Abstract
Tropical and subtropical dry forests make up the world’s largest terrestrial ecosystem. However, these forests have been used to establish several productive activities, such as growing crops, rearing livestock, and using the forest resources, due to their ease of access and climatic conditions, which has led to this ecosystem becoming highly threatened. Therefore, this research assessed the effects of anthropogenic pressures and a number of abiotic variables on natural regeneration in dry forests in the Tumbesian region by addressing three research questions: (a) What is the status of natural regeneration in terms of abundance and diversity? (b) Does livestock grazing and the anthropogenic pressure affect the abundance and diversity of natural regeneration? (c) Does seasonality or grazing have the greatest influence on the regeneration dynamics? Data were obtained from 72 samples (36 fenced and 36 unfenced) during five surveys spanning a 2-year period, and the seedling abundance, mortality, recruitment, species richness and diversity were evaluated using linear mixed models. Natural regeneration was most positively affected by rainy season precipitation, but soil conditions also played an important role. Short-term fences had a major effect on reducing mortality but did not improve the abundance or diversity, whereas cattle grazing significantly affected the abundance of seedlings.
Similar content being viewed by others
Introduction
Scientists have been depicting the threats that tropical dry forests are exposed to for many years1,2,3,4,5,6 and have identified some parts of this ecosystem as places of tremendous diversity and endemism7,8,9. These insights have supported the establishment of new protected areas in several regions of the world–for instance, the Brazilian Minister of the Environment identified 52 priority areas for conservation in the Caatinga in 2002, 27 of which were catalogued as an extreme priority10; Koleff et al.11 stated in 2012 that 42% of the Mexican tropical dry forests should be protected; several private and state-owned areas were added to the list of protected areas in Ecuador, including the ‘Bosques de Paz’ Biosphere Reserve, in 201712; and one of the most representative areas of Peru was placed under protection by the Amazonas Department in 201813. However, although the number of protected areas has increased and a large amount of research has been undertaken on the biophysical aspects of tropical dry forests of Latin America, Caribbean and Africa14,15, little is known about the ecology and regeneration of tropical dry forests16 or the effects of domestic animals and other threats on the natural regeneration of these forests, which are still diminishing in size14.
The tropical dry forest that extends along the Pacific coast from the southwest of Ecuador to the northwest of Peru covers approximately 64,500 km2 17 and faces similar issues18. This region, which is named the ‘Tumbesian region’, is known for its high level of endemism among woody species7,19 and for being one of the better preserved areas in the region20,21. Some effort has been made to understand the ecosystem functionality and the effects of animals on the forest in this region, examples of which include the studies of Jara-Guerrero et al.22, Espinosa et al.18,23,24, Piana & Marsden25 and Cueva et al.26, who evaluated the influence of environmental conditions, anthropogenic disturbances or soil characteristics on seed dispersal or composition of mature dry forest. However, little attention has been paid to the natural regeneration, which has rarely been studied in the region (neither that generated by sprouts nor by seeds), despite this being a key component of the sustainability of the forest27. Studies that have been conducted include an assessment of the effect of goats in one village of Ecuador by Rodriguez in 200628, a very short report on a protected area in Peru by Abou et al. in 201029, and a structural and compositional characterisation of regeneration in three dry forest types in Ecuador by Aguirre et al. in 201330.
As with most tropical dry forests, natural regeneration in the Tumbesian region is affected by seasonality, which is characterised by a long period of drought for 7 months of the year31, as well as by human activities, particularly the grazing of domestic animals20. However, the extent to which natural regeneration is affected by these biotic and abiotic factors remains poorly understood. Therefore, in this study, we attempted to fill these knowledge gaps by addressing the following research questions: (a) What is the status of natural regeneration in the dry forest in terms of abundance and diversity, taking into account seasonality and grazing? (b) Do livestock grazing and other anthropogenic pressures affect the abundance and diversity of natural regeneration in the dry forest? (c) Does seasonality or grazing have the greatest influence on the dynamics of natural regeneration in the dry forest?
Results
Status of natural regeneration
Measurements were made across a total of 1,152 m2 of dry forest during five surveys (four in fenced plots), that were carried out over a nearly 2-year period. The total number of seedlings recorded in fenced plus unfenced plots in the evaluated area were: 6,280; 4,751; 5,069 and 4,572 individuals in surveys 2, 3, 4 and 5 respectively. The average number of seedlings per plot projected to one hectare and computed for each survey varied from 21,900 to 57,200 individuals in unfenced plots and from 43,500 to 51,800 individuals per ha in fenced plots (Fig. 1a). The average abundance decreased slightly from the rainy season to the dry seasons, but remained relatively constant when measurements from the same season and treatment were compared. This seasonal effect was less pronounced in the fenced plots than the unfenced plots due to the lower dry season mortality (Fig. 1b) resulting in similar numbers of individuals occurring in the two seasons (median number of individuals per ha = 35,300–41,200 in fenced plots and 26,900–40,600 in unfenced plots).
Changes in the natural regeneration parameters through time and under different treatments. (a) Abundance, (b) mortality, (c) recruitment, (d) species richness and (e) diversity of seedlings on each survey date in unfenced and fenced plots. Fenced plots were not assessed in survey 1 because they were selected based on the first evaluation of the unfenced plots. Numbers from 1 to 5 correspond to consecutive surveys, which spanned a 2-year period. Yellow = dry season, blue = rainy season.
In the first rainy season, Simira ecuadorensis (Standl.) Steyerm and Erythroxylum glaucum O. E. Schulz had the highest abundances in the study area (1,447 and 1,371 individuals, respectively). Simira ecuadorensis showed good resistance to the dry season, with 1,561 individuals being recorded in the final survey. By contrast, most E. glaucum individuals died during the dry season, with only 265 individuals being recorded alive in the final survey.
During the monitoring period, the mortality of plants was influenced by seasonality, increasing from an average of 9.0% in the rainy season to 35.8% in the dry season, with the same pattern being observed across both treatments (Fig. 1b). However, mortality was slightly lower in the fenced plots than in the unfenced plots. Substantial recruitment was recorded in the fenced plots in surveys 3 and 4 (21.4% and 16.6%, respectively), whereas recruitment was very low in the final survey (3.1%). By contrast, recruitment decreased over time from 16.1% to 3.7% in the unfenced plots, even during the rainy season (Fig. 1c).
