution and stored at − 80 °C. For histological measurements, yellow and white plumage chickens at 11 weeks of age were bled to death, and the newly emerged feather follicles, along with the nearby skin, were removed and fixed in 4% paraformaldehyde at 4 °C. Animal treatment protocols were approved by The Beijing Municipal Committee of Animal Management and comply with guidelines of The Ethics Committee of the China Agricultural University.
Identification of known genes associated with plumage pigment
In all of the transcriptome samples, three loci that cause white plumage were identified; these are the Dominant white locus on the PMEL gene; the sex-linked Silver locus, caused by a mutation of SLC45A2 on the Z chromosome; and the recessive white locus, which results from the insertion of a complete avian retroviral sequence of 7.7 kb in intron 4 of the TYR gene6,7,8,9. Blood samples were collected from the brachial veins of chickens using standard venepuncture. Genomic DNA was extracted using the phenol and chloroform method72. The Dominant white and recessive white loci were identified using patented methods. PCR of the target region and Sanger sequencing were used to verify the base mutation of SLC45A2. Prior to this, the identification of the three loci was carried out in individuals of the F0, F1, and F2 populations, to elucidate the heredity of the yellow plumage trait.
Histology of the feather follicles
The feather follicles, fixed at 4 °C overnight, were dehydrated in ethanol, cleaned using xylene, and embedded in paraffin. Longitudinal sections of 7 μm thickness were sliced and stained with haematoxylin using standard protocols; eosin was not used because it interferes with the visualisation of melanin. After haematoxylin staining, the sections were fixed with neutral resin and observed under a microscope.
RNA extraction, library construction, and sequencing
Three white and three yellow feather follicle samples from chickens aged 7 and 11 weeks were selected for transcriptome sequencing. Total RNA was extracted from the feather follicles, including epithelium and pulp, using an RNAprep Pure Tissue Kit (Tiangen, Dalian, China) following the manufacturer’s instructions. RNA purity was checked using the NanoDrop 2000 spectrophotometer, and the concentration and integrity of RNA were assessed using the Agilent 2100 Bioanalyzer and Agilent RNA 6000 Nano Kit, respectively. In total, 2 μg RNA per sample was used as input material for RNA sample preparation. The sequencing libraries were prepared using the NEBNext® Ultra™ RNA Library Prep Kit for Illumina® (#E7530L, NEB, USA) following the manufacturer’s recommendations; the libraries were sequenced on an Illumina Hiseq 4000 platform using 150 bp paired-end reads. All RNA sequence data were deposited in the NCBI Gene Expression Omnibus under Accession GSE146956.
Analysis of RNA-seq data
A Perl script was used to filter the original raw data. Contaminated adapter reads, reads with > 5% ambiguous “N” bases, and low quality reads were removed. The reference genomes (ftp://ftp.ensembl.org/pub/release-96/fasta/gallus_gallus/dna/Gallus_gallus.GRCg6a.dna.toplevel.fa) and the annotation file (ftp://ftp.ensembl.org/pub/release-96/gtf/gallus_gallus/Gallus_gallus.GRCg6a.96.gtf.gz) were downloaded from the ENSEMBL database (https://www.ensembl.org/index.html). The reference genome index was built using Bowtie2 v2.2.3, and the clean data were then aligned to the reference genome using TopHat272. Reads for each gene were counted using HTSeq v0.6.0; the expression levels of genes per sample, expressed as FPKM, were based on sequence lengths and on the read counts mapped to them71.
Screening and functional annotation of DEGs
Differential expression analysis between the yellow and white feather groups was performed using the package DEGseq, and DEGs were screened using a significance criterion of false discovery rate (FDR) ≤ 0.05 and |log2(fold change)| ≥ 1 between the two groups73.
To further elucidate the functional roles of DEGs, GO and KEGG pathway enrichment analysis were performed using the DAVID database (https://david.abcc.ncifcrf.gov/). GO enrichment analysis can reveal the biological functions of DEGs, and KEGG is a database resource containing a collection of manually drawn pathway maps. GO terms that were significantly annotated (P < 0.05) were considered significantly enriched.
