Abstract
To provide the real-world outcomes of people with proliferative diabetic retinopathy (PDR) in India and highlight opportunities for improvement of their disease status and to evaluate their visual acuity (VA) status. A multicenter retrospective study in which ten centers in India with established vitreoretinal services for over 10 years were invited to provide long-term data on PDR. This study population were of Indian nationality. Patients with a diagnosis of type 1 or 2 diabetes with a clinical diagnosis of active PDR in any or both eyes, who had long term follow-up for up to 10 years were included. Baseline data collected included age, sex, duration of diabetes, source of referral and best-corrected visual acuity, diabetic retinopathy status in both eyes. Available follow-up data on VA were collected at 6 months post baseline, 5 years and 10 years within a ± 3 months window. Evaluating the presenting VA of people with PDR, short-term outcomes at 6 months and the incidence of visual impairment (VI) at 5 and 10 years are the main outcome of the study. Data was available for 516, 424 and 455 patients at baseline, 5 years and 10 years respectively. Gender and duration of diabetes did not have statistically significant effect on VI outcomes. Eyes receiving treatment early in the disease course (i.e. baseline VA ≥ 6/12) had significantly better VA outcomes at 10 years versus eyes treated at a later stage (i.e. baseline VA < 6/12) (p = <0.0001). On comparing eyes with stable treated PDR and persistent PDR at end of 10 year follow up, a significantly higher percentage of eyes in the stable treated group maintained VA of ≥ 6/12 (55.1% vs. 24.2%) (p = < 0.0001), indicating persistent disease activity due to inadequate treatment results in worse VA outcomes. We found no trend in VI or blindness with increasing levels of age at both 5- and 10-year time points (p > 0.05). The age standardized incidence for VI was 11.10% (95% CI 8.1, 14.2) and for blindness was found to be 7.7% (95% CI 5.2, 10.3). Our results suggest that despite robust recent clinical trial results showing that pan retinal photocoagulation is an excellent treatment for PDR, people with diabetes in India need to be made aware of annual screening and treatment of their eyes to avoid vision impairment and blindness.
Similar content being viewed by others
Introduction
Proliferative diabetic retinopathy (PDR) is a treatable cause of severe visual loss in people with diabetes. If left untreated, most eyes with low risk PDR characterized by mild to moderate retinal or optic disc neovascularization progress to high risk PDR with increasing retinal or disc neovascularization. These eyes remain symptomless until the onset of complications such as vitreous hemorrhage, tractional retinal detachment or diabetic macular edema (DME)1. Systematic screening and timely treatment of PDR in countries with established screening programs have resulted in a decrease in the rate of blindness and the incidence of Advanced Diabetic Eye Disease (ADED) over time2. Screening for diabetic retinopathy (DR) is still at its infancy in most low and middle-income countries (LMIC)3. There is limited literature on the presenting visual acuity (VA) of patients with PDR and their mode of referral to eye care centers in LMIC.
Pan retinal photocoagulation (PRP) has been the standard of care for PDR, with vitreo-retinal surgery advocated for patients with complications1,4,5. Recent studies have reported anti-vascular endothelial growth factor (anti-VEGF) therapy as alternative first line treatment for PDR6,7,8. It is unclear, however whether these protocols for treatment can be implemented well across hospitals in LMIC. In countries such as India, where most patients are dependent on out of pocket expenses for their healthcare, the management of diabetic eye disease is influenced by cost of care, lack of screening programs and the lack of public awareness of diabetic eye disease and the need for regular follow-up for ongoing treatment. There is also a wide variation in provision of healthcare in India, with some centers providing world-class services to others that do not have basic facilities or personnel to provide treatment. Therefore, in order to assess the visual outcomes and incidence of visual impairment (VI) in chronic conditions such as PDR in LMIC, it is best to focus on some of the best treatment centers in the country that provide a comprehensive treatment package for PDR and are more likely to have the data to provide the treatment accounts and the prevalence and incidence of VI in people being treated for this condition.
The aims of this study were to evaluate the presenting VA of people with PDR, short-term outcomes at 6 months and the incidence of VI at 5 and 10 years to highlight opportunities for improvement in India and other LMICs.
Methods
The study protocol was approved by the Institutional Review Board (Ethics committee) at Vision Research Foundation, Sankara Nethralaya, Chennai. As it is a retrospective study, the informed consent was waived by the ethics committee and it adhered to the tenets of the Declaration of Helsinki. Twenty centres in India with established vitreoretinal services for over 10 years were invited to provide long-term data on PDR based on the availability of either electronic patient records or paper registers that enabled retrieval of long-term data. Only 10 centres were able to provide this data.