A total of 85 species from 31 families were recorded in the study area (see Supplementary Table S1), which included 37 tree species, 36 shrub species and 12 species that could not be identified. The number of species remained almost the same across surveys 2 to 5 in the unfenced plots (mean = 8.8, 8.6, 9.1 and 8.7 species per plot, respectively) but showed a slight increase over time in the fenced plots (mean = 8.4, 8.8, 9.6 and 9.4 species per plot) (Fig. 1d). A similar pattern was also observed for Simpson’s index, which had mean values ranging from 0.62 in survey 1 to 0.67 in survey 4 for the unfenced plots and 0.62 in survey 3 to 0.66 in survey 4 for the fenced plots (Fig. 1e). Surprisingly, during the evaluation period no seedlings were recorded for some of the characteristic species32 of the study area, including Ceiba trischistandra (A.Gray) Bakh., Cavanillesia platanifolia (Bonpl.) Kunth, Eriotheca roseorum (Cuatrec.) A.Robyns and Myroxylon balsamum (L.) Harms. Furthermore, only a few individuals of other species were recorded, such as Centrolobium ochroxylum Rose ex Rudd (one individual) and Loxopterygium huasango Spruce ex Engl. (two individuals).
Effects of biotic and abiotic factors on natural regeneration
Model selection revealed that the amount of variance in abundance that could be explained by the best models ranged from 10% to 13% for the fixed effects and from 56% to 60% for the fixed + random effects. Abundance appears to be more influenced by pressure predictors (Table 1). By contrast, the amount of variance in species richness that could be explained by the best models ranged from 51% to 63% for the fixed effects and equated to 72% for the fixed + random effects. The diversity was explained only by the soil predictors; just one model was selected, which explained 40% of the variance using the fixed effects and 53% of the variance using both fixed + random effects.
Four models were identified as best explaining the abundance of natural regeneration (Table 1). All of these models included rainy season precipitation (SPrec) and three included the interaction between the presence of horses and donkeys (Equine) and treatment (Treat). Only the best model included the presence of cows and bulls (Cattle) as an independent predictor, while the second and third best model include this predictor as a term of interaction. The four-way interaction between the presence of goats (Goats), Cattle, Equine and Treat was included in the second-best model, and the interaction between Cattle and Treat was included in the third-best model.
Both Equine and SPrec had significant positive effects on the abundance of natural regeneration, with the latter having the most evident positive effect (Table 2; Fig. 2a,b). By contrast, Cattle had a significant negative effect on abundance (Table 2; Fig. 2c), indicating that natural regeneration is affected by the grazing of cows and bulls. While fences (Treatopen) did not affect the number of individuals when it was used as main predictor (Fig. 2d), the interaction Treat and Equine had a significant negative effect (Table 2; Fig. 2e), demonstrating that the abundance of regenerating seedlings was lower in unfenced sites where horses were present than in fenced sites where horses had previously been present.
Species richness was best explained by five models, all of which included SPrec, four of which included soil depth (SDepth) and drainage (Drain), and three of which included Treat (Table 1). All of these predictors were included in the best model.
SPrec had a significant positive influence on species richness (Table 2), indicating that species richness was higher in the rainy season. Species richness was also significantly higher in sites with deeper soils than in sites with shallow soils (<10 cm) and in sites with moderately and well-drained soils than in sites with compacted or non-permeable soils (Table 2). As seen for abundance, fences did not affect the species richness in our study area.
Only one model was selected for diversity, which included soil drainage and texture (Text) as predictors (Table 1). Moderately drained soils had a significant positive effect on the diversity compared with poorly drained soils, and sites with clay loam and loam soils had a significantly higher diversity than those with clay–sandy loam soils (Table 2).
Regarding the dynamics of natural regeneration, 36% and 56% of the variance in mortality was explained by the fixed and fixed + random effects, respectively, that were included in the best model, while 30–35% and 60–61% of the variance in recruitment was explained by the fixed and fixed + random effects, respectively (Table 3).
Only one model was identified as best explaining mortality, which included SPrec, Treat and elapsed time (Time) as fixed effects (Table 3). Both SPrec and Time had significant negative effect on mortality (Table 4), indicating that mortality was lower in the rainy season than in the dry season and also decreased with elapsed time through the monitoring period. In addition, the mortality of natural regeneration was significantly higher in unfenced plots than in the fenced plots.
The two best models explaining recruitment both included SPrec and Time as predictors (Table 3). While both of these predictors had a positive influence on recruitment, only SPrec was significant (Table 4), indicating that the recruitment of new individuals increases during the rainy season. There was also a significant negative interaction between these predictors on the recruitment of new seedlings, demonstrating that the effect of the rainy season precipitation decreased as time elapsed or the effect of time decreased as rainfall increased. Fences did not affect the rate of recruitment.
Discussion
State of natural regeneration
Seasonality had a large influence on the number of individuals throughout the study period, with the abundance increasing during the rainy season and decreasing during the dry season, as would be expected (Fig. 1a). This difference was particularly evident in the unfenced plots. By contrast, Rodriguez28 found that the number of individuals decreased over time in both unfenced and fenced plots in a smaller part of the same study area, even during the rainy season. Our finding that there was an almost constant number of individuals in the fenced plots showed that a fencing effect mainly occurred during the dry season, when food availability was lower and animals needed to be meticulous in searching for food. Similarly, in an evaluation of the effects of ungulates (cattle and goats) in Hawaii, Cabin et al.33 found that there was a much higher abundance of seedlings in a year that was catalogued as being rainy (1997) compared with a year that was exceptionally dry (1998), when there was a marked decrease in the number of seedlings across both treatments and no additional individuals of the most abundant species [Diospyros sandwicensis (A.DC.) Fosberg] throughout the year.
Previous studies have also found that mortality is highly seasonal28,33,34. For example, Rodriguez28 reported maximum mortalities of 20% in the rainy season and 67% in the dry season (across 4 and 2 months, respectively), and Lieberman & Li34 reported maximum mortalities of 12% in the rainy season and 33% in the dry season (across 2 months in both cases). Similarly, in the present study, we obtained maximum mortality values of 23% for the rainy season and 85% for the dry season (across 4 and 6 months, respectively). If we make the same comparison for the fenced plots, the maximum values were 23% for the rainy season and 36% for the dry season (across 2 months for each) in the study of Rodriguez28 and 35% in the rainy season and 87.5% in the dry season (across 4 and 6 months, respectively) in the present study. These findings indicate that mortality during the dry season was much higher in the present study than has previously been reported, which may have resulted from the low number of individuals in some samples resulting in most or all of the seedlings (e.g. two from a total of three seedlings) dying before the next evaluation.
