Abstract
Patients with cervical myelopathy may manifest impairments in functional activities and balance control caused by compression of the spinal cord. The objective of the current study was to determine long-term changes in the upright balance control of patients with cervical myelopathy who had undergone cervical decompression surgery. This is a prospective cohort study from the preoperative phase to 3 months, 6 months, and 1 year postsurgery. Fifty-three patients with cervical myelopathy were recruited for the cervical myelopathy group and 22 age-matched healthy controls were recruited for the control group. Functional assessments including Japanese Orthopedic Association Cervical Myelopathy Evaluation Questionnaire-Lower Extremity Function (JOACMEQ-LEF) and 10-second step test; as well as balance assessments including postural sway (center-of-pressure: COP) were performed for both groups. The JOACMEQ-LEF (p = 0.036) scores of the myelopathy group improved postoperatively, and a significant decrease in COP variables of postural sway was observed. The upright posture was less stable in the myelopathy group than in the control group (p < 0.05) both before and after surgery. The effect size and standard response mean of the COP variables ranged from −0.49 to 0.03 at 3 months, 6 months, and 1 year postsurgery. The upright balance control had improved significantly 6 months after decompression surgery. However, the balance control of the patients who had undergone decompression surgery remained less stable than that of the age-matched healthy controls. Balance training should be initiated before 6 months postsurgery to accelerate balance control recovery in patients with cervical myelopathy.
Similar content being viewed by others
Introduction
Cervical myelopathy is a condition caused by compression of the spinal cord and can cause direct injury to nerves1. Symptoms of cervical myelopathy, including loss of hand dexterity, standing imbalance, gait impairment, sensory loss, and bladder dysfunction, correspond to the level of cervical spine injury2.
Cervical decompression surgery is a common invasive intervention for cervical myelopathy3. The effectiveness of decompression surgery is usually evaluated based on functional outcomes, which are related to the effects of clinical manifestations on daily activities4. Although decompression surgery can alleviate the symptoms of myelopathy, surgical outcomes may be affected by age and symptom duration5. Long symptom duration may be associated with poor outcomes in functional activities that require stability in upright positions such as standing and walking6.
Gait parameters such as velocity, step length, cadence, stance phase, and single-stance phase have been reported to improve after decompression surgery7,8. However, in a recent study, no measured spatiotemporal parameters changed significantly postsurgery, except for electromyography findings9. Inconsistent results have been reported regarding postoperative effects on gait in patients with myelopathy. Moreover, increased signal intensity in T2-weighted cervical spine magnetic resonance images was correlated with impaired gait performance but not with scores of the modified Japanese Orthopedic Association (JOA) scale and Nurick scale10. Therefore, functional parameters other than gait such as upright balance control, which might be correlated with disease severity11, should be considered for postsurgical assessment.
Center-of-pressure (COP) movement is a simple biomechanical variable that can reflect an individual’s ability to maintain upright balance control12,13. COP movement parameters such as sway area, mean velocity, and range of traveling can quantify postural sway during movement14,15. Moreover, collecting data for COP movement is simple, and thus such data can easily be used in clinical settings. The present study investigated long-term changes in upright balance control (i.e., COP movement) in patients with myelopathy who had undergone cervical decompression surgery.
Methods
Study design
This observational prospective cohort study study determined improvements in upright balance control in patients with cervical myelopathy at 3 months, 6 months, and 1 year postsurgery. This study is part of as part of an ongoing research register in ClinicalTrial.gov (Identifier: NCT03396055). The study was approved by the Research Ethics Committee of National Taiwan University Hospital (201505093RIN).
Study procedure
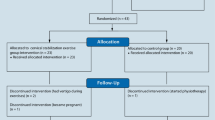
The study flowchart is shown in Fig. 1. Patients with myelopathy and age-matched healthy controls were assessed for eligibility. The study purpose was explained to all recruited participants, and written informed consent was obtained from all participants.