Study population
This study population were of Indian nationality from different regions of India. Patients with a diagnosis of type 1 or 2 diabetes with a clinical diagnosis of active PDR in any or both eyes, who presented to these centres in 2008 and had long term follow-up for up to 10 years were included in the study. Clinical practice in India for treatment of PDR involves PRP for low and high risk PDR. Anti-vascular endothelial growth factor (VEGF) injections are given in cases with DME. For advanced PDR appropriate surgical intervention is performed when indicated. The patients could have prior treatment in another hospital before being seen in these centres so the study population consisted of treatment naïve PDR and those with persistent PDR post initial PRP. The patients could also have concomitant DME.
Study design
This is a multi-centre retrospective cohort study. The patient data were identified either from electronic patient records or manually from registers maintained since 2008 to allow for outcome measurements at 6 months, 5 and 10 years. Consecutive patients who met the inclusion criteria were included in the study.
Baseline data
Baseline data collected included age, sex, duration of diabetes, source of referral and best-corrected visual acuity (BCVA), DR status in both eyes, presence of DME. PDR status was defined as per the Early Treatment Diabetic Retinopathy Study (ETDRS) classification9. Low risk PDR included retinal neovascularization (NVE) < 1.27 mm2 (level 61) or disc neovascularization (NVD) < 0.74 mm2 and/or NVE ≥ 1.27 mm2 (level 65). High risk PDR included NVD < 0.74 mm2 and/or NVE ≥ 1.27 mm2 and/or vitreous haemorrhage/pre retinal haemorrhage (VH/PRH) ≥ 2.5 mm2 (level 71) or NVD ≥ 0.75 mm2 and or VH/PRH not obscuring the macula (level 75). Advanced PDR (level 81, 85) was defined as level 75 with VH/PRH obscuring macula. Presence of VH, retinal detachment, fibrovascular proliferation, neovascular glaucoma were also recorded. At final follow up, active/persistent PDR was defined as eyes with new features suggesting reactivation of proliferation or potentially sight threatening complications of fibrous proliferation. Stable treated PDR was defined as eyes with evidence of photocoagulation, regressed neovascularization and absence of features of active disease.
Follow-up data
Available follow-up data on VA were collected at 6 months post baseline, 5 years and 10 years within a ± 3 months window. The numbers of PRP sessions, cataract surgery, treatment of DME and vitrectomy with or without retinal surgeries were recorded within the first 6 months when most PDR eyes should stabilize if adequate PRP is given and total number of concomitant procedures over 10 years collected to understand the long-term treatment requirements.
Visual acuity
It is routine practice to perform BCVA using Snellen charts in these centres. However, the optometrists recorded the BCVA measurements in busy clinic settings. Under these circumstances, it is possible that the optometrists had not spend enough time to encourage the patients to read as far as possible. As a result, the BCVA may have been underestimated at times.
Definition of visual impairment and blindness
There are no universally accepted criteria for VI, blindness (severe VI) or changes in VI. Thus, VI was defined using both the United States (US) and World Health Organisation (WHO) criteria. Incidence of VI applied only to those with no VI at baseline. In the US criteria, no VI was defined as eyes with BCVA 6/12 or better, VI was defined as worse than 6/12 but better than 6/60 Blindness was defined as 6/60 or worse. The WHO criteria defined no VI as 6/18 or better, VI was worse than 6/18 but no worse than or equal to 3/60 and blindness was defined as worse than 3/60. These definitions were also used for both eyes, better-seeing eye and worse seeing eye10,11.
Statistical analyses
Descriptive statistics was used to analyse the baseline characteristics. The visual outcomes at 6 months, 5 and 10 years were analysed to report mean and median outcomes at each time points for all eyes, better eye and worse eye at individual levels. The last observation was not carried forward in this study as it is long-term study. The 5-year and 10-year incidence of monocular VI and blindness in different age groups were calculated as the proportion of the number of new eyes with VI and blindness to the number of eyes with no VI at baseline. Chi square (Cochran Armitage) for trend was used to look at incidence of VI and blindness with increasing age. Multivariable regression was used to analyse the effect of baseline factors on VI and blindness. One way ANOVA was used to compare VA outcomes based on type of referral.