Real-time quantitative reverse transcription PCR (qRT-PCR) verification of RNA-seq data
Four genes (MFSD12, TYR, NR1H4 and THRSP) were selected to measure expression levels in feather follicles of 7- and 11-week old chickens, in order to verify the RNA-seq data using qRT-PCR. Five key pigment genes (TYRP1, DCT, PMEL, MLANA and HPGDS) were specifically chosen to assess their function in melanogenesis; to this end, we measured their expression levels in all samples of 3-, 5-, 7-, and 11-week old chickens. The primers for these genes are described in Table S5.
The qRT-PCR was performed on a CFX96TM Real-Time System (Bio Rad, Hercules, CA, USA) using Real Master Mix SYBR Green I (Tiangen). GAPDH was used as a housekeeping gene in all qRT-PCR experiments. Five biological replicates per group and three technical replicates per sample were used for qRT-PCR. The 2−ΔΔCT method was used to calculate gene expression levels74. Significant differences between the two groups were evaluated using unpaired Student’s t tests. All results are shown as the means ± standard error (SE). P < 0.05 was considered significant and P < 0.01 extremely significant.
References
Cooke, T. F. et al. Genetic mapping and biochemical basis of yellow feather pigmentation in budgerigars. Cell 171, 427–439 (2017).
Lopes, R. J. et al. Genetic basis for red coloration in birds. Curr. Biol. 26, 1427–1434 (2016).
Cone, R. D. et al. The melanocortin receptors: agonists, antagonists, and the hormonal control of pigmentation. Recent Prog. Horm. Res. 51, 287–317, 318 (1996).
Wilczek, A., Kondoh, H. & Mishima, Y. Composition of mammalian eumelanins: analyses of DHICA-derived units in pigments from hair and melanoma cells. Pigment Cell Res. 9, 63–67 (1996).
Land, E. J. & Riley, P. A. Spontaneous redox reactions of dopaquinone and the balance between the eumelanic and phaeomelanic pathways. Pigment Cell Res. 13, 273–277 (2000).
Kerje, S. et al. The dominant white, dun and smoky color variants in chicken are associated with insertion/deletion polymorphisms in the PMEL17 gene. Genetics 168, 1507–1518 (2004).
Chang, C. et al. Complete association between a retroviral insertion in the tyrosinase gene and the recessive white mutation in chickens. BMC Genomics 7, 19 (2006).
Sato, S., Otake, T., Suzuki, C., Saburi, J. & Kobayashi, E. Mapping of the recessive white locus and analysis of the tyrosinase gene in chickens. Poult. Sci. 86, 2126–2133 (2007).
Gunnarsson, U. et al. Mutations in SLC45A2 cause plumage color variation in chicken and japanese quail. Genetics 175, 867–877 (2007).
Kinoshita, K. et al. Endothelin receptor B2 (EDNRB2) is responsible for the tyrosinase-independent recessive white (mo(w)) and mottled (mo) plumage phenotypes in the chicken. PLoS ONE 9, e86361 (2014).
Schwochow Thalmann, D. et al. The evolution of Sex-linked barring alleles in chickens involves both regulatory and coding changes in CDKN2A. PLoS Genet. 13, e1006665 (2017).
Brunberg, E. et al. A missense mutation in PMEL17 is associated with the Silver coat color in the horse. BMC Genet. 7, 46 (2006).
Ishishita, S. et al. Nonsense mutation in PMEL is associated with yellowish plumage colour phenotype in Japanese quail. Sci. Rep. 8, 16732 (2018).
Schiöth, H. B. et al. Remarkable synteny conservation of melanocortin receptors in chicken, human, and other vertebrates. Genomics 81, 504–509 (2003).
Levy, C., Khaled, M. & Fisher, D. E. MITF: Master regulator of melanocyte development and melanoma oncogene. Trends Mol. Med. 12, 406–414 (2006).
Vickrey, A. I. et al. Introgression of regulatory alleles and a missense coding mutation drive plumage pattern diversity in the rock pigeon. ELife. 7, e34803 (2018).
Domyan, E. T. et al. SOX10 regulates multiple genes to direct eumelanin versus pheomelanin production in domestic rock pigeon. Pigment Cell Melanoma Res. 32, 634–642 (2019).
