Abstract
Prior pooled analysis of eribulin studies (301 and 305) indicated eribulin prolonged overall survival (OS) in patients with locally advanced/metastatic breast cancer (MBC) regardless of visceral or nonvisceral disease. This hypothesis-generating post hoc analysis examined the efficacy of eribulin according to the location of metastatic sites at baseline in 1864 pretreated patients with locally advanced/MBC from studies 301 and 305. Analyses included OS, progression-free survival (PFS), and objective response rate; OS and PFS were also analyzed according to estrogen-receptor status. Eribulin appeared efficacious in patients with locally advanced/MBC, irrespective of the location of metastases at baseline. A nominally significant difference in OS in favor of patients randomized to eribulin compared with control in patients with bone, lymph node, and chest wall/breast/skin metastases at baseline was observed. Additionally, a difference in OS was also seen in patients with liver metastases randomized to eribulin versus control (median: 13.4 versus 11.3 months, respectively; hazard ratio, 0.84 [95% CI: 0.72, 0.97]). Results of this exploratory analysis suggest that eribulin may be efficacious for the treatment of locally advanced/MBC for patients with bone, liver, lung, lymph node, and chest wall/breast/skin metastases.
Similar content being viewed by others
Introduction
Metastatic breast cancer (MBC) is a heterogenous disease, with metastases commonly located in soft tissue (ie, skin, lymph nodes, and the contralateral breast), bone, and visceral organs (ie, lungs, pleura, peritoneum, liver, and brain)1. Although treatment for MBC has steadily improved, few agents have been shown to prolong survival and MBC remains essentially incurable with a median overall survival (OS) of 2–3 years2,3. Moreover, patients with visceral metastases typically have a worse prognosis than patients with nonvisceral metastases2,4,5,6.
Eribulin is a synthetic analogue of halichondrin B, originally isolated from the marine sponge Halichondria okadai7. The antitumor activity of eribulin is driven by a distinct mode of interaction with microtubules, in which eribulin inhibits the growth phase but not the shortening phase8,9,10,11. In vitro and preclinical studies suggest that eribulin may also have nonmitotic mechanisms including vascular remodeling, reversal of the epithelial-to-mesenchymal (EMT) transition, and inhibition of cancer cell migration/invasion12,13,14; some of these noncytotoxic effects have also been demonstrated clinically15. In the United States, eribulin is indicated for the treatment of patients with MBC who have previously received ≥2 chemotherapeutic regimens for the treatment of metastatic disease—prior therapy should have included an anthracycline and a taxane in either the adjuvant or metastatic setting. Additionally, approval for eribulin in MBC has been extended to the second-line metastatic setting in other geographic areas, including the European Union.
The efficacy of eribulin has been demonstrated in several phase 3 studies (Studies 305, 301, and 304) in patients with locally advanced or MBC that had been more heavily pretreated (Studies 305 and 304)16,17 or less heavily pretreated (Study 301)18. In the studies included in our analysis (Studies 305 and 301), pretreatment was required to include a taxane and an anthracycline16,18. In Study 305 (EMBRACE; NCT00388726), the primary end point was achieved with eribulin significantly improving OS compared with treatment of physicians’ choice (TPC; hazard ratio [HR], 0.81 [95% CI: 0.66, 0.99]; P = 0.041)16; an updated OS analysis confirmed this benefit in OS for eribulin (13.2 months versus 10.5 months; HR, 0.81 [95% CI: 0.67, 0.96]; P = 0.014)16. Study 301 compared eribulin with capecitabine (NCT00337103); eribulin was associated with a nonsignificant increase in OS compared to capecitabine (15.9 months versus 14.5 months, respectively; HR, 0.88 [95% CI: 0.77, 1.00]; P = 0.056)18. In both studies, the toxicity of eribulin was manageable and comparable to that of other chemotherapeutic agents used in this setting16,18.