Lieberman & Li34, Cabin et al.33 and Vieira & Scariot35 stated that both mortality and recruitment are highly seasonal in tropical dry forests, with higher mortality in the dry season and higher recruitment in the rainy season. By contrast, we found that there was a continuous decrease in recruitment in the unfenced plots, even during the rainy season (Fig. 1c). However, recruitment in the fenced plots was closer to their prediction – for example, there was similar recruitment between the first dry period and the next wet period, followed by a sharp decrease in the second dry period (Fig. 1c). The unclear seasonal influence in our study area could be explained by the unusual climatic situation during the previous rainy season (survey 2), when precipitation was restricted to only two or three relevant events in January, almost no rainfall in February and a short rainy period from March to May, followed by some light showers even in June, which contrasts with the continuous rains that usually occur from December to March. Therefore, it is likely that several seedlings that established during the wet period (after our assessment) were recorded in the dry period in survey 3.
It has previously been noted that seed banks in tropical dry forests contain low numbers of seeds and species18,36. Ceccon et al.36 and Ray & Brown37 argued that the low number of species with high seed germination rates and high mortality rates was mainly caused by environmental stress, predation and dispersal restrictions and, according to Espinosa et al.18, our study area is also affected by these conditions. Therefore, since the low availability of seeds negatively affects the species richness and abundance, shoot propagation is considered more important than seed propagation in the study area18,30.
The total number of species we recorded was considerably higher than the 21 species reported by Aguirre et al.30 for the Ecuadorian part of the same study area and the 53 species reported by Lieberman & Li34 in a small 120-ha tropical dry forest patch located in Ghana. As in the present study, Rodriguez28 detected a small difference in species richness between treatments, with a slightly higher number of species and diversity index in fenced plots. However, unlike our results, Rodriguez28 also found that the number of species decreased over time in fenced plots, which could be related to the fact that recruitment was not accounted for. Similarly, Cabin et al.33 found that plots in a preserved area that had been fenced for 40 years had a larger number of species than unfenced plots, as well as a larger number of native than exotic species.
Species that were characteristic of the study area32, such as Ceiba trischistandra, Cavanillesia platanifolia, Eriotheca roseorum and Myroxylon balsamum, were previously recorded in a mature forest inventory26 and, according to Aguirre & Kvist20, populations of the first two of these species have been maintained or may even have recovered. However, we did not record seedlings of these species in our study, and seedlings of Centrolobium ochroxylum and Loxopterygium huasango were scarce. This situation was also pointed out by Aguirre et al.30, who attributed it to a few species having abundant regeneration in dry forests and strong perturbations. However, it may also be caused by the low diversity in the seed bank in this region18 or the presence of selective seed or seedling predators.
Predictors affecting the natural regeneration
One factor that has been shown to determine the establishment, survival and development of natural regeneration in dry forest is the availability of water18,38. Similarly, in the present study, seasonal precipitation (the rainy season) had the highest positive influence on all of the parameters analysed except diversity, with the abundance of seedlings, the number of species and recruitment increasing significantly and mortality decreasing significantly during the rainy season (Tables 2 and 4). Similar results were also presented by Espinosa et al.18, Lieberman & Li34, Cabin et al.33 and Vieira & Scariot35. These effects can be attributed to the higher water availability, the accelerated decomposition of organic matter18, and the higher concentration and uptake of nutrients that accumulate in the soil through the dry season when the uptake by vegetation is lower39.
It is well known that unusual events such as El Niño bring major changes in the atmosphere, with temperatures that can exceed the normal average by 2 °C, while the rainfall over Peru and Ecuador can greatly increase the vegetation cover of dry lands40. Therefore, a longer assessment could allow the effects of these events on natural regeneration to be analysed.
Although treatment was included in the best models for all of the parameters except diversity, fencing only had a significant positive effect on mortality and by interaction with Equine on abundance. By contrast, Rodriguez28 and Cabin et al.33 found a meaningful difference between unfenced and fenced samples for aspects such as species richness and abundance. In the present study, species richness and recruitment exhibited only small differences between fenced and unfenced plots (Tables 2 and 4). Furthermore, although well-established young individuals between 2–3 m of height were found on private land with longer exclusion times (approximately 6 years), these were not recorded in any of our plots and only a few have been reported in the study area30. Therefore, we believe that our observation time of 2 years may not have been sufficient to reveal direct fencing effects.
Fences were found to be associated with decreased mortality (Table 3), which is consistent with the findings of Rodriguez28. This is because fences reduce the impact of animals that eat and trample seedlings20 and of people that damage individual plants when they walk through the forests or carry out extractive activities.
Animals affected only the abundance of seedlings, with cattle having significant negative effects and horses and donkeys having significant positive effects (Table 2). This difference may have been caused by the current numbers of these animals in the area. It has previously been shown that cattle exert a negative effect on the structure of mature forests, particularly in terms of the abundance of individuals41,42,43, but there is little information about the effects of horses or donkeys on mature and young plants. Since these groups of animals have similar physical traits and food preferences, we believe that they are highly likely to have a similar impact on the forest, suggesting that the number of animals is a critical factor for the level of impact, i.e. a high number of horses or cows would lead to a high impact. Horses and donkeys were once an essential means of transport, but this function has decreased since the introduction of motorcycles. By contrast, cattle are used for meat, milk or sale, leading to a considerable increase in number (Fig. 2f). The reduced number of horses could have favoured regeneration through the contribution of manure, the scarification or distribution of seeds, reduced trampling and the low number of seedlings being eaten. When considering these results, it is important to note that our evaluation of the influence of animals was based on faecal samples, which decompose following exposure to environmental conditions such as sun, wind and water. Therefore, the effects of animals should be interpreted with caution.
Wild animals could also affect natural regeneration as well as leaf litter covering the seedlings. However, they were not considered in this study, because of the difficulty to obtain suitable data and the limited budget.