Yang, J., Qu, Y., Huang, Y. & Lei, F. Dynamic transcriptome profiling towards understanding the morphogenesis and development of diverse feather in domestic duck. BMC Genomics 19, 316–391 (2018).
Li, A. et al. Diverse feather shape evolution enabled by coupling anisotropic signalling modules with self-organizing branching programme. Nat. Commun. 8, s14139 (2017).
Ng, C. S. et al. Transcriptomic analyses of regenerating adult feathers in chicken. BMC Genomics 16, 756 (2015).
Wang, X. et al. Combined transcriptomics and proteomics forecast analysis for potential genes regulating the Columbian plumage color in chickens. PLoS ONE 14, e210850 (2019).
Yu, S., Wang, G., Liao, J., Tang, M. & Sun, W. Transcriptome profile analysis of mechanisms of black and white plumage determination in Black-Bone chicken. Cell. Physiol. Biochem. 46, 2373–2384 (2018).
Li, S., Wang, C., Yu, W., Zhao, S. & Gong, Y. Identification of genes related to white and black plumage formation by RNA-Seq from white and black feather bulbs in ducks. PLoS ONE 7, e36592 (2012).
Guo, Y. et al. A complex structural variation on chromosome 27 leads to the ectopic expression of HOXB8 and the muffs and beard phenotype in chickens. PLoS Genet. 12, e1006071 (2016).
Nie, C. et al. Genome-wide association study revealed genomic regions related to white/red earlobe color trait in the Rhode Island Red chickens. BMC Genet. 17, 115 (2016).
Gunnarsson, U. et al. The Dark brown plumage color in chickens is caused by an 8.3-kb deletion upstream of SOX10. Pigment Cell Melanoma Res. 24, 268–274 (2011).
Yeo, J. et al. Detection of exonic variants within the melanocortin 1 receptor (MC1R) gene in Black Silky, White Leghorn and Golden duckwing Araucana chicken. Mol. Biol. Rep. 41, 4843–4846 (2014).
Nadeau, N. J., Mundy, N. I., Gourichon, D. & Minvielle, F. Association of a single-nucleotide substitution in TYRP1 with roux in Japanese quail (Coturnix japonica). Anim. Genet. 38, 609–613 (2007).
China National Commission of Animal Genetic Resources. Animal Genetic Resources in China Indigenous Breeds Vol. 2, 151 (China Agriculture Press, Beijing, 2015).
Baxter, L. L., Ling, H., Loftus, S. K. & Pavan, W. J. Spotlight on spotted mice: a review of white spotting mouse mutants and associated human pigmentation disorders. Pigment Cell Res. 17, 215–224 (2010).
Raposo, G. & Marks, M. S. Melanosomes—dark organelles enlighten endosomal membrane transport. Nat. Rev. Mol. Cell Biol. 8, 786–797 (2007).
Andrzej, S., Tobin, D. J., Shigeki, S. & Jacobo, W. Melanin pigmentation in mammalian skin and its hormonal regulation. Physiol. Rev. 84, 1155–1228 (2004).
Sturm, R. A., Teasdale, R. D. & Box, N. F. Human pigmentation genes: identification, structure and consequences of polymorphic variation. Gene 277, 49–62 (2001).
Tomohisa, H., Shosuke, I. & Kazumasa, W. The mouse pink-eyed dilution allele of the P-gene greatly inhibits eumelanin but not pheomelanin synthesis. Pigment Cell Melanoma Res. 24, 241–246 (2011).
Hellstrom, A. R. et al. Inactivation of Pmel alters melanosome shape but has only a subtle effect on visible pigmentation. PLoS Genet. 7, e1002285 (2011).
Watt, B. et al. Mutations in or near the transmembrane domain alter PMEL amyloid formation from functional to pathogenic. PLoS Genet. 7, e1002286 (2011).
Clark, L. A., Wahl, J. M., Rees, C. A. & Murphy, K. E. Retrotransposon insertion in SILV is responsible for merle patterning of the domestic dog. Proc. Natl. Acad. Sci. U. S. A. 103, 1376–1381 (2006).
Kwon, B. S. et al. Mouse silver mutation is caused by a single base insertion in the putative cytoplasmic domain of Pmel 17. Nucleic Acids Res. 23, 154–158 (1995).