Although eribulin is widely used in patients with previously treated MBC, its efficacy according to metastatic organ sites has not been well characterized. However, in one earlier subgroup analysis of Studies 305 and 301 combined19, eribulin improved OS compared with TPC/capecitabine in patients with visceral (median OS, 14.3 versus 12.2 months; HR 0.89; P = 0.037) and nonvisceral (median OS, 18.8 versus 16.6 months; HR 0.72; P = 0.045) disease; moreover, greater benefit was seen with eribulin compared with TPC/capecitabine in patients with >2 involved metastatic organ sites (median OS, 13.1 versus 10.5 months; HR 0.77; P < 0.001)19. In this current post hoc pooled exploratory analysis of Studies 30516 and 30118 we assess OS, progression-free survival (PFS), and objective response rate (ORR) according to organ sites of metastases at baseline for patients randomized to receive eribulin versus TPC/capecitabine. Additionally, we present the percentage change in the sum of the diameters of target lesions from baseline to postbaseline nadir according to sites of metastases (ie, waterfall plots) and OS and PFS according to metastatic site and estrogen-receptor (ER) status.
Methods
Patients
Patient eligibility criteria for Study 30516 and Study 30118 have been published. Briefly, eligible patients were women ≥18 years of age with histologically or cytologically confirmed breast cancer, an Eastern Cooperative Oncology Group performance status of 0–2, and adequate bone marrow, liver, and renal function. In Study 305, patients were previously treated with 2–5 chemotherapy regimens (of which ≥2 were for locally recurrent breast cancer or MBC)16, and in Study 301, patients were previously treated with ≤3 chemotherapy regimens (of which ≤2 were for advanced and/or metastatic disease)18.
The source Studies 305 and 301 were conducted in accordance with the World Medical Association Declaration of Helsinki, ethical standards of the responsible committee on human experimentation (institutional and national), and guidelines of the International Conference for Harmonization/Good Clinical Practice. Informed consent was obtained from all individual participants included in the studies and both studies were approved by ethics committees (Study 305: The New York Presbyterian Hospital-Weil Medical College of Cornell University Committee on Human Rights in Research [for the lead investigator in the United States]; Study 301: Committee for the Protection of Human Subjects [for the lead investigator in the United States]).
Study design and treatment
Studies 305 and 301 were phase 3, multicenter, open-label, randomized trials that compared the efficacy of eribulin with TPC (Study 305) or capecitabine (Study 301) in women with locally advanced/MBC16,18. In Study 305, patients were randomly assigned (2:1) to either eribulin mesylate (1.4 mg/m2 [equivalent to eribulin 1.23 mg/m2 when expressed as a free base]; administered intravenously over 2–5 minutes on days 1 and 8 of a 21-day cycle) or TPC (any single-agent chemotherapy, or hormonal or biological therapy approved for the treatment of cancer and administered according to local practice; radiotherapy; or symptomatic treatment alone). In Study 301, patients were randomly assigned (1:1) to either eribulin mesylate (1.4 mg/m2; intravenously over 2–5 minutes on days 1 and 8 of a 21-day cycle) or capecitabine (1.25 g/m2; orally twice daily on days 1–14 of a 21-day cycle). Treatment in both studies continued until disease progression, unacceptable toxicity, or patient/investigator request to discontinue.
Study end points
The primary end point of Study 305 was OS; secondary end points included PFS, ORR, and duration of response16. Tumor response was assessed with Response Evaluation Criteria In Solid Tumors (RECIST) version 1.020 and all secondary end points were carried out by independent masked review of tumor assessments. Additionally, sensitivity analyses were completed based on investigator review.
In Study 301, OS and PFS were co-primary end points18. Secondary end points included ORR, duration of response, safety, quality of life, and population pharmacokinetic/pharmacodynamic relationships. Tumor response was assessed with RECIST version 1.020 by independent radiology review (primary analysis) and investigator review (secondary analysis).
Findings for the primary and secondary end points of both studies have been published16,18.
Post Hoc subgroup analyses
Subgroup analyses of data pooled from Studies 305 and 301 were conducted to compare the efficacy of eribulin with TPC/capecitabine according to the organ sites of metastases. Patients were divided into subgroups according to metastatic sites. The following sites were observed in >5% of all patients at baseline and were included in this analysis: bone, liver, lymph node, chest wall/breast/skin, or lung. Target and nontarget lesions were included. Of note, patients with metastases in more than 1 organ site were included in the analyses for multiple subgroups.
Analyses included OS, PFS, ORR, and percentage change in the sum of diameters of target lesions from baseline to postbaseline nadir. Moreover, analyses of OS and PFS were conducted according to ER status in each subgroup.
Statistical methods
Analyses were conducted with pooled data and included patients in the intention-to-treat population (all patients randomly assigned to treatment groups). To determine median OS and PFS (including OS and PFS by ER status), estimates of survival were adjusted by study using methodology previously outlined in a separate pooled analysis of Studies 305 and 30119.