Soil depth and drainage were important predictors of species richness, with soils that were >10 cm deep and better drained hosting larger numbers of species (Table 2). Alban et al.44 previously reported that Prosopis pallida seeds that were sown superficially (5 cm depth) were damaged or eaten by ants and lizards, and surface seeds will also be accessible to selective birds and rodents. Furthermore, nutrients are transported by leaching rainwater towards deeper soils39, leading to a better nutritive status of deeper seeds compared to those at the surface. Since this leaching will depend on the soil drainage capacity, the lower species richness and diversity that occurs in poorly drained soils can also be explained by lower nutrient movement into the soil.
Soil texture was a significant predictor of diversity, with clay loam and loam soils having higher levels of diversity than clay–sandy loam soils (Table 2). This can be explained by the high nutrient and water storage capacity, good aeration, and good root penetration of loam soils compared with sandy soils, all of which favour germination and plant development45.
Elapsed time was an important predictor of mortality and recruitment (Table 3). Similarly, Rodriguez28 showed that mortality exhibited a constant decrease over time that was disrupted only by the rainy season. This effect was expected because the number of individuals decreases throughout the dry season and the surviving individuals will have adapted to those conditions, thus decreasing mortality.
In contrast to our findings in the mature forest26, there was no evidence that the human pressure index (HPI) affected natural regeneration. One likely reason for this is that fencing restricted the activities of humans as well as animals within the plots. We also found that there was a high correlation between HPI and 10 of the other 13 predictors we used [all except SPrec, Treat and stoniness (Ston); see Supplementary Table S2], which may have limited the inclusion of this predictor in the models.
Lieberman & Li34 identified canopy closure as an important predictor of forest density. However, as for HPI, canopy closure was highly correlated with several of the predictors considered in our study and was considered in only 12 models (see Supplementary Table S3). However, we found that both the number of species and the abundance of individuals tended to increase with canopy closure. This would be expected if we consider that a closed canopy favours seed germination and seedling growth because it helps to prevent the seeds of species that are dispersed by wind and gravity, which are usually produced in the dry season46,47, from becoming desiccated by high temperatures. Furthermore, a closed canopy also helps to prevent fresh fruits and seeds that are dispersed by animals, which mainly occur in the rainy season47, from being washed away during heavy rainfall.
Conclusions
Seasonal precipitation is a highly influential predictor of natural regeneration in the dry forest. However, unfortunately, the rainy season is also the period with the greatest pressures due to the high availability of food for animals. Therefore, suitable management strategies should be introduced to help halt the loss of abundance and richness due to aspects not related to drought. These programmes could be coordinated by local government and supported by farmers’ organisations and communities and could include compensation for farmers for losses during the temporary closure of certain areas for 5 years or more.
Since an almost constant species richness was recorded during the 2 years of assessment, we assume that this parameter is not affected by anthropogenic activities or abiotic factors in our study area in the medium term. Furthermore, a substantial number of seedlings was recorded during the rainy period. However, more accurate information on the mortality, recruitment and abundance of individuals during the distinct phases of regeneration is essential for determining whether the forest structure will be affected in the near future.
While the use of fences to exclude all types of activity is important in the study area, this will not affect the diversity and structure of the future forest if they are only established for a short period of time. Therefore, fences must be maintained on a medium- to long-term basis.
Cattle currently deserve special attention, as they are affecting the abundance of seedlings. Control measures have already been introduced at some sites in the study area, such as regulating the number of cows per family that are allowed in the forest. However, this research shows that this rule is not working either because it is not being complied with or because the proposed limit is still too high. Since our approach of estimating the intensity of animal impact from faeces has several weaknesses, more precise animal inventories are recommended in the future, as well as determination of the carrying capacity of the forest.
Soil conditions have a much larger effect on natural regeneration than on the mature forest in the study area. Texture, drainage and soil depth are all important predictors that can generate changes in the species richness and diversity of natural regeneration. Thus, areas with suitable conditions should be identified, fenced and used as seedling production sites.
Finally, our results indicate that mortality is vulnerable to both seasonality and the actions of animals and humans, whereas recruitment is mostly affected by the former.
Methods
Study area
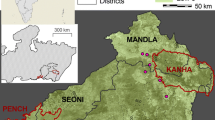
This research was conducted in the central part of the Tumbesian region, in southwestern Ecuador and north-western Peru (Fig. 3a). This area ranges from 200 to 1,100 m above sea level (a.s.l.)48,49 and has a mean annual temperature of 20–26 °C, although this can exceed 35 °C during the rainy season50. The annual precipitation ranges from 300 to 700 mm20, but nearly 85% of this is registered in the 4 months from January to April and there are two or three critically dry months (September–November). Consequently, two seasons can be clearly identified according to the presence or absence of rainwater. The region is affected by El Niño events, which occur every 3–7 years and bring intense rains that cause a change in the climate40.
Geographic location of the study area. The maps were built using QGIS Desktop v. 2.8.776 (http://qgis.osgeo.org) (a) Locations of the study area and protected areas within the Tumbesian region. ‘ACR/ABVP’, areas of regional conservancy of Perú and area of protective forests and other vegetation of Ecuador. Modified from “La Región Tumbesina–una riqueza compartida77” (b) Distribution of clusters in the study area.
Colonisation of the area dates back to the pre-Columbian civilisations51, which means that there has been quite a long period of intervention in the dry forest ecosystem, with increasingly harmful management practices being undertaken. This induced a shift in the landscape until it reached its current state. A period of intensive logging of Handroanthus chrysanthus (Jacq.) S.O.Grose and H. billbergii (Bureau & K.Schum.) S.O.Grose that had occurred for almost two decades was stopped in 1978 through the declaration of ‘closed areas’ in lands below 1,000 m a.s.l in southwest Ecuador52. However, timber extraction for domestic use (generally of selected species) continues today because it is a permitted activity on state lands52, and illegal extraction has not been completely eliminated in either country20,50. Furthermore, livestock management is deficient or inexistent, with goats, cattle, horses and donkeys being released to graze freely in the forest.
Data collection
Natural regeneration
Based on the dry forest types described by Lozano53 and Aguirre et al.49, we considered two formations (deciduous and semi-deciduous) and following Cueva & Chalán54 three density levels (dense, semi-dense and sparse). This resulted in six different types of forest, which were considered to adequately cover most of the forest variability.