Participant recruitment
Fifty-three patients with myelopathy were recruited. The inclusion criteria for the myelopathy group were age between 20–80 years, presence with one or more neurological sign/symptom of cervical myelopathy and a diagnosis of cervical myelopathy according to relevant imaging examination. All patients in the myelopathy group were assessed by the same neurosurgeon to determine the requirement of decompression surgery. Participants were excluded if the participants were not able to stand upright for 1 minute, were unable to communicate or follow instructions, were unsuitable candidates for cervical decompression surgery because of other medical conditions, had traumatic spinal injury, had previous neurological dysfunction of the central nervous system (CNS), had recent musculoskeletal injury to the lower extremities, had vestibular dysfunction, or had infection or metastasis in the spine or lower extremities.
Twenty-two age-matched healthy controls were recruited. The inclusion criteria for the healthy control group were age between 20–80 years and absence of neck or back pain, severe musculoskeletal injury to the lower extremities or spine, vestibular dysfunction, and neurological dysfunction.
Functional assessments
Functional assessments were conducted in the myelopathy group. The JOA Cervical Myelopathy Evaluation Questionnaire (JOACMEQ) is a self-reported instrument used to evaluate the severity of cervical myelopathy. The total score of each item on this scale is generally high because patients’ self-reported conditions usually improve following treatment16. In this study, the JOACMEQ-Lower Extremity Function (JOACMEQ-LEF), which examines an individual’s ability to walk on a flat surface, stand on one leg, climb stairs, bend forward, kneel or stoop, and walk for more than 15 minutes, was used16,17.
The 10-second step test is another instrument to evaluate the severity of cervical myelopathy18. The participants were asked to take a step by lifting the thighs parallel to the floor in the same place without support at maximum speed18. The number of steps performed in 10 seconds was counted. Each participant was asked to perform the test at maximum speed18. For safety purposes, the examiner supervised all participants to prevent fall incidents.
Upright balance assessment
The upright balance assessment was conducted at four time points for the myelopathy group and on the recruitment day for the control group. Participants were asked to stand on a force platform (Kistler 9286 A, Kistler Instrumente AG, Winterthur, Switzerland) for 30 seconds at a sampling rate of 1000 Hz in each standing trial. All participants adopted a neutral stance (feet shoulder width apart) with eyes open and eyes closed. The same standing balance assessment was conducted for the remaining 37 participants on a different force platform (AMTI OR6, Advanced Medical Technology Inc., Watertown, MA, USA) for 30 seconds at a sampling rate of 1000 Hz. The participants were allowed to rest if they felt tired or soreness in the legs.
Data processing
Force platform signals were converted from analog to digital at a sampling rate of 1000 Hz. LabVIEW (National Instruments Corp., Austin, TX, USA) software was used to compute COP movement based on ground reaction force and moment in anteroposterior (AP) and mediolateral (ML) directions. Subsequently, collected data were processed and filtered through a second-order Butterworth low-pass filter of 5 Hz by using Matlab R2010a software. COP movement was further analyzed for time-domain measures, namely COP excursion, sway velocity, and sway area14. The COP sway area is defined as the area of the 95% of all points on the COP path. COP sway velocity is defined as the mean average velocity of the COP sway. The COP range is defined as the greatest distance between any two points on the COP movement path in anterior-posterior (AP) and medial-lateral (ML) direction. The root mean square (RMS) distance of COP was defined as RMS value of the resultant distance time series, which was also calculated in AP and ML direction respectively.
COP displacement reflects how the body moves to maintain balance stability. The previous study14 illustrated the time domain measures of COP movement among healthy young adults (21–35 years old) and healthy elderly (66–80 years old) in eyes open and eyes closed condition respectively. Besides, COP movement was used to evaluate balance performance in patients with cervical19,20,21 and lumbar13 disorder. However, the comprehensive time-domain COP normative data has not been published before for patients myelopathy for different level of severity.
Statistical analysis
The sample size in this study was determined by G* Power Software version 3.1 based on the pilot study on the COP data in patients with myelopathy group. Statistical analysis was performed using PASW Statistics 18 for Macintosh (SPSS, Chicago, IL, USA). Nonparametric tests were used for data analysis because functional outcomes and COP movement variables were not normally distributed. A p value of less than 0.05 (alpha, α) was considered statistically significant.