Watt, B., van Niel, G., Raposo, G. & Marks, M. S. PMEL: A pigment cell-specific model for functional amyloid formation. Pigment Cell Melanoma Res. 26, 300–315 (2013).
Sun, L. et al. Silencing of PMEL attenuates melanization via activating lysosomes and degradation of tyrosinase by lysosomes. Biochem. Biophys. Res. Commun. 503, 2536–2542 (2018).
Mariat, D., Taourit, S. & Guérin, G. A mutation in the MATP gene causes the cream coat colour in the horse. Genet. Sel. Evol. 35, 119–133 (2003).
Lin, S. J. et al. Topology of feather melanocyte progenitor niche allows complex pigment patterns to emerge. Science 340, 1442–1445 (2013).
Prota, G. The chemistry of melanins and melanogenesis. Fortschr. Chem. Org. Naturst. 64, 93–148 (1995).
Potterf, S. B. et al. Cysteine transport in melanosomes from murine melanocytes. Pigment Cell Res. 12, 4–12 (2010).
Schallreuter, K. U., Lemke, K. R., Hill, H. Z. & Wood, J. M. Thioredoxin reductase induction coincides with melanin biosynthesis in brown and black guinea pigs and in murine melanoma cells. J. Investig. Dermatol. 103, 820–824 (1994).
Schmidt-Kuntzel, A., Eizirik, E., O’Brien, S. J. & Menotti-Raymond, M. Tyrosinase and tyrosinase related protein 1 alleles specify domestic cat coat color phenotypes of the albino and brown loci. J. Hered. 96, 289–301 (2005).
Tsukamoto, K., Jackson, I. J., Urabe, K., Montague, P. M. & Hearing, V. J. A second tyrosinase-related protein, TRP-2, is a melanogenic enzyme termed DOPAchrome tautomerase. EMBO J. 11, 519–526 (1992).
Kobayashi, T. et al. The Pmel 17/silver locus protein. Characterization and investigation of its melanogenic function. J. Biol. Chem. 269, 29198–29205 (1994).
Chasseaud, L. F. The role of glutathione and glutathione S-transferases in the metabolism of chemical carcinogens and other electrophilic agents. Adv. Cancer Res. 29, 175–274 (1979).
Hoashi, T. et al. MART-1 is required for the function of the melanosomal matrix protein PMEL17/GP100 and the maturation of melanosomes. J. Biol. Chem. 280, 14006–14016 (2005).
Schmutz, S. M., Berryere, T. G. & Goldfinch, A. D. TYRP1 and MC1R genotypes and their effects on coat color in dogs. Mamm. Genome 13, 380–387 (2002).
Li, J. et al. A missense mutation in TYRP1 causes the chocolate plumage color in chicken and alters melanosome structure. Pigment Cell Melanoma Res. 32, 381–390 (2019).
Utzeri, V. J., Ribani, A. & Fontanesi, L. A premature stop codon in the TYRP1 gene is associated with brown coat colour in the European rabbit (Oryctolagus cuniculus). Anim. Genet. 45, 600–603 (2014).
Berryere, T. G., Schmutz, S. M., Schimpf, R. J., Cowan, C. M. & Potter, J. TYRP1 is associated with dun coat colour in Dexter cattle or how now brown cow?. Anim. Genet. 34, 169–175 (2003).
Guibert, S., Girardot, M., Leveziel, H., Julien, R. & Oulmouden, A. Pheomelanin coat colour dilution in French cattle breeds is not correlated with the TYR, TYRP1 and DCT transcription levels. Pigment Cell Res. 17, 337–345 (2004).
Zhang, X. T. et al. Molecular cloning and expression analysis of tyr and tyrp1 genes in normal and albino yellow catfish Tachysurus fulvidraco. J. Fish Biol. 92, 979–998 (2018).
Hirobe, T. et al. The slaty mutation affects eumelanin and pheomelanin synthesis in mouse melanocytes. Eur. J. Cell Biol. 85, 537–549 (2006).
Costin, G. E. et al. Mutations in the mouse DCT gene induce a reduction of eumelanin and an increase in the level of pheomelanin produced by melanocytes in culture. Pigment Cell Res. 17, 435 (2004).