Four clusters were installed in each type of forest to give 24 clusters in total (Fig. 3b). Each of these clusters comprised three 60 × 60 m plots, within of which there were four 2 × 2 m sub-plots. After the first regeneration inventory, 12 clusters were selected from a matrix of high, medium and low regeneration abundances and species diversities. Additional sub-plots were then installed and fenced in each of these 12 clusters (four per large plot) (Fig. 4) to give four unfenced and four fenced sub-plots per plot (or 288 sub-plots in total: 144 fenced and 144 unfenced). Since the distance between the sub-plots was only 16 m and no variability was perceptible, they were grouped and treated as a single sample (Fig. 4). Thus, 12 clusters (8 in Ecuador and 4 in Peru) containing 72 samples (36 per treatment) were assessed over a total area of 1,152 m2.
Over the almost 2-year study period, five surveys of the unfenced plots were carried out in Dec. 2014, Jul. 2015, Jan. 2016, May 2016 and Nov. 2016, and four surveys of the fenced plots were carried out at the same times with the exception of Dec. 2014. During each survey, all of the seedlings of trees and shrubs that were >5 cm tall were identified and recorded. In addition, from survey 2 onwards, any dead seedlings were recorded and all live seedlings were labelled. Thus, mortality and recruitment were computed from survey 3 onwards.
Biotic and abiotic factors
To estimate the number of grazing animals, in the first survey the faeces of goats, cows, horses and donkeys were collected along a 1 m × 60 m transect placed along the eastern border of the larger plots (Fig. 4) and separated according to the animal group. The dry weights of these samples were then obtained, combining the values for horses and donkeys.
To estimate the intensity of general anthropogenic pressure caused by activities such as firewood or timber extraction, trampling, grazing, etc., Hegyi’s competition index was adapted55, using the expression defined in Cueva et al.26 to compute the HPI.
To characterise the light conditions in the plots, photographs of the canopy (Canp) were captured with a Canon EOS 300D DIGITAL camera using an 8-mm fisheye lens. One photograph was taken at the internal corners of each 2 × 2 m sub-plot (Fig. 4) in both the rainy and dry seasons. The camera was always placed 1.30 m above the ground facing north.
We included the available abiotic variables that are considered to have high influence on the structure and species composition of the tropical dry forest ecosystem1,2.
Climatic information gridded to a resolution of 30 arc-seconds (approximately 1 km2) was obtained from Fick & Hijmans31. The seasonal precipitation (SPrec) was obtained by summing separately the rainfall from January to April to obtain the amount of precipitation in the rainy season and the rainfall from May to December to obtain the amount of rainfall in the dry season. The mean annual temperature (MTemp) was obtained as an average. The altitude (Alt) was measured in the field for each plot.
For the Ecuadorian part of the study area, soil data were obtained from the Geopedological Map of Zapotillo and Celica of the Instituto Espacial Ecuatoriano56. The soil parameters used were organic material (OM), soil depth (SDepth), drainage (Drain), stoniness (Ston) and texture (Text). For the Peruvian part of the study area, the Soil Classification Map of the Oficina Nacional de Evaluación de Recursos Naturales (ONERN)57 was used to identify the soil type. Unfortunately, this work does not include specific information about the soil characteristics, so they were assumed to be identical to those of the same soil type in the nearest localities on the Ecuadorian side.
Time was considered as the number of elapsed months since the first survey. Treatment (Treat) comprised two groups: open for unfenced plots and exclusion for fenced plots.
The ranges or levels of all predictors can be found in Supplementary Table S4, the full database used to evaluate structure and diversity is provided in the Supplementary Table S5 and the dataset to evaluate dynamics in the Supplementary Table S6.
Data analysis
Data processing
Abundance (Ind) and species richness (Spp) were calculated by counting the number of live individuals and species, respectively, of tree and shrub seedlings in each sample, treatment and survey period. Mortality (Mort) was computed by considering the percentage of dead individuals in relation to the number of individuals in the previous survey. The recruitment rate (Recr) was obtained by calculating the percentage of new individuals in a survey in relation to the total number of individuals in the same survey. Diversity was computed by calculating Simpson’s index (Simp), as this is considered an effective and robust diversity measure58.
The canopy images were processed by Geigl59 using Gap Light Analyzer v2.0 software60 (available on https://www.caryinstitute.org/science/our-scientists/dr-charles-d-canham/gap-light-analyzer-gla). Canopy coverage was computed as a percentage, and the average among the four sub-plots (unfenced and fenced plots were averaged separately) was used for each sample.
Statistical analysis
Generalized linear mixed models (GLMMs)61,62,63 were applied to identify the effects of various predictors on the parameters evaluated. The response variables were the abundance of regeneration as a structure indicator; mortality and recruitment as indicators of the dynamics; and species richness and Simpson’s index as indicators of the diversity of natural regeneration.
A total of 14 predictors were used: five biotic indicators (Goats, Cattle, Equine, HPI and Canp); eight abiotic variables related to climate, geography and soil (SPrec, MTemp, Alt, OM, SDepth, Drain, Ston and Text); and treatment (Treat). To improve the model fitting the predictor variables were transformed as shown in the Supplementary Table S4.
Correlations among all of the predictor variables were tested (Supplementary Table S2) and models were then built by considering that strongly correlated predictors could not be included in the same model to avoid collinearity. In addition, a maximum of five predictors were included in each model to avoid overfitting64, resulting in 168 candidate models. A further 18 models were also built that included interactions between variables representing anthropogenic pressure and those that Cueva et al.26 identified as having a relevant influence on the structure and diversity of the mature forest. Thus, a total of 186 candidate models were tested (see Supplementary Table S3). The asterisk (*) between interaction terms represents both the higher-order interaction term as well as the lower-order main effects.
The predictors SPrec, Treat and Time and their interactions were chosen to predict mortality and recruitment. In this instance, we only evaluated the effect of these three predictors because our goal was to assess if animals are more influential than seasonality, which would allow us to say if management policies are required to improve conditions in the dynamic of natural regeneration. Thirteen models were built for this purpose (see Supplementary Table S7). In all cases, formation and cluster were considered as random effects (plots were nested within clusters, and clusters were nested within formations), and Time was considered as a random slope to account for the repeated measures.