Results
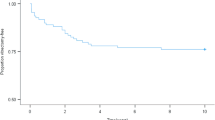
The baseline characteristics of the study cohort from 10 centres are summarised in (Table 1). Data was available on a total of 519 patients; VA data was available in 516 patients at baseline, 431 patients at 6 months, 424 patients at 5 years and 455 patients at 10 years. In our cohort, gender and duration of diabetes did not have a statistically significant effect on the final VA outcomes.
Using US criterion for VI, the odds ratios (OR) for the age categories (reference < 40 years) were as follows: 1.35 (0.55–3.33), 1.04 (0.44–2.47), 1.02 (0.40–2.59) and 1.63 (0.13–20.36); OR for sex (reference female) was 0.99 (0.61–1.60). Based on WHO criterion for VI, the OR for age were as follows (reference age < 40): 0.97 (0.37–2.51), 0.83 (0.34–2.05), 0.70 (0.25–1.95) and there were no cases for age 70 +, therefore no OR reported for this group. The OR for sex (reference female) was 1.08 (0.62–1.88). For all comparisons, p values were not statistically significant at the 5% level.
Although PRP was the main treatment done for all eyes with active PDR, other interventions were also required over 10 years. Eyes with PDR and DME were mainly treated with macular laser although the records on numbers of sessions of PRP or macular laser were incomplete and were not used in any analysis. Among the methods for patient referral, most PDR eyes identified and referred from screening programs were in the early course of disease (VA ≥ 6/12) when compared to self-referral where most patients presented quite late in the disease course (VA < 6/12) (p = 0.0002). At 10 years, higher number of eyes diagnosed at via screening ended up maintaining VA ≥ 6/12 than other modes of referral (p = 0). (Table 2) shows the proportions of eyes that underwent the interventions at 6 months and 10 years.
The change in visual outcome at 6 months, 5 and 10 years are shown in (Table 3).
The PDR status of the eyes at 10 years was also analyzed based on their baseline status. Of the 499 eyes with low risk PDR, 306 (77.3%) were stable PDR, 26 (5.2) % progressed to high risk PDR and 36 (7.2%) developed advanced PDR. Out of 299 eyes with baseline high risk PDR, 122 (40.8%) were stable treated PDR, 28 (9.3%) progressed to advanced PDR. Only 36/182 (19.7%) of the eyes with baseline advanced PDR were stable and treated.
Of the 1,032 eyes at baseline, 877 eyes had data on the PDR status at 10 years. All eyes with no PDR at baseline had developed PDR by 10 years. Of the 645 (73.5%) eyes with stable treated PDR at 10 years, 356 (55.1%) had VA of 6/12 or better while 199 (30.8%) and 90 (13.9%) eyes had VA of worse than 6/12 and better than 6/60 and 6/60 or worse respectively. Of the 95 (10.8%) eyes with persistent high-risk PDR at 10 years, 23 (24.2%), 49(51.6%) and 23(24.2%) eyes had a VA of 6/12 or better, worse than 6/12 and better than 6/60 and 6/60 or worse respectively. Almost 3/4th (75.8%) eyes in the persistent PDR group had VA < 6/12 as against 45% in the stable treated group (p = <0.0001).
Among the treatment naïve eyes at baseline with early diagnosis in disease course (i.e. VA better than 6/12) and early treatment initiation resulted in 65.7% (267) eyes to maintain VA better than 6/12 at 10 year follow up. Also only 26.1% (106) eyes among these ended up with VA worse than 6/12 at end of 10 years. This was in contrast to treatment naïve eyes at baseline with VA worse than 6/12 i.e. eyes that were diagnosed late and thus treated late in the course of the disease. Only 31.7% (53) eyes were able to achieve and maintain VA better than 6/12, and as high as 46.7% (78) eyes had VA worse than 6/12 at 10 year follow up (p < 0.0001).
New onset VI and blindness by 5 years is shown in (Table 4).
335 eyes with baseline VA of 6/12 or better (based on US criterion) and 368 eyes with VA 6/18 or better (based on WHO criterion) were at risk for vision loss over the study period. 61/335 (18.2%) eyes at baseline worsened from 6/12 or better and 48/368 (13%) worsened from 6/18 or better. Over 75% eyes that worsened from 6/12 and 70% that worsened from 6/18 were aged between 40 and 59 years. Moreover, according to WHO criteria, 409 eyes with VA better than 3/60 at baseline in the better seeing eyes had the potential to become blind by 5 years. By 5 years, 37/393 (9.4%) and 23/409 (5.6%) eyes became blind according to US and WHO criterion respectively.