Differences in functional outcomes (JOACMEQ-LEF and 10-second step test) and in COP variables in the four phases served as the main effect and were examined using the Friedman test. If the main effect of phase difference was detected, pairwise comparisons of variables between the phases were performed using the Wilcoxon signed-rank test with Bonferroni adjustment. The difference in COP variables between the myelopathy and age-matched control groups over the four phases was determined using the Mann–Whitney U test.
Results
The basic of information of the participants are illustrated in Table 1. Figure 2 shows the results of the Friedman test for the phase factor in functional assessments (i.e., the JOACMEQ-LEF and 10-second step test). We observed a significant difference among the phases in JOACMEQ-LEF scores (p = 0.036). The results of pairwise comparison revealed a significant difference in the JOACMEQ-LEF between 3 months and 1 year postsurgery (p = 0.002).
Comparison of Japanese Orthopaedic Association Cervical Myelopathy Evaluation Questionnaire-lower extremities function (JOACMEQ-LEF) and 10 second step test after 3 months, 6 months and 1 year of surgery (preoperative phase as baseline). 0 M: preoperative phase; 3 M: postoperative 3 months; 6 M: postoperative 6 months; 1Y: postoperative 1 year. *Significant difference between 0 M and 1Y (p < 0.05/6 after Bonferroni’s adjustment). #Significant difference between 3 M and 1Y (p < 0.05/6 after Bonferroni’s adjustment).
Figure 3 presents the results of the Friedman test for the phase factor of COP variables in the eyes-open stance. Significant differences were found in all COP variables among the phases of decompression surgery in the eyes-open stance (95% confidence ellipse area: p = 0.022; mean velocity: p = 0.019; range-AP: p = 0.007; range-ML: p = 0.017; RMS distance-AP: p = 0.023; RMS distance-ML: p = 0.028). The pairwise comparison results revealed significant differences in the 95% confidence ellipse area (p = 0.006), mean velocity (p = 0.008), and RMS distance-AP (p = 0.004) between the preoperative phase and 6 months postsurgery. Moreover, the pairwise comparison results also showed significant differences in the mean velocity (p = 0.004), range-AP (p = 0.001), and RMS distance-AP (p = 0.004) between the preoperative phase and 1 year postsurgery. However, the COP variables did not differ significantly among the phases of decompression surgery in the eyes-closed stance (Fig. 4).
Comparison between phases and groups in COP variables during eyes-open stance in (A) 95% confidence ellipse area, (B) mean velocity, (C) range-AP, (D) range-ML, (E) RMS distance-AP and (F) RMS distance-ML. 0 M: myelopathy group at preoperative phase; 3 M: myelopathy group at postoperative 3 months; 6 M: myelopathy group at postoperative 6 months; 1Y: myelopathy group at postoperative 1 year; Control: age-matched control group. *Significant difference between phases of surgery (p < 0.05). §Significant difference between myelopathy group and age-matched control group (p < 0.05).
Comparison between phases and groups in COP variables during eyes-closed stance in (A) 95% confidence ellipse area, (B) mean velocity, (C) range-AP, (D) range-ML, (E) RMS distance-AP and (F) RMS distance-ML. 0 M: myelopathy group at preoperative phase; 3 M: myelopathy group at postoperative 3 months; 6 M: myelopathy group at postoperative 6 months; 1Y: myelopathy group at postoperative 1 year; Control: age-matched control group. *Significant difference between phases of surgery (p < 0.05). §Significant difference between myelopathy group and age-matched control group (p < 0.05).
Figures 3 and 4 show the results of the Mann–Whitney U test for the four phases of surgery in the eyes-open and eyes-closed stances, respectively, for the control and myelopathy groups. The 95% confidence ellipse area differed significantly between the control and myelopathy groups in the preoperative phase and at 3 months and 1 year postsurgery (p < 0.05) in the eyes-open and eyes-closed stances. The mean velocity, range-AP, and RMS distance-AP differed significantly among the four phases of surgery (p < 0.05) in the eyes-open and eyes-closed stances between the control and myelopathy groups.