For all OS and PFS analyses, HR values and 2-sided 95% CIs were computed using Cox models with treatment as a covariate and stratified by study, region, prior capecitabine use, and HER2/neu status. Because these post hoc analyses involved multiple comparisons, all P values should be considered nominal and should not be used to directly determine statistical significance; nominal P values were based on log-rank tests and stratified as noted above. For ORR, odds ratios (95% CIs) of treatments were computed using Cochran-Mantel-Haenszel test, stratified by study. Tumor responses were based on investigator review per RECIST version 1.0.
Results
Patients
Patient dispositions for Study 305 and Study 301 have been reported elsewhere16,18. Overall, 1864 patients (Study 305, n = 762; Study 301, n = 1102) were included in this pooled post hoc subgroup analysis. Of these patients, 1062 were assigned to receive eribulin (Study 305, n = 508; Study 301, n = 554), 254 to receive TPC (Study 305), and 548 to receive capecitabine (Study 301)16,18. The mean dose intensity of eribulin in Study 305 was 0.78 mg/m2/week (standard deviation, 0.166); in Study 301, the mean dose intensity of eribulin was 0.81 mg/m2/week (standard deviation, 0.14), and the mean dose intensity of capecitabine was 9983.86 mg/m2/week (standard deviation, 1814.27).
In the pooled analysis, the median duration of treatment with eribulin was 119 days (range: 21–1372 days) and the median duration of treatment with TPC/capecitabine was 93 days (range: 1–1442 days).
Overall, the most common metastatic sites were in bone (eribulin, 57.0%; TPC/capecitabine, 58.1%) and liver (eribulin, 51.1%; TPC/capecitabine, 53.6%). Additional details regarding patient demographics and baseline characteristics have been published previously16,18.
Efficacy
Subgroup analyses of survival by metastatic site
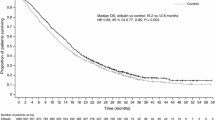
In the overall pooled analysis, patients randomized to receive eribulin had a nominally significant difference in OS compared with patients randomized to receive TPC/capecitabine (median: 14.9 versus 12.9 months; HR, 0.86 [95% CI: 0.77, 0.96]) (Fig. 1a). In addition, OS for the eribulin group differed from the TPC/capecitabine group across all metastatic sites (Fig. 1a). Of note, patients with liver metastases who were randomized to receive eribulin had a nominally significant difference in OS compared with patients who were randomized to receive TPC/capecitabine (median:13.4 versus 11.3 months; HR, 0.84 [95% CI: 0.72, 0.97]) (Fig. 2); a nominally significant difference in OS was also observed in patients with metastases in bone (median: 14.6 versus 12.5 months; HR, 0.76 [95% CI: 0.66, 0.88]), lymph nodes (median: 14.4 versus 11.8 months; HR, 0.82 [95% CI: 0.70, 0.97]), and chest wall/breast/skin (median: 15.5 versus 11.2 months; HR, 0.81 [95% CI: 0.66, 0.98]) (Fig. 1a).
Summary of overall survival (a) and progression-free survival (b) in patients with locally advanced/MBC, by site of lesions at baseline (pooled population). (a) Patients randomized to eribulin had a nominally significant difference in overall survival compared with control in the overall pooled population. Patients with bone, liver, lymph node and chest wall/breast/skin metastases had a nominally significant difference in overall survival with eribulin compared with TPC/capecitabine. (b) Overall, patients treated with eribulin had a nominally significant difference compared with control in progression-free survival. Assessment by baseline lesion location, revealed only patients with baseline bone metastases randomized to eribulin had a nominally significant difference in progression-free survival compared with TPC/capecitabine. Control includes TPC and capecitabine; HR values and 2-sided 95% CIs were computed using Cox models with treatment as a covariate and stratified by study, region, prior capecitabine use, and HER2/neu status; nominal P values were based on log-rank tests and stratified as noted above. Medians were based on survival curves adjusted by study. CI, confidence interval; HR, hazard ratio; MBC, metastatic breast cancer; TPC, treatment of physician’s choice.
