Abstract
In functional imaging, accumulating evidence suggests that spontaneous activity decreases during the resting state in the core brain regions of the default-mode network [e.g. medial prefrontal cortex (mPFC)] in schizophrenia. However, the significance of this decreased activity has not been clarified in relation to its clinical symptoms. In this study, near-infrared spectroscopy (NIRS), which is a simple imaging modality suitable for resting state paradigm, was used to evaluate the intensity of the spontaneous activity during the resting state in chronic schizophrenia. Consistent with previous findings of fMRI studies, spontaneous activity decreased in the mPFC of patients with schizophrenia. In addition, the decreased spontaneous activity was associated with severe hallucinations in this region where reality monitoring is fundamentally engaged. These results may encourage additional application of NIRS with the resting state paradigm into daily clinical settings for addressing the broad phenotypes and unstable course of schizophrenia.
Similar content being viewed by others
Introduction
Since default-mode activity was termed for the brain regional activity that is enhanced during the resting state1,2, numerous research fields have been opened up to identify the significance of this activity in human brains and brain diseases3,4,5. The default-mode activity is characterized by spontaneous low-frequency (<0.1 Hz) fluctuations of the brain blood flow6. Such fluctuations are synchronized among specific brain regions called the default-mode network whose core regions consist of the medial prefrontal cortex (mPFC) and PCC/precuneus1,4. While most of the recent studies about disturbances of this activity have examined the impact on the network changes in psychiatric illnesses, less attention has been paid to quantify the intensity of this activity, which may provide an additional valid marker for mental illnesses.
An index to measure the intensity of the resting-state activity in functional MRI (fMRI) is the amplitude of low-frequency fluctuations (ALFF), which appear dominantly in the core regions of the default-mode network7,8. The ALFF indicates the sum of the power across low-frequency range (0.01–0.08 Hz) fluctuations of the brain blood flow, whereas the fractional ALFF (fALFF) indicates the ratio of the ALFF to the total power. Although previous fMRI studies have shown that both the ALFF and fALFF have reliable signals from the grey matter of the brain, the ALFF is believed to be more sensitive to the differences among groups and individuals owing to its higher test-retest reliability8. On the other hand, the fALFF is reported to generate lesser noise from physiological sources7,8. Therefore, it is recommended to use both analyses to maximize the reliability of examining spontaneous regional brain activity during the resting state8.
Several lines of evidence using fMRI suggest that the ALFF or fALFF is decreased during the resting state in the core regions of the default-mode network in schizophrenia9,10,11,12,13,14,15,16. However, the extended analysis to explore the relevance of the decreased activity with clinical phenotypes of schizophrenia has shown inconsistent results. Among the five studies that reported the reduction of the ALFF or fALFF in the mPFC of schizophrenia10,11,14,15,16, one reported that ALFF was associated with a general severity of the disease15, and another reported an association between fALFF and disorganized symptoms16. Another study also reported that the decreased ALFF was recovered with the improvement of positive symptoms in the first episode of schizophrenia11. However, the other two studies reported no association between the ALFF and clinical symptoms10,14. To overcome such a complexity of pathophysiology in the broad phenotypes of schizophrenia, simple neuroimaging modalities suitable for measurements in routine clinical psychiatry may be warranted.
Near-infrared spectroscopy (NIRS) is a non-invasive imaging device that is easy to use and economically efficient. It demands less physical constraint due to the tolerance for small movements, and measurements can be taken without the acoustic scanner noise that is inevitable in fMRI17,18. Because these advantages can provide relatively relaxed circumstances for examinees, NIRS is suitable for resting-state measurements. In addition, test–retest studies have validated the stability of NIRS measurements in the brain network during the resting state19,20,21. Our previous study using wearable NIRS examined the resting-state activity in the mPFC as a region of interest (ROI) and detected a decrease of the fALFF in patients with schizophrenia22. However, the significance of the decreased activity in relation to the clinical symptoms of schizophrenia has not been elucidated. This study analyses the ALFF and fALFF using NIRS systematically on the individual channels in the prefrontal regions. It investigates possible associations between the ALFF/fALFF and clinical symptoms in patients with schizophrenia.