Discussion
The present study investigated changes in upright balance control in patients with myelopathy who had undergone decompression surgery. The findings of this study indicated that upright balance control had improved at 6 months postsurgery. However, compared with the age-matched healthy controls, the upright posture of the patients with myelopathy remained less stable even after surgery.
The findings of the JOACMEQ-LEF, a subjective assessment, showed improvements at 1 year postsurgery which was in line with those of previous studies22,23,24. However, the results of the 10-second step test, an objective functional assessment, did not show significant improvements after surgery. Movement in the 10-second step test depends on the loading and unloading of the lower extremities in the ML plane by the hip abductor or adductor25. In our study, the patients with myelopathy exhibited AP balance control impairment. Thus, the 10-second step test may not be able to reflect the recovery in these patients.
In our study, upright balance control exhibited gradual improvement in the patients with myelopathy at 6 months and 1 year postsurgery. The slow healing of injured neural tissues in the spinal cord may have delayed the improvement of upright balance control in the early stage of the postoperative phase. A previous animal study on spinal cord injury reported that sprouting of corticospinal tract fibers occurred between 3 weeks and 3 months after injury, with penetration of the axons of this tract into the lesion matrix occurring over a long period26. Patients are usually asked to wear a neck collar to limit movement in their necks for the first 3 months after surgery. Patients may also become postoperatively inactive because of fear of movement in the initial 3 months postsurgery27. Thus, improvement of balance steadiness started after the termination of the immobilization phase. Postoperative motor relearning and balance training should be initiated before 6 months postsurgery to accelerate balance control recovery. Patients with shorter periods between symptom onset and rehabilitation are expected to achieve greater improvements in functional outcomes28.
In our study, upright balance control gradually improved in the patients with myelopathy in the eyes-open stance only. Sensorimotor impairment should be considered because ascending (sensory) and descending (motor) fibers in the spinal cord may be injured or damaged after compression20. Patients with myelopathy may be more mobile and active in daily functions performed in an upright position with eyes open. To maintain balance stability, various sensory inputs may be reweighted by the CNS29,30. In this study, visual inputs may have outweighed the proprioceptive input of the lower extremities to regulate balance control in the patients with myelopathy during the eyes-open stance31. Delayed recovery of proprioception may increase the balance sway of patients with myelopathy during the eyes-closed stance because of inefficient sensory integration for balance control32. These factors explain the delayed postoperative recovery of balance stability in the patients in the eyes-closed stance.
Either before or after decompression surgery, the balance steadiness of the patients with myelopathy (particularly in the AP direction) was less stable than that of the age-matched healthy controls in both the eyes-open and eyes-closed stances. Several assumptions can explain the poor upright balance control observed in the patients with myelopathy.
First, the sensorimotor deficit may not be completely recovered at 1 year postsurgery. Decompression surgery expanded the transverse area of the cervical canal for reversible cord injury and facilitated the morphological recovery of injured nerve tissues33. However, various pathological progressions might decrease the viscoelasticity of the cervical spinal cord, thereby indicating the delay and low degree of recovery33. The delayed recovery of injured cord tissues may impair ankle muscles for dorsiflexion and plantarflexion control, which are responsible for AP balance control, during upright standing12,34.
Second, some patients with myelopathy may have irreversible injury to a part of the spinal cord, particularly the corticospinal tract, after undergoing prolonged compression35,36. Surgical outcomes of decompression surgery are reportedly affected by the severity of histological changes secondary to spinal cord compression35,36. Histological changes in neuronal cells such as gliosis35, microcavities35, demyelination35, myelomalacia36,37, spongiform changes36,37, and necrosis36,37 may be caused by severe spinal compression or repetitive microtrauma in the spinal cord38. Irreversible pathological alterations in the spinal cord lead to permanent impairment of the proprioception of the lower extremities and the control of distal muscles (e.g., the tibialis anterior). Thus, a patient’s recovery may not progress further after 1 year postsurgery.