Estimated overall survival in patients with locally advanced/MBC with liver (a) and lung (b) metastases at baseline (pooled population). (a) Patients with baseline liver metastases randomized to eribulin had a nominally significant difference in overall survival compared with patients randomized to TPC/capecitabine. (b) Patients with baseline lung metastases randomized to eribulin had no significant difference in overall survival compared to control. Control includes TPC and capecitabine; HR values and 2-sided 95% CIs were computed using Cox models with treatment as a covariate and stratified by study, region, prior capecitabine use, and HER2/neu status; nominal P values were based on log-rank tests and stratified as noted above. Medians were based on survival curves adjusted by study. CI, confidence interval; HR, hazard ratio; MBC, metastatic breast cancer; TPC, treatment of physician’s choice.
The median overall PFS was 4.0 months for patients randomized to eribulin versus 3.4 months for patients randomized to TPC/capecitabine; the HR for progression-free survival was 0.89 (95% CI: 0.80, 0.99) (Fig. 1b). In analyses of specific subgroups, only patients with bone metastases randomized to receive eribulin had a nominally significant difference in PFS compared with patients randomized to receive TPC/capecitabine (median: 4.1 versus 3.1 months; HR, 0.77 [95% CI: 0.67, 0.89]) (Fig. 1b). PFS results were similar in the eribulin group and TPC/capecitabine group for patients with other sites of metastases, including patients with metastases in the liver (median: 3.7 versus 2.8 months; HR, 0.87 [95% CI: 0.75, 1.02]) (Figs. 1b and 3a), lung (median: 3.7 versus 3.0 months; HR, 0.95 [95% CI: 0.81, 1.11]) (Figs. 1b and 3b), lymph nodes (median: 3.8 versus 3.2 months; HR, 0.96 [95% CI: 0.82, 1.12] (Fig. 1b), and chest wall/breast/skin (median: 3.6 versus 2.9; HR, 0.86 [95% CI: 0.71, 1.05]) (Fig. 1b).
Estimated progression-free survival in patients with locally advanced/MBC with liver (a) and lung (b) metastases at baseline (pooled population). (a) Progression-free survival was similar in patients with baseline liver metastases regardless of treatment. (b) Similar results were seen in patients with baseline lung metastases. Control includes TPC and capecitabine; HR values and 2-sided 95% CIs were computed using Cox models with treatment as a covariate and stratified by study, region, prior capecitabine use, and HER2/neu status; nominal P values were based on log-rank tests and stratified as noted above. Medians were based on survival curves adjusted by study. CI, confidence interval; HR, hazard ratio; MBC, metastatic breast cancer; TPC, treatment of physician’s choice.
The results of these pooled analyses are generally consistent with the results of the analyses of OS and PFS by metastatic organ site at baseline in the individual Studies 305 and 301 (Online Resource Supplemental Figs. 1 and 2).
Subgroup analyses of survival by metastatic site and ER status
In patients with ER-positive disease, nominally significant differences in OS were observed with the eribulin group compared with the TPC/capecitabine group in patients with metastases to the bone (median: 14.7 versus 13.5 months, respectively; HR, 0.79 [95% CI: 0.66, 0.96]) or liver (median: 14.4 versus 12.5 months, respectively; HR, 0.81 [95% CI: 0.66, 0.99]) (Fig. 4a); nominally significant differences in PFS were also seen with metastases to the bone (median: 4.2 versus 3.4 months, respectively; HR, 0.76 [95% CI: 0.64, 0.91]) or liver (median: 4.1 versus 2.8 months, respectively; HR, 0.79 [95% CI: 0.65, 0.96]) (Fig. 4b).
Overall survival (a) and progression-free survival (b) in estrogen-receptor–positive patients with locally advanced/MBC, by site of lesions at baseline (pooled population). (a) Overall survival was nominally significantly different in estrogen-receptor-positive patients with bone or liver metastases at baseline randomized to eribulin compared with TPC/capecitabine. (b) In the overall population, progression-free survival was nominally significantly different in estrogen-receptor-positive patients randomized to eribulin compared with control. Patients with bone or liver metastases at baseline randomized to eribulin had a nominally significant difference in progression-free survival compared with TPC/capecitabine. Control includes TPC and capecitabine. HR values and 2-sided 95% CIs were computed using Cox models with treatment as a covariate and stratified by study, region, prior capecitabine use, and HER2/neu status; nominal P values were based on log-rank tests and stratified as noted above. Medians were based on survival curves adjusted by study. CI, confidence interval; HR, hazard ratio; MBC, metastatic breast cancer; TPC, treatment of physician’s choice.