The effects of the predictors on abundance were assessed using the maximum likelihood approach with a negative binomial error distribution to deal with overdispersion65. The effects of the predictors on species richness were assessed using a GLMM with a Poisson error distribution and the Laplace approximation to get a true likelihood62,66. The effects of the predictors on diversity were assessed using the REML approach62. All of these analyses were performed using the lme4 package61 v1.1–21. The effects on mortality were assessed using the REML approach and Bayesian fitting to deal with singular fitting67 using the blme package68 v1.0–4. Finally, the effects of the predictors on recruitment were assessed using a GLMM with a Gaussian error distribution and link identity because 22% of the recruitment values were zeros. The glmmTMB package69 was used, as this is able to deal with zero-inflated model70.
Model selection was carried out using the delta Akaike information criterion (∆AIC < 2)71. For all models, we computed the marginal and conditional variance (R2m and R2c, respectively)72 to determine the proportion of the variance that was explained by each model. R2m and R2c were calculated using the MuMIn package73 v1.42.1 when the lme4 and blme packages were used and the sjstats package74 v0.17.4 when the glmmTMB package was used.
All analyses were performed in the R programming environment v3.5.375.
Data availability
The datasets are available as supplementary information. R scripts available upon request. Please email to jorge.cueva@tum.de for R scripts derived from freely available R packages: see material and methods for more details.
References
Janzen, D. H. Tropical dry forests: The most endangered major tropical ecosystem. Biodiversity, https://doi.org/10.17226/989 (National Academy Press 1988).
Gentry, A. H. Changes in Plant Community Diversity and Floristic Composition on Environmental and Geographical Gradients. Ann. Missouri Bot. Gard. 75, 1–34 (1988).
Best, B. J. & Kessler, M. Biodiversity and Conservation in Tumbesian Ecuador and Peru. (BirdLife International, 1995).
Olson, D. M. et al. The Global 200: A Representation Approach to Conserving the Earth’s Distinctive Ecoregions. (2000).
Miles, L. et al. A global overview of the conservation status of tropical dry forests. J. Biogeogr. 33, 491–505 (2006).
Semper-Pascual, A. et al. Mapping extinction debt highlights conservation opportunities for birds and mammals in the South American Chaco. J. Appl. Ecol. 55, 1218–1229 (2018).
Myers, N., Mittermier, R. A., Mittermier, C. G., da Fonseca, G. A. & Kent, J. Biodiversity hotspots for conservation priorities. Nature 403, 853–858 (2000).
Linares-Palomino, R., Oliveira-Filho, A. & Pennington, R. T. Neotropical Seasonally Dry Forests: Diversity, Endemism, and Biogeography of Woody Plants. in Seasonally Dry Tropical Forests: Ecology and Conservation (eds. Dirzo, R., Young, H. S., Mooney, H. A. & Ceballos, G.) 3–21, https://doi.org/10.5822/978-1-61091-021-7 (Island Press 2011).
Banda-R., K. et al. Plant diversity patterns in neotropical dry forests and their conservation implications. Science (80-.). 353, 1383–1387 (2016).
Ministério do Meio Ambiente. Biodiversidade Brasileira: Avaliação e identificação de áreas e ações prioritárias para conservação, utilização sustentável e repartição dos benefícios da biodiversidade nos biomas brasileiros. http://www.icmbio.gov.br/revistaeletronica/index.php/BioBR/article/view/140/115 (2002).
Koleff, P., Urquiza-Haas, T. & Contreras, B. Prioridades de conservación de los bosques tropicales en México: reflexiones sobre su estado de conservación y manejo. Ecosistemas 21, 6–20 (2012).
UNESCO. Bosques de Paz Transboundary Biosphere Reserve (Ecuador/Peru). UNESCO, https://en.unesco.org/biosphere/transboundary/bosques-de-paz (2017).
Ministerio del Ambiente del Perú. Decreto Supremo que establece el Área de Conservación Regional Bosques Tropicales Estacionalmente Secos del Marañón. 34–38 (El Peruano, 2018).
Blackie, R. et al. Tropical dry forests: The state of global knowledge and recommendations for future research. CIFOR 30, https://doi.org/10.17528/cifor/004408 (2014).
Sunderland, T. et al. Global dry forests: a prologue. Int. For. Rev. 17, 1–9 (2015).
Stoner, K. E. & Sánchez-Azofeifa, G. A. Ecology and regeneration of tropical dry forests in the Americas: Implications for management. For. Ecol. Manage. 258, 903–906 (2009).
Dinerstein, E. et al. Una Evaluación del estado de conservación de las eco-regiones terrestres de América Latina y el Caribe. (Banco Internacional de Reconstrucción y Fomento/Banco Mundial, 1995).
Espinosa, C. I., De La Cruz, M., Luzuriaga, L. & Escudero, A. Bosques tropicales secos de la región pacífico ecuatorial: diversidad, estructura, funcionamiento e implicaciones para la conservación. Ecosistemas 21, 167–179 (2012).
Linares-Palomino, R., Kvist, L. P., Aguirre, M. Z. & Gonzales-Inca, C. Diversity and endemism of woody plant species in the Equatorial Pacific seasonally dry forests. Biodivers. Conserv. 19, 169–185 (2009).
Aguirre, M. Z. & Kvist, L. P. Floristic composition and conservation status of the dry forests in Ecuador. Lyonia 8, 41–67 (2005).
Aguirre, M. Z. & Geada-Lopez, G. Estado de conservación de los bosques secos de la provincia de Loja, Ecuador Conservation status of the dry forests of the province of Loja. Arnaldoa 24, 207–228 (2017).
Jara-Guerrero, A., De la Cruz, M. & Méndez, M. Seed Dispersal Spectrum of Woody Species in South Ecuadorian Dry Forests: Environmental Correlates and the Effect of Considering Species Abundance. Biotropica 43, 722–730 (2011).
Espinosa, C. I. Estructura y funcionamiento de ecosistemas secos del Sur de Ecuador. Tesis Doctoral (Universidad Politécnica de Madrid, 2012).
Espinosa, C. I., Cabrera, O., Luzuriaga, A. & Escudero, A. What factors affect diversity and species composition of endangered tumbesian dry forests in southern Ecuador? Biotropica 43, 15–22 (2011).