Third, cortical reorganization plasticity may trigger the recovery of upright balance control39,40, because decompression surgery can terminate the injury mechanism in the spinal cord. However, patients who undergo decompression surgery may be observed lack of steadiness or develop a fear of falling because of their adaptation to the previous compensatory strategy as a “safer way” to stand9,41. Patients with lumbar spinal fusion may exhibit a shorter forward reach distance because of fear avoidance42,43; this may cause them to stand in a compensatory pattern, even when the sensorimotor spinal pathway is recovering. Consequently, although the sensorimotor spinal pathway is recovering, the recovery of cortical reorganization of patients may be slowed because of a lack of task-specific practice39,44.
Finally, the adaptive or compensatory mechanism may be present in the musculoskeletal system45. The cervical muscle dysfunction and the pathological changes in the spinal tract may trigger an altered movement strategy in the lower extremities to compensate for sensorimotor dysfunction41,46. For instance, to maintain balance stability, the delayed antagonist reaction of the tibialis anterior20 may trigger corrective responses in the trunk and proximal joint47. Fatigability with incomplete spinal cord lesions may be associated with changes in muscle properties and characteristics of poor control such as muscle weakness, muscle atrophy, and delayed activation48 in the distal end of the lower extremities. The habitual compensatory pattern may persist after surgery if patients have not learned the correct movement pattern.
The findings of the current study demonstrated the progression of functional outcomes and upright balance control in individuals with cervical myelopathy who had undergone cervical decompression surgery up until 1 year postsurgery. The findings provide biomechanical evidence of balance control after decompression surgery. Incomplete recovery of upright balance control indicates that early mobilization should be initiated as soon as possible after surgery49. Postoperative rehabilitation should be initiated after termination of the postoperative neck immobilization phase. Motor relearning programs and customized balance training should be introduced in postoperative rehabilitation as soon as possible. In addition, COP variables can be used to examine the upright balance control of patients with myelopathy in clinical settings because balance control indicates recovery of the sensorimotor function3.
Study limitations
First, the lifestyles and exercise habits of our participants were not controlled for and may have resulted in various surgical outcomes. Second, the assessment was performed only once in the control group without continuous follow-up; thus, the aging effect in the control group within 1 year could not be excluded from this study.
Conclusions
The upright balance control of the patients with cervical myelopathy had improved 6 months after cervical decompression surgery. This postoperative improvement of upright balance control was observed only during the eyes-open stance. The upright posture of the patients with myelopathy remained less stable than that of the age-matched healthy controls after surgery. This result may be attributed to incomplete recovery of cervical spinal cord injury, permanent damage to spinal cord cells, and ongoing cortical reorganization and musculoskeletal adaptation changes in the patients who had undergone decompression surgery. Balance training could be initiated as early as 3 months postsurgery to accelerate balance control recovery in patients with cervical myelopathy.
References
Matz, P. G. Does nonoperative management play a role in the treatment of cervical spondylotic myelopathy? Spine J 6, S175–S181 (2006).
Tracy, J. A. & Bartleson, J. D. Cervical spondylotic myelopathy. Neurologist 16, 176–187, https://doi.org/10.1097/NRL.0b013e3181da3a29 (2010).
Yoshikawa, M. et al. Impaired postural stability in patients with cervical myelopathy: evaluation by computerized static stabilometry. Spine (Phila Pa 1976) 33, E460–464, https://doi.org/10.1097/BRS.0b013e318178e666 (2008).
Singh, A. et al. A summary of assessment tools for patients suffering from cervical spondylotic myelopathy: a systematic review on validity, reliability and responsiveness. Eur Spine J 24(Suppl 2), 209–228, https://doi.org/10.1007/s00586-013-2935-x (2015).
Tetreault, L. A., Karpova, A. & Fehlings, M. G. Predictors of outcome in patients with degenerative cervical spondylotic myelopathy undergoing surgical treatment: results of a systematic review. Eur Spine J 24(Suppl 2), 236–251, https://doi.org/10.1007/s00586-013-2658-z (2015).
King, J. T. Jr., Moossy, J. J., Tsevat, J. & Roberts, M. S. Multimodal assessment after surgery for cervical spondylotic myelopathy. J Neurosurg Spine 2, 526–534, https://doi.org/10.3171/spi.2005.2.5.0526 (2005).