Results
Alteration of the frontal ALFF/fALFF in patients with schizophrenia
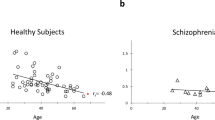
Compared with the control subjects, the ALFF was significantly decreased in patients with schizophrenia on five channels [ch1 (control 8.58 ± 0.93, schizophrenia 4.77 ± 0.52, t = 3.6, p = 0.001), ch2 (control 6.00 ± 0.74, schizophrenia 3.38 ± 0.41, t = 3.0, p = 0.005), ch4 (control 8.74 ± 0.98, schizophrenia 4.37 ± 0.40, t = 4.1, p = 0.0002), ch5 (control 5.43 ± 0.64, schizophrenia 3.19 ± 0.34, t = 3.1, p = 0.004), ch6 (control 7.61 ± 1.09, schizophrenia 3.996 ± 0.43, t = 3.1, p = 0.004)], but not on the other channels (p > 0.006) (Fig. 1).
The fALFF was significantly decreased in patients with schizophrenia on five channels [ch1 (control 2.44 ± 0.17, schizophrenia 1.56 ± 0.16, t = 3.8, p = 0.0005), ch4 (control 2.53 ± 0.15, schizophrenia 1.63 ± 0.22, t = 3.3, p = 0.002), ch5 (control 1.70 ± 0.14, schizophrenia 1.13 ± 0.12, t = 3.0, p = 0.004), ch6 (control 2.26 ± 0.24, schizophrenia 1.39 ± 0.16, t = 3.1, p = 0.004), ch9 (control 1.98 ± 0.11, schizophrenia 4.41 ± 0.15, t = 3.1, p = 0.004)], but not on the other channels (p > 0.006) (Fig. 2).
As such, both the ALFF and fALFF commonly showed significant decreases on four channels (ch1, ch4, ch5, and ch6) in patients with schizophrenia. These channels are highlighted in yellow in Fig. 3a.
(a) Locations for 10 channels of NIRS. The channels where both the ALFF and fALFF showed significant decreases in schizophrenia are highlighted in yellow. The ALFF was significantly associated with severity of hallucinations on the channel circled in red. (b) A significant association between the ALFF and severity of hallucinations on ch6 (*p = 0.001).
Association of the ALFF/fALFF with the clinical symptoms
In the multiple regression analysis for detecting clinical relevance with decreased ALFF or fALFF in schizophrenia, the ALFF was found to be significantly associated with hallucination scores of CRDPSS on ch6 (β = –0.69, P = 0.001) (Fig. 3b). No other significant association of the clinical variables were observed with the ALFF (p > 0.01) nor with the fALFF (p > 0.01) on any channel.
Discussion
This is an initial study to show the applicability of NIRS in detecting the significance of decreased ALFF in the symptomatology of schizophrenia. Both the ALFF and fALFF revealed significant decreases in the patients on four channels, which were annotated in the anterior part of the mPFC (ch5 and ch6) and the extended area to the right frontal pole (ch1 and ch4). These results are consistent with those of previous fMRI studies that have shown a decreased ALFF or fALFF in the mPFC of schizophrenia10,11,14,15,16, though NIRS is technically unable to precisely delineate the affected brain region. In the region of ch6 located at the ventral part of the anterior mPFC, a significant association emerged between the ALFF and severity of hallucinations that was evaluated based on the patients’ behaviours, which were influenced by their persistent auditory hallucinations. This finding agrees with the fMRI study which reported an association of improvement of positive symptoms with the restoration of the decreased ALFF in the mPFC11. Combined with our results, the decreased ALFF may be continuously observed in the mPFC of patients with schizophrenia who have sustained severe positive symptoms, such as hallucinations.