In patients with ER-negative disease, the eribulin group had nominally significant differences in OS compared with the TPC/capecitabine group in patients with metastases to the bone (median: 13.8 versus 8.6 months, respectively; HR, 0.62 [95% CI: 0.47, 0.83]), lung (median: 11.3 versus 8.7 months, respectively; HR, 0.77 [95% CI: 0.60, 0.99]), lymph nodes (median: 11.6 versus 8.8 months, respectively; HR, 0.69 [95% CI: 0.55, 0.88]), or chest wall/breast/skin (median: 12.2 versus 8.7 months, respectively; HR, 0.70 [95% CI: 0.52, 0.94]); a numerical difference in OS was observed in patients with liver metastases (median: 10.8 versus 8.5 months; HR, 0.75 [95% CI: 0.57, 1.00]) (Fig. 5a). Only patients with ER-negative disease and bone metastases had a nominally significant difference in PFS (median: 3.5 versus 2.8 months; HR, 0.72 [95% CI: 0.54, 0.96]) with randomization to eribulin versus TPC/capecitabine (Fig. 5b).
Overall survival (a) and progression-free survival (b) in estrogen-receptor–negative patients with locally advanced/MBC, by site of lesions at baseline (pooled population). (a) Overall survival was nominally significantly different in estrogen-receptor-negative patients in the overall population, and in patients with bone, lung, lymph node or chest wall/breast/skin metastases at baseline randomized to eribulin compared with TPC/capecitabine. (b) Progression-free survival was nominally significantly different only in estrogen-receptor-negative patients with bone metastases at baseline randomized to eribulin compared with TPC/capecitabine. Control includes TPC and capecitabine; HR values and 2-sided 95% CIs were computed using Cox models with treatment as a covariate and stratified by study, region, prior capecitabine use, and HER2/neu status; nominal P values were based on log-rank tests and stratified as noted above. Medians were based on survival curves adjusted by study. CI, confidence interval; HR, hazard ratio; MBC, metastatic breast cancer; TPC, treatment of physician’s choice.
Tumor response according to sites of metastases at baseline
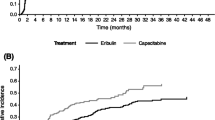
ORR was similar for patients randomized to eribulin (14.3% [95% CI: 12.3, 16.6]) and TPC/capecitabine (15.6% [95% CI: 13.1, 18.3] in the overall pooled population (Fig. 6). Individual analyses of ORR by site of metastases at baseline in Studies 305 and 301 are included in Online Resource Supplemental Figs. 3 and 4. A nominally significant difference in ORR between the treatment groups was observed for the overall population, bone metastases, and liver metastases in Study 305 (Online Resource Supplemental Fig. 3). For Study 301, none of the observed differences in ORR were nominally significant (Online Resource Supplemental Fig. 4).
Summary of tumor response as assessed by investigators per RECIST version 1.0, by site of lesions at baseline (pooled population). The overall response rates were similar across the pooled population regardless of location of metastases at baseline. Control includes TPC and capecitabine; odds ratio (95% CI) was calculated using Cochran-Mantel-Haenszel test, stratified by study. CI, confidence interval; RECIST, Response Evaluation Criteria In Solid Tumors; TPC, treatment of physician’s choice.
Analyses of percentage change in sum of diameters of target lesions from baseline to postbaseline nadir by metastatic site
Among patients with both baseline and postbaseline measurements of target lesions, 63% (544/857) randomized to receive eribulin and 63% (403/638) randomized to receive TPC/capecitabine experienced a reduction in tumor size in postbaseline nadir imaging studies (ie, percentage change in the sum of the diameters of the target lesions from baseline to postbaseline nadir imaging studies showed a reduction in tumor size). Of patients with liver metastases randomized to receive eribulin or TPC/capecitabine, 66% (n = 325/492) and 63% (n = 231/367) respectively, had reductions in tumor size on postbaseline nadir imaging studies (Fig. 7). Similar results for eribulin versus TPC/capecitabine were seen for patients with lung metastases (63% [n = 252/401] versus 61% [n = 190/309]), lymph node metastases (68% [n = 294/431] versus 65% [220/339]), and chest wall/breast/skin metastases (66% [n = 173/264] versus 62% [n = 131/212]) (Fig. 7).