Piana, R. P. & Marsden, S. J. Impacts of cattle grazing on forest structure and raptor distribution within a neotropical protected area. Biodivers. Conserv. 23, 559–572 (2014).
Cueva, O. J. et al. Influence of anthropogenic factors on the diversity and structure of a dry forest in the central part of the Tumbesian region (Ecuador-Perú). Forests 10, 1–22 (2019).
Castro, M. G., Tigabu, M., González, R. B. & Odén, P. C. Natural regeneration dynamics of three dry deciduous forest species in Chacocente Wildlife Reserve, Nicaragua. J. For. Res. 20, 1–6 (2009).
Rodriguez, M. T. Influencia del ganado caprino en el sotobosque del ecosistema bosque seco de la comunidad Cabeza de Toro-cantón Zapotillo (Loja-Ecuador). (Universidad del Azuay, 2006).
Abou Rajab, Y. et al. Stand structure, natural regeneration and tree growth in the seasonally dry forest of El Angolo, Northern Peru. (University of Hohenheim, Universidad Nacional Agraria La Molina, Forest Ecology and Remote Sensing, 2010).
Aguirre, M. Z., Betancourt Figueras, Y. & Geada López, G. Regeneración natural en los bosques secos de la provincia de Loja y utilidad para el manejo local. Cedamaz 3, 54–65 (2013).
Fick, S. E. & Hijmans, R. J. WorldClim 2: new 1-km spatial resolution climate surfaces for global land areas. Int. J. Climatol. 37, 4302–4315 (2017).
Aguirre, M. Z. & Kvist, L. P. Composición florística y estructura de bosques estacionalmente secos en el sur-occidental de Ecuador, provincia de Loja, municipios de Macara y Zapotillo. Arnaldoa 16, 87–99 (2009).
Cabin, R. J. et al. Effects of Long‐Term Ungulate Exclusion and Recent Alien Species Control on the Preservation and Restoration of a Hawaiian Tropical Dry Forest. Conserv. Biol. 14, 439–453 (2000).
Lieberman, D. & Li, M. Seedling Recruitment Patterns in a Tropical Dry Forest in Ghana. J. Veg. Sci. 3, 375–382 (1992).
Vieira, D. L. M. & Scariot, A. Principles of natural regeneration of tropical dry forests for restoration. Restor. Ecol. 14, 11–20 (2006).
Ceccon, E., Huante, P. & Rincón, E. Abiotic factors influencing tropical dry forests regeneration. Brazilian Arch. Biol. Technol. 49, 305–312 (2006).
Ray, G. J. & Brown, B. J. Seed Ecology of Woody Species in a Caribbean Dry Forest. Restor. Ecol. 2, 156–163 (1994).
Ruthenberg, H. Farming Systems in the Tropics. (Clarendon Press, 1971).
Roy, S. & Singh, J. S. Seasonal and Spatial Dynamics of Plant-Available N and P Pools and N-Mineralization in Relation to Fine Roots in a Dry Tropical Forest Habitat. Soil Biol. Biochem. 27, 33–40 (1995).
Trenberth, K. E. El Niño Southern Oscillation (ENSO). In Encyclopedia of Ocean Sciences (eds. Cochran, J. K., Bokuniewicz, H. J. & Yager, P. L.) 420–432, https://doi.org/10.1016/B978-0-12-409548-9.04082-3 (Elsevier Inc. 2019).
Stern, M., Quesada, M. & Stoner, K. E. Changes in composition and structure of a tropical dry forest following intermittent cattle grazing. Rev. Biol. Trop. 50, 1021–34 (2002).
Cierjacks, A. & Hensen, I. Variation of Stand Structure and Regeneration of Mediterranean Holm Oak along a Grazing Intensity Gradient along a grazing intensity gradient. Plant Ecol. 173, 215–223 (2004).
Schulz, K. et al. Grazing deteriorates the soil carbon stocks of Caatinga forest ecosystems in Brazil. For. Ecol. Manage. 367, 62–70 (2016).
Albán, L., Matorel, M., Trías, J. & Vera, J. Reforestación extensiva con algarrobo (Prosopis pallida) en la Región Desértica de Piura. Perú. Rev. Zo. áridas 7, 244–252 (2002).
Andrades, M. & Martínez, M. E. Fertilidad del suelo y parámetros que la definen. Agricultura y alimentación (Publication service, Universidad de la Rioja, 2014).
Bullock, S. H. Plant reproduction in neotropical dry forests. In Seasonally Dry Tropical Forests (eds. Bullock, S. H., Mooney, H. A. & Medina, E.) 277–303, https://doi.org/10.1017/CBO9780511753398.011 (Cambridge University Press, 1995).
Griz, M. L. S. & Machado, I. C. Fruiting phenology and seed dispersal syndromes in Caatinga, a tropical dry forest in the northeast of Brazil. J. Trop. Ecol. 17, 303–321 (2001).
Gentry, A. H. Diversity and floristic composition of neotropical dry forests. In Seasonally Dry Tropical Forests (eds. Bullock, S. H., Mooney, H. A. & Medina, E.) 146–194 (Cambridge University Press, 1995).
Aguirre M. Z., Kvist, L. P. & Sánchez, T. O. Bosques secos en Ecuador y su diversidad. In Botánica Económica de los Andes Centrales (eds. Morales R., M., Øllgaard, B., Kvist, L. P., Borchsenius, F. & Balslev, H.) 162–187 (Universidad Mayor de San Andrés, 2006).
Leal-Pinedo, J. M. & Linares-Palomino, R. Los bosques secos de la reserva de biosfera del noroeste (Perú): Diversidad arbórea y estado de conservación. Caldasia 27, 195–211 (2005).
Hocquenghem, A. M. Para Vencer la Muerte: Piura y Tumbes. Raíces en el Bosque Seco y en la Selva Alta—Horizontes en el Pacífico y en la Amazonia. (Centro Nacional de la Investigación Científica (CNRS)/Instituto Francés de Estudios Andinos (IFEA)/Instituto de la Naturaleza y el Conocimiento Ambiental Humano (INCAH), 1998).
Ministerio de Agricultura y Ganadería, Ministerio de Industrias Comercio e Integración & Ministerio de Defensa Nacional. Declaratoria de veda total a la explotación forestal en los bosques de las provincias de Loja y El Oro. Registro Oficial 595-0162 3–4 (1978).