Over 10 years, 373 eyes with baseline VA of 6/12 or better (based on US criterion) and 405 eyes with VA 6/18 or better (based on WHO criterion) were at risk for vision loss over the study period. Incident VI defined as worsening from 6/12 or better according to US criterion was 66/373 (17.6%). Based on WHO criterion it was 45/405 (11.11%) with majority of these incident cases seen in the age-group 40–59 years (Table 5). Moreover, according to WHO criteria of blindness, 441 eyes with VA better than 3/60 at baseline in the better seeing eye had the potential to become blind by 10 years. By 10 years, 55/425 (12.9%) and 34/441 (7.7%) eyes became blind according to US and WHO criterion respectively.
The age standardized incidence (standardized to the study population) of VI was 17.7% (95% CI 13.9, 21.6) using US criterion and 11.10 (95% CI 8.1, 14.2) based on WHO criterion at 10 years. The age standardized incidence (standardized to study population) for blindness was 12.9 (95% CI 9.7, 16.1) based on US criterion and 7.7% (95% CI 5.2, 10.3) with WHO criterion at 10 years. The age standardized incidence (standardized to India census population 2001) of VI was 14.2 (95% CI 7.1, 21.3) and 9.3 (95% CI 3.6, 14.9) using US and WHO criterion respectively. The age standardized incidence (standardized to India census population 2001) of blindness was 14.6 (95% CI 7.9, 21.4) based on US and 14.6 (95% CI 7.7, 21.5) using WHO criterion at 10 years. Additionally, we found no trend in VI or blindness with increasing levels of age at both 5- and 10-year time points.
Discussion
Contemporary data from clinical trials on PDR patients conducted in high-income countries show that PRP remains an ideal treatment option for PDR with good short and long-term visual outcomes despite a 40% drop-out of patients by 5 years6,7,8. However, our 10-year study results from India reveal a few important points that may be of relevance to all LMIC.
Firstly, the baseline characteristics show that although the demographic features of these patients are similar to those reported from Western countries, only a third of patients are referred from DR screening programs. Inadequate screening contributes to poor presenting vision12. Nearly 20% of the study cohort presented with VI or blindness in the better-seeing eye. However, the patients diagnosed through screening had better presenting VA and final visual outcomes, reinforcing the importance of screening programs.
Moreover, at presentation, approximately a third of patients presented with high risk PDR and 8% of the better seeing eye had ADED explaining why vitreoretinal surgery was required in a third of individuals in the first 6 months. This point further highlights the late presentation of a significant number of patients for treatment. Presenting VA and severity of PDR are both predictors of visual outcome13. Therefore, it is imperative that policies are in place for systematic screening and care pathways be designed for timely treatment and follow-up of this high-risk group. The challenges include the costs of laser devices and expertise required at the treatment centers.
Although our baseline data is obtained in 2008, the reports from the Vision Loss Expert Group of the Global Burden of Disease Study show that the prevalence of blindness due to DR has not decreased from 1990 to projected figures in 202014. Whilst the prevalence of PDR in people with diabetes is about 3% in India, PDR and its complications are the most common cause of DR related blindness. As PDR is initially asymptomatic, another barrier to treatment is the lack of public and patient awareness of the need for timely treatment. Therefore, more patient education and advocacy programs have to be initiated to ensure these changes.
After initial PRP, most patients will require fill-in sessions. In Protocol S, 38% required further laser in the first 6 months6. In our study, 1,070 interventions for PDR were required by 6 months and a further 283 procedures were required over the 10 years indicating that PRP is not a one off procedure and that the patients need to be monitored regularly over a prolonged period15. In addition, only 73.5% had stable treated PDR. Over 10 years, 12% of eyes progressed to high risk PDR and a further 10% high risk PDR to advanced eye disease and 10% remained high risk, substantiating the fact that PDR eyes need to monitored closely over 10 years and treatment given as required.
This study also shows that despite PRP, nearly 50% of the patients had VI at 10 years, suggesting that patients may not be lasered sufficiently or may be monitored less frequently than required.
When we consider incident cases of VI, we found that on average, 17% develop VI 10% become blind by 5 years and these figures worsen marginally by 10 years. In contrast, only 9% and 6% of participants showed 10 or 15 letters worsening after 5 years in Protocol S8.