While previous neuroimaging studies have shown that hyperactivity is observed in the sensory cortex during hallucinatory experiences23,24,25, task-induced brain activity changes validate the mPFC as the additional domain of the hallucinations in schizophrenia23,25,26. Psychological fMRI studies have shown that the mPFC is involved in self-monitoring27 and that its anterior part specifically works for reality monitoring, which discriminates between self-generated and externally presented information28. Additionally, reduced activities have been reported in the mPFC during reality-monitoring tasks in patients with schizophrenia29,30,31. Based on these findings, it is hypothesized that hyperactivity in the sensory cortex accompanied by hypo-activation of the mPFC results in the impairment of reality monitoring, leading to the failure to recognize sensory activities as self-generated and, ultimately, hallucinatory experiences in schizophrenia28.
Another line of evidence suggests that the default-mode activity mediates self-referential mental activity. Although the default-mode network is activated during the resting state, it is more potently activated during self-referential tasks2,4. Considering that the mPFC is a core brain region of the default-mode network and that the anterior mPFC is involved in reality monitoring among self-referential processing27, a reduction in the default-mode activity of the anterior mPFC reflects a weakened reality monitoring in schizophrenia. Taken together with the link between impaired reality monitoring and hallucinations28, these conjectures support our results in terms of the association between the severity of hallucinations and reduced spontaneous activity during the resting state in the anterior mPFC in schizophrenia. In short, a decline in the spontaneous activity of the anterior mPFC may be a basis for the weakened reality monitoring that may misattribute self-generated sensory activities as hallucinations.
In the present work, we focused on a limited group of patients with schizophrenia to address the heterogeneous nature of the disease. Because patients with schizophrenia usually have unstable disease courses, chronic patients at a relatively stable phase were recruited. These were hospitalised cases with apparent social functioning deficits who would be probably categorized as psychosis biotype 1, the subgroup typically consisting of deteriorated cases of schizophrenia, as defined in previous studies32,33. In addition, only male subjects were examined to reduce clinical variability. Our finding of the significance of decreased ALFF in this adjusted group may encourage the application of NIRS analysis to follow-up studies with larger cohorts to address the broad variety of phenotypes in schizophrenia.
There are some limitations associated with this study. First, NIRS can only measure the cortical surface activity18,34, and NIRS signals include contamination of peripheral hemodynamic factors, such as skin perfusion, even in the limited frequency range of blood flow fluctuations for ALFF (0.01–0.08 Hz)35. Second, the possible influence of antipsychotic medication cannot be fully excluded on ALFF and fALFF. Although the chlorpromazine-equivalent antipsychotic dose was not significantly associated with the ALFF nor fALFF on any channels, the sample size of our cohort was too small to manage the variation of the antipsychotics. Third, some probe errors were noted on the channels around the side edge of the headset of the wearable NIRS in patients with schizophrenia. This may be due to i) their body motions, and/or ii) their unfitness to the semi-structured headset, which possibly accounts for the minor physical anomalies of the craniofacial measurements that have been reported in schizophrenia36,37,38,39.
In conclusion, the results of the current study suggest that NIRS is a valid modality to examine the intensity of resting-state activity, such as the ALFF, for evaluating the pathophysiology of schizophrenia. The resting-state paradigm has the advantage of allowing the assessment of patients who may have difficulties adhering to attention-demanding tasks, as is often the case with schizophrenia patients. Given that NIRS is a safe and convenient modality that can offer comfortable circumstance for neurophysiological measurements, its further clinical application should be explored within the paradigm of resting state for psychiatry diseases, such as schizophrenia.