Percentage change in sum of diameters of target lesions from baseline to postbaseline nadir, by metastatic site at baseline in patients randomized to receive eribulin (a) or control (b) (pooled population). Patients with liver, lung, lymph node or chest wall/breast/skin metastases showed decreased tumor sizes when treated with (a) eribulin or (b) TPC/capecitabine. n is the number of patients with both baseline and postbaseline measurements of target lesions.
Discussion
The efficacy of eribulin in locally advanced/MBC has been previously established in Studies 305 and 301, which demonstrated a statistically significant improvement in OS compared with TPC (Study 305: P = 0.041)16, and similar OS compared with capecitabine (Study 301: P = 0.056)18. The results from this post hoc exploratory pooled analysis are largely consistent with these findings. Our current analyses provide insights into the efficacy of eribulin with regard to the organ sites of metastases at baseline. Such insights are of potential relevance because a patient’s prognosis is dependent on the location of metastases at baseline. Specifically, patients with visceral disease (eg, metastases in the lung, liver, brain) are known to have reduced survival duration4, and among patients with visceral disease, metastases to the liver (median OS, 13 months) or brain (median OS, 7 months) generally portend the worst prognosis21.
The pooled analysis presented here suggests that eribulin may be an effective treatment option in patients with locally advanced/MBC, irrespective of their organ sites of metastatic disease at baseline (ie, bone, liver, lung, lymph nodes, and chest wall/breast/skin). In this analysis, nominal differences in OS (P = 0.02) were observed in favor of eribulin versus TPC/capecitabine in patients with liver metastases at baseline. ORRs were similar with eribulin (17.1%) and TPC/capecitabine (16.5%) in this population. Given the typically poor prognosis of patients with chemotherapy-pretreated bone, liver, lung, lymph nodes, and chest wall/breast/skin metastases, the efficacy of eribulin in these patients is noteworthy. Moreover, the ability of eribulin to reduce target lesion tumor size may be clinically significant in reducing metastases-associated symptoms and organ dysfunction.
The consistent efficacy of eribulin across metastatic sites is likely the result of its mechanism of action (ie, its unique interaction with microtubules)8,9,10,11. Eribulin’s efficacy against difficult-to-treat liver metastases, in particular, may be the result of a multifaceted interplay between eribulin’s mechanism of action and the complex microenvironment of the liver. Specifically, the potential for eribulin to reverse EMT and inhibit cancer cell migration/invasion may provide a mechanistic advantage for eribulin in the treatment of liver metastases, given that liver sinusoidal endothelial cells have been shown to induce EMT in colorectal cancer cells12,13,14,22.
Of interest, the absolute prolonged median OS time difference with eribulin compared with control was 2.0 months (eribulin, 14.9 months; control, 12.9 months), whereas the absolute prolonged time of median PFS difference with eribulin compared to control was 0.6 months (eribulin, 4.0 months; control, 3.4 months). Previous studies with eribulin have suggested that the discordance between OS and PFS improvement may be partly due to eribulin’s ability to remodel the tumor microenvironment and vasculature; such remodeling may improve the antitumor activity of eribulin, as well as positively impact the efficacy of subsequent anticancer therapies, resulting in improved OS13,23. Additionally, a previous study by Twelves et al.24 indicated that the development of new metastasis is a risk factor for shorter OS. As both growth of existing metastasis and development of new metastasis are considered “progression” in terms of PFS, whereas the more aggressive form of progression (ie, development of new metastasis) is considered a risk factor for shorter OS, the difference between these forms of progressive disease may not be captured by the PFS end point but instead may be reflected in the OS end point. Moreover, in support of this theory, Twelves et al. showed that new-metastasis-free survival was longer in the eribulin arm than in the comparator arm in EMBRACE, which could account for the improved OS seen with eribulin treatment24. This may provide a rationale for the more prolonged OS, but not PFS, observed in eribulin-treated patients in the current analysis.
This analysis is limited by the relatively small number of patients in each subgroup, its post hoc nature, and the multiple testing comparisons; as such, P values are considered nominal. Despite these limitations, the results of this post hoc analysis are consistent with previous findings of eribulin’s efficacy in locally advanced/MBC16,18, and suggest that eribulin can be an effective treatment option in patients with bone, liver, lung, lymph node, and chest wall/breast/skin metastases.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available, but are available from the corresponding author on reasonable request. However, information that could be used to identify patients are not available, nor was any such information available to any of the authors.
References
Kamby, C. et al. Pattern of spread and progression in relation to the characteristics of the primary tumour in human breast cancer. Acta Oncol. 30, 301–308 (1991).