Kuhtz-Buschbeck, J. P., Johnk, K., Mader, S., Stolze, H. & Mehdorn, M. Analysis of gait in cervical myelopathy. Gait Posture 9, 184–189 (1999).
Maezawa, Y., Uchida, K. & Baba, H. Gait analysis of spastic walking in patients with cervical compressive myelopathy. J Orthop Sci 6, 378–384 (2001).
Malone, A., Meldrum, D. & Bolger, C. Three-dimensional gait analysis outcomes at 1 year following decompressive surgery for cervical spondylotic myelopathy. European Spine Journal 24, 48–56 (2015).
Kim, C. R., Yoo, J. Y., Lee, S. H., Lee, D. H. & Rhim, S. C. Gait analysis for evaluating the relationship between increased signal intensity on t2-weighted magnetic resonance imaging and gait function in cervical spondylotic myelopathy. Archives of physical medicine and rehabilitation 91, 1587–1592, https://doi.org/10.1016/j.apmr.2010.07.008 (2010).
Hsu, W. L., Chen, C. Y., Tsauo, J. Y. & Yang, R. S. Balance control in elderly people with osteoporosis. Journal of the Formosan Medical Association. 113, 334–339, https://doi.org/10.1016/j.jfma.2014.02.006 (2014).
Winter, D. A., Prince, F., Frank, J. S., Powell, C. & Zabjek, K. F. Unified theory regarding A/P and M/L balance in quiet stance. J Neurophysiol 75, 2334–2343, https://doi.org/10.1152/jn.1996.75.6.2334 (1996).
Wong, W.-J., Lai, D.-M., Wang, S.-F., Wang, J.-L. & Hsu, W.-L. Changes of balance control in individuals with lumbar degenerative spine disease after lumbar surgery: a longitudinal study. The Spine Journal 19, 1210–1220, https://doi.org/10.1016/j.spinee.2019.02.015 (2019).
Prieto, T. E., Myklebust, J. B., Hoffmann, R. G., Lovett, E. G. & Myklebust, B. M. Measures of postural steadiness: differences between healthy young and elderly adults. IEEE Trans Biomed Eng 43, 956–966, https://doi.org/10.1109/10.532130 (1996).
Edwards, C. C. 2nd, Riew, K. D., Anderson, P. A., Hilibrand, A. S. & Vaccaro, A. F. Cervical myelopathy. current diagnostic and treatment strategies. Spine J 3, 68–81 (2003).
Tanaka, N. et al. An outcome measure for patients with cervical myelopathy: the Japanese Orthopaedic Association Cervical Myelopathy Evaluation Questionnaire (JOACMEQ): an average score of healthy volunteers. J Orthop Sci 19, 33–48, https://doi.org/10.1007/s00776-013-0494-y (2014).
Chien, A. et al. Translation, cross-cultural adaptation, and validation of a Chinese version of the Japanese Orthopaedic Association Cervical Myelopathy Evaluation Questionnaire. Spine (Phila Pa 1976) 39, 963–970, https://doi.org/10.1097/BRS.0000000000000313 (2014).
Yukawa, Y. et al. “Ten second step test” as a new quantifiable parameter of cervical myelopathy. Spine 34, 82–86, https://doi.org/10.1097/BRS.0b013e31818e2b19 (2009).
Yoshikawa, M. et al. Impaired postural stability in patients with cervical myelopathy: evaluation by computerized static stabilometry. Spine 33, E460–E464 (2008).
Nardone, A., Galante, M., Grasso, M. & Schieppati, M. Stance ataxia and delayed leg muscle responses to postural perturbations in cervical spondylotic myelopathy. Journal of Rehabilitation Medicine 40, 539–547, https://doi.org/10.2340/16501977-0214 (2008).
Lin, I. S. et al. Reweighting of the sensory inputs for postural control in patients with cervical spondylotic myelopathy after surgery. Journal of neuroengineering and rehabilitation 16, 96, https://doi.org/10.1186/s12984-019-0564-2 (2019).