Methods
Subjects
Twenty hospitalised male patients (25–68 years, mean ± SEM = 50.6 ± 3.0) with chronic schizophrenia and their case-matched healthy controls (26–66 years, 50.1 ± 2.5) participated in this study. All of them were right-handed. Each patient was diagnosed by two or more psychiatrists on the basis of the DSM-5 criteria for schizophrenia, and the diagnosis was verified based on detailed clinical observations during hospitalisation. All the patients were medicated with antipsychotics at doses that had been largely unchanged for> 3 months because of the persistent symptoms accompanying their chronic disease. Three patients were medicated using the first generation antipsychotics, seven using the second generation, and 10 using both. None had a history of substance/alcohol abuse. The patients were having apparent deficits in social functioning based on the Global Assessment of Functioning (GAF) score of ≤35. The clinical variables of the patients were as follows: GAF score, 24.2 ± 1.4; illness duration, 27.9 ± 3.5 years; onset age, 23.9 ± 1.6 years; and chlorpromazine-equivalent antipsychotic dose, 804.5 ± 143.7 mg/day. The patients’ symptoms were assessed based on the Clinician-Rated Dimensions of Psychosis Symptom Severity (CRDPSS)40, which is an eight-item scale for dimensional assessment proposed in the DSM-5 to objectively rate the severity of the primary symptoms of psychosis, by two research psychiatrists who were blinded to the NIRS data, along with the information obtained from the clinical psychiatrist in charge and the ward nurses. The mean scores of the CRDPSS among the patients were as follows: hallucinations (2.5 ± 0.3), delusions (2.6 ± 0.2), disorganized speech (3.0 ± 0.2), abnormal psychomotor behaviour (2.5 ± 0.2), negative symptoms (3.3 ± 0.2), impaired cognition (3.2 ± 0.2), depression (0.9 ± 0.1), and mania (0.3 ± 0.2). The controls had no history of neurological or psychiatric disorders. A complete description of the study was provided, and written informed consent was obtained from all the subjects. The study was approved by the Ethics Committee of the Kindai University Faculty of Medicine, and carried out in accordance with the ethical principles of the Declaration of Helsinki and its later amendments.
Near-infrared spectroscopy
NIRS measurements were performed using a 10-channel wearable NIRS device (WOT-100 system; Hitachi High-Technologies Corporation, Tokyo, Japan), as previously described22,41,42. Briefly, the device measured relative changes in oxygenated-(oxy-) and deoxygenated-(deoxy-) hemoglobin (Hb) concentrations using two wavelengths (705 and 830 nm) with a sampling rate of 200 ms. Optical data were analysed using the modified Beer–Lambert law to calculate the signals reflecting changes in Hb levels expressed as arbitrary units (mM–mm)41,42. The NIRS probe unit has a 2 × 4 alternating arrangement of irradiation and detection positions. The distance between the pairs of emission and detector probes was set at 30 mm, and the measurement area between the probes was defined as a channel. The lowest probes were positioned along the Fp1–Fp2 line, according to the International 10–20 system41,43. The arrangement of channels covered the entire forehead to monitor the activation in the prefrontal regions41,42. Three-dimensional coordinates of the channels were obtained using a three-dimensional digitizer (Patriot, POLHEMUS, Inc., Colchester, Vermont, USA), as previously described22. The estimate for the spatial registration of the channels using the probabilistic-determination method43,44 was mapped onto the Montreal Neurological Institute space based on NIRS-SPM45,46 (http://bispl.weebly.com/nirs-spm.html#/) (Fig. 3a). A previous NIRS study of the resting-state paradigm was referenced to designate the mPFC region for the mapped channels47.
Resting-state paradigm
The NIRS signals were acquired during the resting state as previously described22. The subjects were seated in a comfortable chair in a silent room to achieve a resting-state condition. They were instructed to focus on the central fixation point displayed on a monitor during the NIRS measurement while keeping their eyes open and remaining still. The NIRS measurement commenced when the subject continually focused on the fixation point on the monitor for 3 min while at rest. The resting paradigm was of relatively short duration, thus enabling patients to continue following the instructions without falling asleep.
Data analysis
The time courses of oxy- and deoxy-Hb signals were plotted using the BRainSuite_Analyzer (BRSystems. Inc., Kanagawa, Japan) with low pass filter (<0.1 Hz) to eliminate physiological noises, such as those of the heartbeat, respiration, and quick body movements35,48. Relative changes in oxy-Hb signals were analysed according to a previous report that demonstrated a strong correlation between oxy-Hb NIRS measurements and blood oxygenation level-dependent signals measured using fMRI49. The ALFF and fALFF were analysed using the ALFF/fALFF software (BRSystems Inc.; Brainsuit ALFF; Kanagawa, Japan) according to previous reports8,50. This software first transformed the time series of the NIRS data to a frequency domain using the fast Fourier transform, and the power spectrum was obtained for each channel. Then, the square root was calculated at each frequency of the power spectrum. The ALFF was calculated as the sum of the square root across the low-frequency range (0.01–0.08 Hz), and the value of the ALFF was divided by the square root across the total frequency range between 0 and 0.25 Hz to calculate the fALFF.