Our study results are similar to those reported from short term studies in other LMIC highlighting the challenges in the management of PDR in resource constrained countries. A population based study also revealed similar prevalence of 6.3% of blindness due to DR and reinforced that baseline VA is an important predictor of visual outcome in PDR16. Other hospital based studies from LMIC show similar prevalence of clinic based blindness due to PDR17.
Monitoring of the systemic parameters such as glycemic control, blood pressure control and the renal parameters during the follow-up of patients treated for PDR would be invaluable for sustaining improvement in visual outcomes and reducing the burden of VI17.
As PDR is the most common cause of blindness, the resource requirements for these individuals are higher. Around 60% require cataract surgery and a third require vitreo-retinal surgery. There are approximately 3 million people with PDR in India and there are less than 1,000 practicing vitreo-retinal surgeons equating to one surgeon for every 3,000 patients. With the lack of trained human resources for vitreo-retinal surgery, it is obvious that the LMIC countries are in a vicious cycle of limited resources, lack of screening, poor presenting VA and need for vitreoretinal surgery. The only opportunity to break this cycle is to implement screening programs, strengthen the primary care system to control the risk factors of retinopathy and improve health seeking behaviors of our patients by increasing their awareness of the need for screening and frequent monitoring of their eyes.
The strength of this study is it provides both short- and long-term outcomes of PDR in a LMIC. There is a paucity of these types of studies globally. The study highlights an urgent need for quality improvement of the care we provide patients with diabetes at high-risk of visual loss. The study incorporated data from 10 centers in India so the results are generalizable and represents the highest quality of care provided in India.
Limitations of this study include the retrospective nature of the study but the clinics have the BCVA measured as a routine so the quality of data is good. The possibility of under-reporting cannot be excluded, nor can its magnitude, if present, be determined. However, the outcomes resemble those from other studies done in LMIC18,19. It is possible that severely ill people are under-represented in this dataset. Selection bias may also influence the outcome of the study. Only patients who were followed up for up to 10 years are included in this study. As these patients should ideally have received the best care compared to those who were lost to follow-up, the study results may have underestimated the prevalent and incident cases of VI and blindness. However, the magnitude of VI in this study cohort indicates the need for implementation of national improvement programs. A further recommendation is the need for electronic medical records to ensure the roll out of frequent service evaluations and quality improvement programs.
In summary, our results reinforce the need for improved public awareness of sight threatening complications of diabetes, systematic DR screening and prompt treatment of PDR to reduce the magnitude of vision impairment and blindness in people with diabetes.
Data availability
The datasets generated during and/or analysed during the current study are not publicly available, as it is against the organization/hospital policy. But are available from the corresponding author on reasonable request.
Abbreviations
- PDR:
-
Proliferative diabetic retinopathy
- DME:
-
Diabetic macular edema
- ADED:
-
Advanced Diabetic Eye Disease
- LMIC:
-
Low and middle-income countries
- PRP:
-
Panretinal photocoagulation
- NVE:
-
Retinal neovascularization
- NVD:
-
Disc neovascularization
- BCVA:
-
Best-corrected visual acuity
- VI:
-
Visual impairment
- VA:
-
Visual acuity
- DR:
-
Diabetic retinopathy
- Anti-VEGF:
-
Anti-vascular endothelial growth factor
- ETDRS:
-
Early Treatment Diabetic Retinopathy Study
- VH:
-
Vitreous haemorrhage
- PRH:
-
Pre retinal haemorrhage
- WHO:
-
World Health Organisation
- US:
-
United States
References
The Diabetic Retinopathy Study Research Group. Photocoagulation treatment of proliferative diabetic retinopathy Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS Report Number 8. Ophthalmology. 88(7), 583–600 (1981).
Scanlon, P. H. The English National Screening Programme for diabetic retinopathy. Acta Diabetol. 54(6), 515–25 (2017).
Thomas, R. L., Halim, S., Gurudas, S., Sivaprasad, S. & Owens, D. R. IDF Diabetes Atlas: a review of studies utilising retinal photography on the global prevalence of diabetes related retinopathy between 2015 and 2018. Diabetes Res Clin Pract. 157, 107840 (2019).
Obeid, A. et al. Loss to follow-up in patients with proliferative diabetic retinopathy after panretinal photocoagulation or intravitreal anti-VEGF injections. Ophthalmology 125(9), 1386–1392 (2018).
Early Treatment Diabetic Retinopathy Study Research Group. Effects of aspirin treatment on diabetic retinopathy. ETDRS report number 8. Ophthalmology 98(5 Suppl), 757–65 (1991).