Sampath, P., Bendebba, M., Davis, J. D. & Ducker, T. B. Outcome of patients treated for cervical myelopathy. A prospective, multicenter study with independent clinical review. Spine 25, 670–676 (2000).
Fehlings, M. G. et al. Efficacy and safety of surgical decompression in patients with cervical spondylotic myelopathy: results of the AOSpine North America prospective multi-center study. J Bone Joint Surg Am 95, 1651–1658, https://doi.org/10.2106/JBJS.L.00589 (2013).
Furlan, J. C., Kalsi-Ryan, S., Kailaya-Vasan, A., Massicotte, E. M. & Fehlings, M. G. Functional and clinical outcomes following surgical treatment in patients with cervical spondylotic myelopathy: a prospective study of 81 cases Clinical article. Journal of Neurosurgery-Spine 14, 348–355, https://doi.org/10.3171/2010.10.Spine091029 (2011).
Gribble, P. A. & Hertel, J. Effect of hip and ankle muscle fatigue on unipedal postural control. J Electromyogr Kinesiol 14, 641–646, https://doi.org/10.1016/j.jelekin.2004.05.001 (2004).
Hill, C. E., Beattie, M. S. & Bresnahan, J. C. Degeneration and sprouting of identified descending supraspinal axons after contusive spinal cord injury in the rat. Exp Neurol 171, 153–169, https://doi.org/10.1006/exnr.2001.7734 (2001).
Archer, K. R. et al. The effect of fear of movement beliefs on pain and disability after surgery for lumbar and cervical degenerative conditions. Spine 36, 1554–1562, https://doi.org/10.1097/BRS.0b013e3181f8c6f4 (2011).
van der Putten, J. J., Stevenson, V. L., Playford, E. D. & Thompson, A. J. Factors affecting functional outcome in patients with nontraumatic spinal cord lesions after inpatient rehabilitation. Neurorehabil Neural Repair 15, 99–104, https://doi.org/10.1177/154596830101500203 (2001).
Peterka, R. J. & Loughlin, P. J. Dynamic regulation of sensorimotor integration in human postural control. J Neurophysiol 91, 410–423, https://doi.org/10.1152/jn.00516.2003 (2004).
Hsu, W. L. Adaptive postural control for joint immobilization during multitask performance. PloS one 9, e108667, https://doi.org/10.1371/journal.pone.0108667 (2014).
Lee, J. H., Lee, S. H. & Seo, I. S. The characteristics of gait disturbance and its relationship with posterior tibial somatosensory evoked potentials in patients with cervical myelopathy. Spine 36, E524–530, https://doi.org/10.1097/BRS.0b013e3181f412d9 (2011).
Hughes, M. A., Duncan, P. W., Rose, D. K., Chandler, J. M. & Studenski, S. A. The relationship of postural sway to sensorimotor function, functional performance, and disability in the elderly. Archives of physical medicine and rehabilitation 77, 567–572 (1996).
Baba, H. et al. Plasticity of the spinal cord contributes to neurological improvement after treatment by cervical decompression. A magnetic resonance imaging study. J Neurol 244, 455–460 (1997).
Winter, D. A. Human balance and posture control during standing and walking. Gait & Posture 3, 193–214 (1995).
Matsuda, Y. et al. Increased MR signal intensity due to cervical myelopathy. Analysis of 29 surgical cases. J Neurosurg 74, 887–892, https://doi.org/10.3171/jns.1991.74.6.0887 (1991).
Morio, Y. et al. Correlation between operative outcomes of cervical compression myelopathy and mri of the spinal cord. Spine 26, 1238–1245 (2001).
Ohshio, I., Hatayama, A., Kaneda, K., Takahara, M. & Nagashima, K. Correlation between histopathologic features and magnetic resonance images of spinal cord lesions. Spine 18, 1140–1149 (1993).
Yagi, M., Ninomiya, K., Kihara, M. & Horiuchi, Y. Long-term surgical outcome and risk factors in patients with cervical myelopathy and a change in signal intensity of intramedullary spinal cord on Magnetic Resonance imaging. J Neurosurg Spine 12, 59–65, https://doi.org/10.3171/2009.5.SPINE08940 (2010).