Laurence, G., Fabien, M., Anita, R., Alexandre, M. & Friedrich, B. Melanocytes and pigmentation are affected in dopachrome tautomerase knockout mice. Mol. Cell. Biol. 24, 3396–3403 (2004).
Mazari, A. M., Hegazy, U. M. & Mannervik, B. Identification of new inhibitors for human hematopoietic prostaglandin D2 synthase among FDA-approved drugs and other compounds. Chem. Biol. Interact. 229, 91–99 (2015).
Habig, W. H. & Jakoby, W. B. Glutathione S-transferases (rat and human). Methods Enzymol. 77, 218–231 (1981).
Meister, A. Selective modification of glutathione metabolism. Science 220, 472–477 (1983).
Galvan, I. & Alonso-Alvarez, C. An intracellular antioxidant determines the expression of a melanin-based signal in a bird. PLoS ONE 3, e3335 (2008).
Benathan, M. et al. Co-regulation of melanin precursors and tyrosinase in human pigment cells: roles of cysteine and glutathione. Cell. Mol. Biol. (Noisy-le-grand) 45, 981–990 (1999).
Benedetto, J. P. et al. Role of thiol compounds in mammalian melanin pigmentation. II. Glutathione and related enzymatic activities. J. Investig. Dermatol. 79, 422–424 (1982).
Granholm, D. E., Reese, R. N. & Granholm, N. H. Agouti alleles alter cysteine and glutathione concentrations in hair follicles and serum of mice (A Y/A, A wJ/A wJ, and a/A). J. Investig. Dermatol. 106, 559–563 (1996).
Martínez-Esparza, M. et al. The mouse silver locus encodes a single transcript truncated by the silver mutation. Mamm. Genome 10, 1168–1171 (1999).
Coulie, P. G. et al. A new gene coding for a differentiation antigen recognized by autologous cytolytic T lymphocytes on HLA-A2 melanomas. J. Exp. Med. 180, 35–42 (1994).
Kawakami, Y. et al. Cloning of the gene coding for a shared human melanoma antigen recognized by autologous T cells infiltrating into tumor. Proc. Natl. Acad. Sci. U. S. A. 91, 3515–3519 (1994).
Aydin, I. T., Hummler, E., Smit, N. P. & Beermann, F. Coat color dilution in mice because of inactivation of the melanoma antigen MART-1. Pigment Cell Melanoma Res. 25, 37–46 (2012).
Cao, G. Q. & Qing-Shan, M. O. Genomic DNA isolation by Phenol/Chloroform extracting method from sheep blood clot. J. Anhui Agric. Sci. 37, 16771–16772 (2009).
Trapnell, C. et al. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 7, 562–578 (2012).
Wang, L., Feng, Z., Wang, X., Wang, X. & Zhang, X. DEGseq: An R package for identifying differentially expressed genes from RNA-seq data. Bioinformatics 26, 136–138 (2010).
Schmittgen, T. D. & Livak, K. J. Analyzing real-time PCR data by the comparative C(T) method. Nat. Protoc. 3, 1101–1108 (2008).
Acknowledgements
This research was supported by the National Natural Science Foundation of China (31972532) and the China Agricultural Research System (ACRS-40-K05).
Author information
Authors and Affiliations
Contributions
H.Z. provided essential protocols and instruments. H.Z., C.X., J.L. and X.Z. designed the experiments and analysis. X.Z., R.N. and H.Z. helped in sample collection. X.Z. performed the molecular biology experiments. X.Z., B.Z. and Y.Z. performed the analysis and interpreted the results. X.Z. drafted the manuscript. All authors reviewed the manuscripts and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zheng, X., Zhang, B., Zhang, Y. et al. Transcriptome analysis of feather follicles reveals candidate genes and pathways associated with pheomelanin pigmentation in chickens. Sci Rep 10, 12088 (2020). https://doi.org/10.1038/s41598-020-68931-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-68931-1
- Springer Nature Limited
This article is cited by
-
Transcriptome analysis of sexual dimorphism in dorsal down coloration in goslings
BMC Genomics (2024)
-
A complex genomic architecture underlies reproductive isolation in a North American oriole hybrid zone
Communications Biology (2023)