Gross, J. G. et al. Panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. Jama 314(20), 2137–46 (2015).
Sivaprasad, S. et al. Clinical efficacy of intravitreal aflibercept versus panretinal photocoagulation for best corrected visual acuity in patients with proliferative diabetic retinopathy at 52 weeks (CLARITY): a multicentre, single-blinded, randomised, controlled, phase 2b, non-inferiority trial. Lancet 389(10085), 2193–2203 (2017).
Gross, J. G. et al. Five-year outcomes of panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA Ophthalmol. 136(10), 1138–48 (2018).
Diabetic retinopathy study. Report Number 6. Design, methods, and baseline results. Report Number 7. A modification of the Airlie House classification of diabetic retinopathy. Prepared by the Diabetic Retinopathy. Invest Ophthalmol Vis Sci. 21(1 Pt 2), 1–226 (1981).
The Diabetic Retinopathy Vitrectomy Study Research Group. Early vitrectomy for severe vitreous hemorrhage in diabetic retinopathy. Two-year results of a randomized trial. Diabetic Retinopathy Vitrectomy Study report 2. Arch Ophthalmol. 103(11), 1644–52 (1985).
Varma, R. et al. Four-year incidence and progression of visual impairment in Latinos: the Los Angeles Latino Eye Study. Am J Ophthalmol. 149(5), 713–727 (2010).
Scanlon, P. H., Aldington, S. J. & Stratton, I. M. Delay in diabetic retinopathy screening increases the rate of detection of referable diabetic retinopathy. Diabet Med. 31(4), 439–442 (2014).
Bressler, S. B. et al. Panretinal photocoagulation versus ranibizumab for proliferative diabetic retinopathy: factors associated with vision and edema outcomes. Ophthalmology. 125(11), 1776–83 (2018).
Flaxman, S. R. et al. Global causes of blindness and distance vision impairment 1990–2020: a systematic review and meta-analysis. Lancet Glob Health 5(12), e1221–e34 (2017).
Gonzalez, V. H., Wang, P. W. & Ruiz, C. Q. Panretinal photocoagulation for diabetic retinopathy in the RIDE and RISE trials: not “1 and done”. Ophthalmology. https://doi.org/10.1016/j.ophtha.2019.08.010 (2019).
Lopez-Ramos, A. et al. Rapid assessment of avoidable blindness: Prevalence of blindness, visual impairment and diabetes in nuevo leon, Mexico 2014. Ophthalmic Epidemiol. 25(5–6), 412–418 (2018).
Rema, M., Sujatha, P. & Pradeepa, R. Visual outcomes of pan-retinal photocoagulation in diabetic retinopathy at one-year follow-up and associated risk factors. Indian J Ophthalmol. 53(2), 93–99 (2005).
Lartey, S. Y. & Aikins, A. K. Visual impairment amongst adult diabetics attending a tertiary outpatient clinic. Ghana Med J. 52(2), 84–87 (2018).
Choovuthayakorn, J. et al. Characteristics and outcomes of pars plana vitrectomy for proliferative diabetic retinopathy patients in a Limited Resource Tertiary Center over an eight-year period. J Ophthalmol. 2019, 9481902 (2019).
Acknowledgements
This work was part of ORNATE India study which is supported by Global Challenges Research Fund and UK Research and Innovation through the Medical Research Council grant number MR/P027881/1. The research was supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Moorfields Eye Hospital NHS Foundation Trust and UCL Institute of Ophthalmology. The views expressed are those of the author(s) and not necessarily those of the NHS, the NIHR or the Department of Health.
Author information
Authors and Affiliations
Contributions
S.S. and R.R.(1) contributed in conception and design of the study. S.S., R.R.(1), R.K., S.C., wrote the main manuscript text and prepared all the tables. R.K. S.G. and S.C. assisted with statistical analyses. G.A., R.R.(3), U.C.B, L.G., drafted the work and substantively revised it. R.R.(3), P.K.R., A.S., A.D., R.R.(1), S.N., L.C., G.C., U.C.B, helped in acquisition of the data. All authors reviewed the manuscript.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the articleΓÇÖs Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the articleΓÇÖs Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Khan, R., Chandra, S., Rajalakshmi, R. et al. Prevalence and incidence of visual impairment in patients with proliferative diabetic retinopathy in India. Sci Rep 10, 10513 (2020). https://doi.org/10.1038/s41598-020-67350-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-67350-6
- Springer Nature Limited