Statistics
The ALFF and fALFF were compared between schizophrenia and control groups using the Student’s t-test on the 10 individual channels of NIRS. For the channels where significant changes of the ALFF/fALFF were observed in the schizophrenia group, stepwise multiple regression analysis was conducted to investigate the association between the ALFF/fALFF and clinical variables, including the CRDPSS scores. The ALFF or fALFF was analysed as the dependent variable, and the independent variables were set with clinical variables, such as CRDPSS scores, GAF score, current age, onset age, illness duration, and the chlorpromazine-equivalent antipsychotic dose. The threshold for the significance of p-values was set at 0.005 (0.05/10) by the Bonferroni correction because 10 channel regions were measured in this study. All statistical tests were two-tailed and were conducted using GraphPad Prism 6.0 for Windows version 6.07 (GraphPad Software, Inc., La Jolla, CA, USA) or SPSS version 25.0 (IBM Inc., New York, USA).
Exclusion of probe error channels
Within the 10 channels measured using NIRS, those judged to be probe errors by the WOT-100 software were excluded from the analysis for each subject. According to this criterion, ch2 was excluded for two patients; ch3, ch7, and ch9 were excluded for one patient; ch8 was excluded for three patients; and ch10 was excluded for seven patients with schizophrenia. There were no probe errors in the control subjects. Because the number of the probe errors on ch10 was significantly different between the schizophrenia and control groups (Fischer’s exact test; p = 0.008), we eliminated the whole dataset of ch10 from the analysis. Notably, ch4, ch5, and ch6 were intact for all patients with schizophrenia and the control subjects.
Data availability
The datasets generated and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Raichle, M. E. et al. A default mode of brain function. Proceedings of the National Academy of Sciences of the United States of America 98, 676–682, https://doi.org/10.1073/pnas.98.2.676 (2001).
Gusnard, D. A., Raichle, M. E. & Raichle, M. E. Searching for a baseline: functional imaging and the resting human brain. Nature reviews. Neuroscience 2, 685–694, https://doi.org/10.1038/35094500 (2001).
Fox, M. D. & Raichle, M. E. Spontaneous fluctuations in brain activity observed with functional magnetic resonance imaging. Nature reviews. Neuroscience 8, 700–711, https://doi.org/10.1038/nrn2201 (2007).
Buckner, R. L., Andrews-Hanna, J. R. & Schacter, D. L. The brain’s default network: anatomy, function, and relevance to disease. Annals of the New York Academy of Sciences 1124, 1–38, https://doi.org/10.1196/annals.1440.011 (2008).
Buckner, R. L. & DiNicola, L. M. The brain’s default network: updated anatomy, physiology and evolving insights. Nature reviews. Neuroscience 20, 593–608, https://doi.org/10.1038/s41583-019-0212-7 (2019).
Biswal, B., Yetkin, F. Z., Haughton, V. M. & Hyde, J. S. Functional connectivity in the motor cortex of resting human brain using echo-planar MRI. Magnetic resonance in medicine 34, 537–541, https://doi.org/10.1002/mrm.1910340409 (1995).
Zou, Q. H. et al. An improved approach to detection of amplitude of low-frequency fluctuation (ALFF) for resting-state fMRI: fractional ALFF. Journal of neuroscience methods 172, 137–141, https://doi.org/10.1016/j.jneumeth.2008.04.012 (2008).
Zuo, X. N. et al. The oscillating brain: complex and reliable. NeuroImage 49, 1432–1445, https://doi.org/10.1016/j.neuroimage.2009.09.037 (2010).