Lobbezoo, D. J. et al. Prognosis of metastatic breast cancer: are there differences between patients with de novo and recurrent metastatic breast cancer? Br. J. Cancer 112, 1445–1451 (2015).
Cardoso, F. et al. 1st International consensus guidelines for advanced breast cancer (ABC 1). Breast 21, 242–252 (2012).
Chang, J. et al. Survival of patients with metastatic breast carcinoma: importance of prognostic markers of the primary tumor. Cancer 97, 545–553 (2003).
Savci-Heijink, C. D. et al. Retrospective analysis of metastatic behaviour of breast cancer subtypes. Breast Cancer Res. Treat. 150, 547–557 (2015).
Khanfir, A. et al. Prognostic factors and survival in metastatic breast cancer: A single institution experience. Rep. Pract. Oncol. Radiother. 18, 127–132 (2013).
Towle, M. J. et al. In vitro and in vivo anticancer activities of synthetic macrocyclic ketone analogues of halichondrin B. Cancer Res. 61, 1013–1021 (2001).
Jordan, M. A. et al. The primary antimitotic mechanism of action of the synthetic halichondrin E7389 is suppression of microtubule growth. Mol. Cancer Ther. 4, 1086–1095 (2005).
Okouneva, T., Azarenko, O., Wilson, L., Littlefield, B. A. & Jordan, M. A. Inhibition of centromere dynamics by eribulin (E7389) during mitotic metaphase. Mol. Cancer Ther. 7, 2003–2011 (2008).
Smith, J. A. et al. Eribulin binds at microtubule ends to a single site on tubulin to suppress dynamic instability. Biochemistry 49, 1331–1337 (2010).
Dybdal-Hargreaves, N. F., Risinger, A. L. & Mooberry, S. L. Eribulin mesylate: mechanism of action of a unique microtubule-targeting agent. Clin. Cancer Res. 21, 2445–2452 (2015).
Agoulnik, S. I. et al. Eribulin mesylate exerts specific gene expression changes in pericytes and shortens pericyte-driven capillary network in vitro. Vasc. Cell 6, 3 (2014).
Funahashi, Y. et al. Eribulin mesylate reduces tumor microenvironment abnormality by vascular remodeling in preclinical human breast cancer models. Cancer Sci. 105, 1334–1342 (2014).
Yoshida, T. et al. Eribulin mesilate suppresses experimental metastasis of breast cancer cells by reversing phenotype from epithelial-mesenchymal transition (EMT) to mesenchymal-epithelial transition (MET) states. Br. J. Cancer 110, 1497–1505 (2014).
Ueda, S. et al. In vivo imaging of eribulin-induced reoxygenation in advanced breast cancer patients: a comparison to bevacizumab. Br. J. Cancer 114, 1212–1218 (2016).
Cortes, J. et al. Eribulin monotherapy versus treatment of physician’s choice in patients with metastatic breast cancer (EMBRACE): a phase 3 open-label randomised study. Lancet 377, 914–923 (2011).
Yuan, P. et al. Eribulin mesilate versus vinorelbine in women with locally recurrent or metastatic breast cancer: A randomised clinical trial. Eur. J. Cancer 112, 57–65 (2019).
Kaufman, P. A. et al. Phase III open-label randomized study of eribulin mesylate versus capecitabine in patients with locally advanced or metastatic breast cancer previously treated with an anthracycline and a taxane. J. Clin. Oncol. 33, 594–601 (2015).
Twelves, C. et al. Efficacy of eribulin in women with metastatic breast cancer: a pooled analysis of two phase 3 studies. Breast Cancer Res. Treat. 148, 553–561 (2014).
Therasse, P. et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J. Natl. Cancer Inst. 92, 205–216 (2000).
Yücel, B. et al. Importance of metastasis site in survival of patients with breast cancer. Austin J. Med. Oncol. 1, 7 (2014).
Brodt, P. Role of the microenvironment in liver metastasis: From pre- to prometastatic niches. Clin. Cancer Res. 22, 5971–5982 (2016).
Cortes, J., Schöffski, P. & Littlefield, B. A. Multiple modes of action of eribulin mesylate: emerging data and clinical implications. Cancer Treat. Rev. 70, 190–198 (2018).