Lozano C. P. E. Los tipos de bosque en el sur del Ecuador. In Botánica Austroecuatoriana: estudios sobre los recursos vegetales en las provincias de El Oro, Loja y Zamora-Chinchipe (eds. Aguirre M., Z., Madsen, J. E., Cotton, E. & Balslev, H.) 29–49 (Abya Yala, 2002).
Cueva O. J. Elaboración y análisis del estado de la cobertura vegetal de la provincia de Loja - Ecuador. (Universidad Internacional de Andalucía, 2012).
Hegyi, F. A simulation model for managing Jack-pine stands. in Growth models for tree and stand simulation (ed. Fries., J.) 74–90 (Royal College of Forestry, 1974).
Instituto Espacial Ecuatoriano. Mapa geopedologico de Celica y Zapotillo. IDE-PORTAL http://www.ideportal.iee.gob.ec (2018).
Oficina Nacional de Evaluación de Recursos Naturales (ONERN). Mapa de Suelos. https://www.arcgis.com/home/webmap/viewer.html?webmap=8eb6a604e5b042bfb41e6373699d2054 (2015).
Magurran, A. E. Measuring Biological Diversity. (Blackwell Publishing 2004).
Geigl, M. J. Hemisphärische Aufnahmen der Überschirmung zur Analyse der Entwicklung der Naturverjüngung im tumbesischen Trockenwald. (Technische Universität München, 2018).
Frazer, G. W., Canham, C. D. & Lertzman, K. P. Gap Light Analyzer: Imaging software to extract canopy structure and gap light transmission indices from true-colour fisheye photographs. Users Manual and Program Documentation, Version 2.0. https://www.caryinstitute.org/science/our-scientists/dr-charles-d-canham/gap-light-analyzer-gla (1999).
Bates, D. M., Mächler, M., Bolker, B. M. & Walker, S. C. Fitting Linear Mixed-Effects Models using lme4. J. Stat. Softw. 67, 48 (2015).
Bolker, B. M. et al. Generalized linear mixed models: a practical guide for ecology and evolution. Trends Ecol. Evol. 24, 127–135 (2009).
Cayuela, L. Modelos lineales mixtos (LMM) y modelos lineales generalizados mixtos (GLMM) en R. Curso de análisis de datos ecológicos en R (2014).
Neo, L., Yee, A. T. K., Chong, K. Y., Kee, C. Y. & Tan, H. T. W. Vascular plant species richness and composition in two types of post-cultivation tropical secondary forest. Appl. Veg. Sci. 20, 692–701 (2017).
Di Rienzo, J. A., Macchiavelli, R. & Casanoves, F. Modelos lineales generalizados mixtos: aplicaciones en InfoStat. 101 http://repositorio.bibliotecaorton.catie.ac.cr/handle/11554/8691?show=full (2017).
Bolker, B. M. et al. GLMMs in action: gene-by-environment interaction in total fruit production of wild populations of Arabidopsis thaliana Revised version, part 1. 1–33 http://glmm.wdfiles.com/local–files/examples/Banta_2011_part1.pdf (2011).
Bolker, B. M. GLMM FAQ. https://bbolker.github.io/mixedmodels-misc/glmmFAQ.html#singular-models-random-effect-variances-estimated-as-zero-or-correlations-estimated-as–1 (2019).
Chung, Y., Rabe-Hesketh, S., Dorie, V., Gelman, A. & Liu, J. A nondegenerate penalized likelihood estimator for variance parameters in multilevel models. Psychometrika 78, 685–709 (2013).
Brooks, M. E. et al. glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J. 9, 378–400 (2017).
Bolker, B. M. Getting started with the glmmTMB package. 9 https://cran.r-project.org/web/packages/glmmTMB/vignettes/glmmTMB.pdf (2019).
Burnham, K. P. & Anderson, D. R. Model selection and multimodel inference: a practical information-theoretic approach. (Springer, 2002).
Nakagawa, S. & Schielzeth, H. A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol. Evol. 4, 133–142 (2013).
Bartoń, K. MuMIn: Multi-model inference. CRAM https://cran.r-project.org/web/packages/MuMIn/index.html (2018).
Lüdecke, D. sjstats: Statistical Functions for Regression Models. Zenodo, https://doi.org/10.5281/zenodo.1284472 (2019).
R Core Team. R: A language and environment for statistical computing. https://www.r-project.org/ (2019).
QGIS Development Team. QGIS Geographic Information System. http://qgis.osgeo.org (2009).
Cueva O., J. & Rodas, F. La Región Tumbesina: una riqueza compartida. (Naturaleza & Cultura Internacional, 2010).
Acknowledgements
We wish to thank to those who funded this research: to German Research Foundation (DFG) project PAK 824/B3; to Universidad Técnica Particular de Loja (UTPL), who leads the projects PROY_CCN_0030 funded for UTPL and PIC-13-ETAPA-005 funded for Secretaria de Eduación Superior, Ciencia, Tecnología e Innovación (SENESCYT). J.C.O. received the support of SENESCYT and Erzbischöfliches Ordinariat München, many thanks. This work was supported by DFG and the Technical University of Munich (TUM) in the framework of the Open Access Publishing Program. Also, we want to thank to Proofreading Service of TUM Graduate School. To Naturaleza y Cultura Internacional, Parque Nacional Cerros de Amotape, and the communities Malvas, El Cardo, and Piedras Blancas for allowing us to conduct this research on their reserves. Furthermore, to Diego González, Jorge Armijos, Geovanny Cango, Angel Gusmán, Johana Gusmán, Israel Medina, Yodán Zapata and Flavio Olaya for their support in the fieldwork.
Author information
Authors and Affiliations
Contributions
J.C.O., P.H. and C.I.E. designed the study. J.C.O. performed the fieldwork, data analyses and wrote the document. J.C.O. and E.G.-M. undertook the data curation. J.C.O., P.H. and M.W. discussed the results. P.H., M.W., C.I.E. and Z.A.M. reviewed the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cueva-Ortiz, J., Espinosa, C.I., Aguirre-Mendoza, Z. et al. Natural Regeneration in the Tumbesian Dry Forest: Identification of the Drivers Affecting Abundance and Diversity. Sci Rep 10, 9786 (2020). https://doi.org/10.1038/s41598-020-66743-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-66743-x
- Springer Nature Limited