Meda, S. A. et al. Frequency-Specific Neural Signatures of Spontaneous Low-Frequency Resting State Fluctuations in Psychosis: Evidence From Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP) Consortium. Schizophrenia bulletin 41, 1336–1348, https://doi.org/10.1093/schbul/sbv064 (2015).
Huang, X. Q. et al. Localization of cerebral functional deficits in treatment-naive, first-episode schizophrenia using resting-state fMRI. NeuroImage 49, 2901–2906, https://doi.org/10.1016/j.neuroimage.2009.11.072 (2010).
Lui, S. et al. Short-term effects of antipsychotic treatment on cerebral function in drug-naive first-episode schizophrenia revealed by “resting state” functional magnetic resonance imaging. Archives of general psychiatry 67, 783–792, https://doi.org/10.1001/archgenpsychiatry.2010.84 (2010).
Hoptman, M. J. et al. Amplitude of low-frequency oscillations in schizophrenia: a resting state fMRI study. Schizophrenia research 117, 13–20, https://doi.org/10.1016/j.schres.2009.09.030 (2010).
Turner, J. A. et al. A multi-site resting state fMRI study on the amplitude of low frequency fluctuations in schizophrenia. Frontiers in neuroscience 7, 137, https://doi.org/10.3389/fnins.2013.00137 (2013).
Ren, W. et al. Anatomical and functional brain abnormalities in drug-naive first-episode schizophrenia. The American journal of psychiatry 170, 1308–1316, https://doi.org/10.1176/appi.ajp.2013.12091148 (2013).
Li, F. et al. Longitudinal Changes in Resting-State Cerebral Activity in Patients with First-Episode Schizophrenia: A 1-Year Follow-up Functional MR Imaging Study. Radiology 279, 867–875, https://doi.org/10.1148/radiol.2015151334 (2016).
He, Z. et al. Aberrant intrinsic brain activity and cognitive deficit in first-episode treatment-naive patients with schizophrenia. Psychological medicine 43, 769–780, https://doi.org/10.1017/s0033291712001638 (2013).
Chou, P. H., Huang, C. J. & Sun, C. W. The Potential Role of Functional Near-Infrared Spectroscopy as Clinical Biomarkers in Schizophrenia. Curr Pharm Des 26, 201–217, https://doi.org/10.2174/1381612825666191014164511 (2020).
Hoshi, Y. Hemodynamic signals in fNIRS. Progress in brain research 225, 153–179, https://doi.org/10.1016/bs.pbr.2016.03.004 (2016).
Niu, H. et al. Test-retest reliability of graph metrics in functional brain networks: a resting-state fNIRS study. PLoS One 8, e72425, https://doi.org/10.1371/journal.pone.0072425 (2013).
Zhang, H. et al. Is resting-state functional connectivity revealed by functional near-infrared spectroscopy test-retest reliable? J Biomed Opt 16, 067008, https://doi.org/10.1117/1.3591020 (2011).
Zhang, H. et al. Test-retest assessment of independent component analysis-derived resting-state functional connectivity based on functional near-infrared spectroscopy. NeuroImage 55, 607–615, https://doi.org/10.1016/j.neuroimage.2010.12.007 (2011).
Hosomi, F. et al. Capturing spontaneous activity in the medial prefrontal cortex using near-infrared spectroscopy and its application to schizophrenia. Scientific reports 9, 5283, https://doi.org/10.1038/s41598-019-41739-4 (2019).
Allen, P., Laroi, F., McGuire, P. K. & Aleman, A. The hallucinating brain: a review of structural and functional neuroimaging studies of hallucinations. Neuroscience and biobehavioral reviews 32, 175–191, https://doi.org/10.1016/j.neubiorev.2007.07.012 (2008).
Jardri, R., Pouchet, A., Pins, D. & Thomas, P. Cortical activations during auditory verbal hallucinations in schizophrenia: a coordinate-based meta-analysis. The American journal of psychiatry 168, 73–81, https://doi.org/10.1176/appi.ajp.2010.09101522 (2011).