Twelves, C. et al. “New” metastases are associated with a poorer prognosis than growth of pre-existing metastases in patients with metastatic breast cancer treated with chemotherapy. Breast Cancer Res 17, 150 (2015).
Acknowledgements
We would like to thank all of the patients, as well as the Investigators and their teams, who participated in EMBRACE (Study 305) and Study 301. Additionally, we would like to thank Vivian Zeng for her support with data analysis. This study was funded by Eisai Inc., Woodcliff Lake, NJ. Medical writing support was provided by Jeffrey Bratz, PhD, Oxford PharmaGenesis, Newtown, PA, USA, and was funded by Eisai Inc., Woodcliff Lake, NJ, USA.
Author information
Authors and Affiliations
Contributions
All authors assisted with the design and conduct of the analysis, writing the first draft, critically reviewing subsequent drafts, and approved of the final draft for submission. J.O’S. participated in all aspects of the analysis and manuscript development and takes responsibility for the final content and decision to submit.
Corresponding author
Ethics declarations
Competing interests
Joyce O’Shaughnessy: Financial: Honoraria from Seattle Genetics. Speaking fees from Blue Earth Diagnostics, and Novartis. Vendor-sponsored travel from AstraZeneca, Blue Earth Diagnostics, Eli-Lilly, Merck, Novartis, and Seattle Genetics. Consultant/advisory role for AstraZeneca, Celgene, Eli-Lilly, LaRoche, Lilly USA, Merck, Novartis, and Pfizer. Nonfinancial: Nothing to disclose. Javier Cortes: Financial: Consulting/Advisor for Roche, Celgene, Cellestia, AstraZeneca, Biothera Pharmaceutical, Merus, Seattle Genetics, Daiichi Sankyo, Erytech, Athenex, Polyphor, Lilly, Servier, and Merck Sharp & Dohme. Honoraria from Roche, Novartis, Celgene, Eisai, Pfizer, Samsung, Lilly, and Merck Sharp & Dohme. Research funding (to the Institution) from Roche, Ariad Pharmaceuticals, AstraZeneca, Baxalta GMBH/Servier Affaires, Bayer Healthcare, Eisai, F. Hoffman-La Roche, Guardant Health, Merck Sharp & Dohme, Pfizer, Piqur Therapeutics, Puma C, Queen Mary University of London, and Seagen. Stock, patents, and intellectual property with MedSIR. Nonfinancial: Nothing to disclose. Chris Twelves: Financial: Personal fees and costs of clinical trials from Eisai during the conduct of the study. Personal fees and costs related to attending academic meetings from Pfizer; personal fees and costs related to clinical trials from Nektar; costs related to attending academic meetings from Roche/Genentech; and costs related to meeting attendance from Amcure, all outside the submitted work. Nonfinancial: Eisai. Lori J. Goldstein: Financial: Consultant/advisor for Merck, Genentech, Syndax, Immunomedics, Aduro, Genomic Health, Amgen, and Mylan; research support for Merck and Genentech; CME lecture for Genomic Health and Teva; DSMB for Daiichi Sankyo. Nonfinancial: Patient education for the Komen Foundation. Karenza Alexis: Financial: Former employee of Eisai Inc. Nonfinancial: Nothing to disclose. Ran Xie: Financial: Current employee of Eisai Inc. Nonfinancial: Nothing to disclose. Carlos Barrios: Financial: Clinical research support from AbbVie, Amgen, Astellas Pharma, AstraZeneca, Bristol-Myers Squibb, Celgene, Covance, Lilly, Medivation, Merck Serono, Merck Sharp & Dohme, Novartis, Pfizer, PharmaMar, and Roche/Genentech. Advisory board and consulting fees from Boehringer-Ingelheim, GSK, Novartis, Pfizer, Roche/Genentech, and Eisai. Nonfinancial: Nothing to disclose. Takayuki Ueno: Financial: Personal fees from Chugai Pharmaceutical Co., Ltd., Eisai Co., Ltd., AstraZeneca K.K., Taiho Pharmaceutical Co., Ltd., and Novartis Pharma K.K., outside the submitted work. Nonfinancial: Nothing to declare.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
O’Shaughnessy, J., Cortes, J., Twelves, C. et al. Efficacy of eribulin for metastatic breast cancer based on localization of specific secondary metastases: a post hoc analysis. Sci Rep 10, 11203 (2020). https://doi.org/10.1038/s41598-020-66980-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-66980-0
- Springer Nature Limited