Holly, L. T., Dong, Y., Albistegui-DuBois, R., Marehbian, J. & Dobkin, B. Cortical reorganization in patients with cervical spondylotic myelopathy. J Neurosurg Spine 6, 544–551, https://doi.org/10.3171/spi.2007.6.6.5 (2007).
Dong, Y. et al. Compensatory cerebral adaptations before and evolving changes after surgical decompression in cervical spondylotic myelopathy. J Neurosurg Spine 9, 538–551, https://doi.org/10.3171/SPI.2008.10.0831 (2008).
Hsu, W. L., Chou, L. S. & Woollacott, M. Age-related changes in joint coordination during balance recovery. Age. 35, 1299–1309, https://doi.org/10.1007/s11357-012-9422-x (2013).
Pao, J. L., Yang, R. S., Hsiao, C. H. & Hsu, W. L. Trunk Control Ability after Minimally Invasive Lumbar Fusion Surgery during the Early Postoperative Phase. J Phys Ther Sci 26, 1165–1171, https://doi.org/10.1589/jpts.26.1165 (2014).
Wang, T. Y., Pao, J. L., Yang, R. S., Jang, J. S. & Hsu, W. L. The adaptive changes in muscle coordination following lumbar spinal fusion. Hum Mov Sci 40, 284–297, https://doi.org/10.1016/j.humov.2015.01.002 (2015).
Raineteau, O. & Schwab, M. E. Plasticity of motor systems after incomplete spinal cord injury. Nat Rev Neurosci 2, 263–273, https://doi.org/10.1038/35067570 (2001).
Hsu, W. L. et al. Fatigue changes neck muscle control and deteriorates postural stability during arm movement perturbations in patients with chronic neck pain. The spine journal., https://doi.org/10.1016/j.spinee.2019.10.016 (2019).
Comerford, M. & Mottram, S. Kinetic control: the management of uncontrolled movement. (Elsevier Australia, 2012).
Bloem, B. R., Allum, J. H., Carpenter, M. G., Verschuuren, J. J. & Honegger, F. Triggering of balance corrections and compensatory strategies in a patient with total leg proprioceptive loss. Exp Brain Res 142, 91–107, https://doi.org/10.1007/s00221-001-0926-3 (2002).
Dobkin, B. H. Fatigue versus activity-dependent fatigability in patients with central or peripheral motor impairments. Neurorehabil Neural Repair 22, 105–110, https://doi.org/10.1177/1545968308315046 (2008).
Cheng, Y. S. et al. Perturbation-based balance training in postoperative individuals with degenerative cervical myelopathy. Frontiers In Bioengineering And Biotechnology, https://doi.org/10.3389/fbioe.2020.00108 (2020).
Acknowledgements
This work was supported by Ministry of Science and Technology (MOST 105-2628-E-002 -006 -MY3; 108-2221-E-002 -077). The patient enrollment and data collection were assisted by Ms. Ling-Zhi Wei, Ms. Jo-En Chien, Ms. Iu-Shiuan Lin, Ms. Yi-Sheng Cheng and Miss Wei-Jin Wong.
Author information
Authors and Affiliations
Contributions
D.M.L., P.L., C.C.H. and W.L.H. wrote the main manuscript. D.M.L., P.L., C.C.H., S.F.W., J.L.W. and W.L.H. designed the study and experiment protocol. C.H.C. and P.L. conducted the experiment. P.L. and A.C. prepared the figure and tables, analysed the data, and performed the statistical analyses. All authors contributed to the conduct of the study and manuscript preparation. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cheng, CH., Lai, DM., Lau, P.Y. et al. Upright Balance Control in Individuals with Cervical Myelopathy Following Cervical Decompression Surgery: A Prospective Cohort Study. Sci Rep 10, 10357 (2020). https://doi.org/10.1038/s41598-020-66057-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-66057-y
- Springer Nature Limited
This article is cited by
-
Relationship Between Body Composition and Balance Performance in Older Adults with Hyperkyphosis
Journal of Medical and Biological Engineering (2021)