Zmigrod, L., Garrison, J. R., Carr, J. & Simons, J. S. The neural mechanisms of hallucinations: A quantitative meta-analysis of neuroimaging studies. Neuroscience and biobehavioral reviews 69, 113–123, https://doi.org/10.1016/j.neubiorev.2016.05.037 (2016).
Goghari, V. M., Sponheim, S. R. & MacDonald, A. W. III The functional neuroanatomy of symptom dimensions in schizophrenia: a qualitative and quantitative review of a persistent question. Neuroscience and biobehavioral reviews 34, 468–486, https://doi.org/10.1016/j.neubiorev.2009.09.004 (2010).
Northoff, G. & Bermpohl, F. Cortical midline structures and the self. Trends in cognitive sciences 8, 102–107, https://doi.org/10.1016/j.tics.2004.01.004 (2004).
Simons, J. S., Garrison, J. R. & Johnson, M. K. Brain Mechanisms of Reality Monitoring. Trends in cognitive sciences 21, 462–473, https://doi.org/10.1016/j.tics.2017.03.012 (2017).
Vinogradov, S., Luks, T. L., Schulman, B. J. & Simpson, G. V. Deficit in a neural correlate of reality monitoring in schizophrenia patients. Cerebral cortex (New York, N.Y.: 1991) 18, 2532-2539, https://doi.org/10.1093/cercor/bhn028 (2008).
Garrison, J. R., Fernandez-Egea, E., Zaman, R., Agius, M. & Simons, J. S. Reality monitoring impairment in schizophrenia reflects specific prefrontal cortex dysfunction. NeuroImage. Clinical 14, 260–268, https://doi.org/10.1016/j.nicl.2017.01.028 (2017).
Subramaniam, K., Ranasinghe, K. G., Mathalon, D., Nagarajan, S. & Vinogradov, S. Neural mechanisms of mood-induced modulation of reality monitoring in schizophrenia. Cortex; a journal devoted to the study of the nervous system and behavior 91, 271–286, https://doi.org/10.1016/j.cortex.2017.01.005 (2017).
Clementz, B. A. et al. Identification of Distinct Psychosis Biotypes Using Brain-Based Biomarkers. The. American journal of psychiatry 173, 373–384, https://doi.org/10.1176/appi.ajp.2015.14091200 (2016).
Reininghaus, U. et al. Transdiagnostic dimensions of psychosis in the Bipolar-Schizophrenia Network on Intermediate Phenotypes (B-SNIP). World psychiatry: official journal of the World Psychiatric Association (WPA) 18, 67–76, https://doi.org/10.1002/wps.20607 (2019).
McCormick, P. W., Stewart, M., Lewis, G., Dujovny, M. & Ausman, J. I. Intracerebral penetration of infrared light. Technical note. Journal of neurosurgery 76, 315–318, https://doi.org/10.3171/jns.1992.76.2.0315 (1992).
Kirilina, E. et al. Identifying and quantifying main components of physiological noise in functional near infrared spectroscopy on the prefrontal cortex. Frontiers in human neuroscience 7, 864, https://doi.org/10.3389/fnhum.2013.00864 (2013).
Compton, M. T. & Walker, E. F. Physical manifestations of neurodevelopmental disruption: are minor physical anomalies part of the syndrome of schizophrenia? Schizophrenia bulletin 35, 425–436, https://doi.org/10.1093/schbul/sbn151 (2009).
McGrath, J. et al. Minor physical anomalies and quantitative measures of the head and face in patients with psychosis. Archives of general psychiatry 59, 458–464, https://doi.org/10.1001/archpsyc.59.5.458 (2002).
Ismail, B., Cantor-Graae, E. & McNeil, T. F. Minor physical anomalies in schizophrenic patients and their siblings. The American journal of psychiatry 155, 1695–1702, https://doi.org/10.1176/ajp.155.12.1695 (1998).
Lin, A. S. et al. Minor physical anomalies and craniofacial measures in patients with treatment-resistant schizophrenia. Psychological medicine 45, 1839–1850, https://doi.org/10.1017/s0033291714002931 (2015).
American Psychiatric Association. & American Psychiatric Association. DSM-5 Task Force. Diagnostic and statistical manual of mental disorders: DSM-5. 5th edn, (American Psychiatric Association, 2013).
Pinti, P. et al. Using Fiberless, Wearable fNIRS to Monitor Brain Activity in Real-world Cognitive Tasks. Journal of visualized experiments: JoVE https://doi.org/10.3791/53336 (2015).
Atsumori, H. et al. Development of wearable optical topography system for mapping the prefrontal cortex activation. The Review of scientific instruments 80, 043704, https://doi.org/10.1063/1.3115207 (2009).
Singh, A. K., Okamoto, M., Dan, H., Jurcak, V. & Dan, I. Spatial registration of multichannel multi-subject fNIRS data to MNI space without MRI. NeuroImage 27, 842–851, https://doi.org/10.1016/j.neuroimage.2005.05.019 (2005).
Tsuzuki, D. et al. Virtual spatial registration of stand-alone fNIRS data to MNI space. NeuroImage 34, 1506–1518, https://doi.org/10.1016/j.neuroimage.2006.10.043 (2007).
Ye, J. C., Tak, S., Jang, K. E., Jung, J. & Jang, J. NIRS-SPM: statistical parametric mapping for near-infrared spectroscopy. NeuroImage 44, 428–447, https://doi.org/10.1016/j.neuroimage.2008.08.036 (2009).
Jang, K. E. et al. Wavelet minimum description length detrending for near-infrared spectroscopy. J Biomed Opt 14, 034004, https://doi.org/10.1117/1.3127204 (2009).
Sakakibara, E. et al. Detection of resting state functional connectivity using partial correlation analysis: A study using multi-distance and whole-head probe near-infrared spectroscopy. NeuroImage 142, 590–601, https://doi.org/10.1016/j.neuroimage.2016.08.011 (2016).
Cordes, D. et al. Frequencies contributing to functional connectivity in the cerebral cortex in “resting-state” data. AJNR. American journal of neuroradiology 22, 1326–1333 (2001).
Strangman, G., Boas, D. A. & Sutton, J. P. Non-invasive neuroimaging using near-infrared light. Biological psychiatry 52, 679–693, https://doi.org/10.1016/s0006-3223(02)01550-0 (2002).
Zang, Y. F. et al. Altered baseline brain activity in children with ADHD revealed by resting-state functional MRI. Brain & development 29, 83–91, https://doi.org/10.1016/j.braindev.2006.07.002 (2007).
Acknowledgements
We appreciate and thank the volunteers who participated in this study and acknowledge the assistance of Ms Shizuka Ishida and Mr Yusuke Gomyo for their technical support in NIRS measurements and statistical analyses. We would also like to thank Enago (www.enago.jp) for the English language review. This work was supported by a Grant-in-Aid for Scientific Research from the Japan Society for the Promotion of Science (No. 16K10229 and 17K10320).
Author information
Authors and Affiliations
Contributions
M.Y. designed the study. M.Y., F.H. and Y.K. collected NIRS data. F.H., Y.K., A.T. and S.O. obtained clinical data of patients with schizophrenia. M.Y. managed the analyses and wrote the manuscript. O.S. supervised this study. All authors contributed to and have approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yanagi, M., Hosomi, F., Kawakubo, Y. et al. A decrease in spontaneous activity in medial prefrontal cortex is associated with sustained hallucinations in chronic schizophrenia: An NIRS study. Sci Rep 10, 9569 (2020). https://doi.org/10.1038/s41598-020-66560-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-66560-2
- Springer Nature Limited
This article is cited by
-
Metabolite differences in the medial prefrontal cortex in schizophrenia patients with and without persistent auditory verbal hallucinations: a 1H MRS study
Translational Psychiatry (2022)
-
Shared increased entropy of brain signals across patients with different mental illnesses: A coordinate-based activation likelihood estimation meta-analysis
Brain Imaging and Behavior (2022)