Abstract
It is demanded to monitor temperature in tissue during oncological hyperthermia therapy. In the present study, we non-invasively measured the temperature elevation inside the abdominal cavity and tumour tissue of a living rat induced by capacitive-coupled radiofrequency heating. In the analysis of ultrasound scattered echoes, the Nakagami shape parameter m in each region of interest was estimated at each temperature. The Nakagami shape parameter m has temperature dependence; hence, the temperature increase inside tissue specimens can be detected with the m values. By carrying out in vivo experiments, we visualized the temperature increase inside the abdominal cavity and tumour tissue of living rats using two-dimensional hot-scale images indicating the absolute values of the ratio changes of the m values. In both the abdominal cavity and tumour tissue, the brightness in the hot-scale images clearly increased with increasing temperature. The increases in brightness in the hot-scale images imply the temperature elevations inside the abdominal cavity and tumour tissue of the living rats. The study results prove that the acoustic method we proposed is a promising method for monitoring changes in the internal temperature of the human body under hyperthermia treatment.
Similar content being viewed by others
Introduction
Non-invasive measurement of internal body temperature distribution has great potential in the medical field. In particular, internal body temperature distribution is the most important parameter for carrying out oncological hyperthermia therapy safely, correctly, and effectively. However, currently, hyperthermia therapy is conducted without monitoring internal body temperature, including the temperature of malignant tumour tissue, in almost all cases. There is no realistic method to non-invasively detect internal body temperature distribution during heating under therapy except for operating a magnetic resonance imaging (MRI) device. Although it is well known that internal body temperature can be detected by an MRI instrument1,2,3,4,5, an MRI device is adopted as an internal body temperature detector by only one hyperthermia device (BSD-2000 3D/MR; Pyrexar Medical, Salt Lake City, UT, USA)6. Unfortunately, this hyperthermia device is not preferred among oncological hyperthermia societies because of its high price. A major trend is that oncological hyperthermia therapy devices require an invasive temperature measurement system using a thermocouple or a fibre optic temperature sensor. However, nobody actually monitors the internal body temperature by inserting a sensor probe into the tumour tissue. A realistic method to non-invasively measure the temperature distribution inside the human body has been demanded by oncological hyperthermia societies for three decades.
In addition, many researchers have proposed a solution using ultrasound technologies instead of using an MRI instrument. There is a major engineering trend to detect internal body temperature based on the temperature dependence of ultrasound propagation speed7,8,9,10,11,12. Those studies have achieved success in detecting a temperature increase inside biological tissue in in vitro and ex vivo situations. However, the temperature increase can not be measured precisely by the acoustic method with ultrasound propagation speed in in vivo situations because body motion and pulsation exist. In this method, the temperature change is estimated with a known ultrasound propagation path length and propagation time. It is unrealistic to estimate precise axial displacement along the ultrasound propagation path using a B-mode image in an in vivo situation.
On the other hand, it was reported that some statistical properties obtained by statistical analysis of ultrasound backscattered echoes can be applied to quantify liver diseases13,14,15,16, detect scatterer size and density in a tumour17,18,19, and classify breast lesions20,21; hence, some research groups found that the statistical parameters obtained from analysis of ultrasound scattered echoes have the potential to detect temperature distribution inside biological tissue specimens22,23,24,25,26. Moreover, a computer simulation study has demonstrated that one of the statistical parameters, the Nakagami shape parameter m, is strongly related to the scatterer concentration in the medium27; thus, it is predicted that the Nakagami shape parameter m changes with a change in the medium volume due to thermal expansion or contraction of medium containing scatterers. Our research group has focused on the possibility of non-invasively monitoring the temperature distribution inside the human body with the temperature dependence of the Nakagami shape parameter m for oncological hyperthermia treatment. We previously reported phantom and ex vivo study results22,23. We presented that it is important to avoid the influence of deformation resulting from a temperature elevation in soft tissue specimens to select a proper size of a region of interest (ROI) in the estimation of the shape parameter of the Nakagami distribution22. In that study, temperature changes inside soft tissue with deformation could be detected as a two-dimensional hot-scale image indicating absolute values of ratio changes of m values, α, estimated with some ROI sizes that were assumed to be larger than the amount of displacement of the soft tissue22. The study result implies that internal temperature changes under in vivo conditions can be expressed as hot-scale images indicating absolute values of ratio changes of m values estimated by selecting proper ROI sizes with consideration of the displacement due to body motion and pulsation. Furthermore, it was shown in our previous study that variations in the Nakagami shape parameter m due to a change in temperature depend on an initial m value23. By taking into account the initial m value dependence, we proposed a new parameter αmod. that was elicited by adding the multiplying factor varying as a function of the initial m value to absolute values of ratio changes of m values, α23. Furthermore, the temperature gradient inside a locally heated real soft tissue specimen with no thermal lesions was visualized with two-dimensional αmod. maps more clearly than that visualized using the previous method with α23. In this study, we present an in vivo study result showing that the temperature elevation inside the abdominal cavity and tumour tissue of a living rat induced with capacitive-coupled radiofrequency (RF) current heating was detected by hot-scale images indicating absolute values of ratio changes of m values, αmod..
Results
Temperature elevation inside tissue detected in vivo by ultrasound scattered echoes in healthy rat
In the healthy rat experiment, an Slc:SD female living rat was heated from 30.0 to 41.0 °C by RF current. Ultrasound scattered echoes from the abdominal cavity of the rat were measured. In addition, temperatures inside the abdominal cavity were detected with fibre optic temperature sensor probes. Figure 1(a,b) show the experimental setup and the schematic of the experimental setup for the healthy rat experiment. In the healthy rat experiment, the temperature measured at Point 2 is defined as a reference temperature. The temporal variations in temperature inside the abdominal cavity and on the surface of the skin under the electrode of the living rat during heating are shown in Fig. 2(a). As seen in Fig. 2(a), temperatures at each point varied with ripples due to the RF current generator being paused briefly each time ultrasound scattered echoes were measured. The temperature increase from the non-induced state ΔT at each point is plotted as a function of the reference temperature in Fig. 2(b).
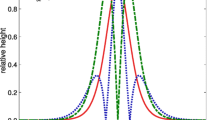
Figure 3 shows typical grey-scale B-mode images obtained from the abdominal cavity of the rat at reference temperatures of 30.0 and 40.0 °C and histograms of envelopes of the ultrasonic echo signal. The red solid line indicates the Nakagami distribution function in Fig. 3. In the B-mode images, there is a non-negligible deformation between each reference temperature. The displacement seen in the B-mode images is thought to be due to body motion or pulsation during heating. In our previous study22, it was presented that the calculation of absolute values of ratio changes of m values can be done correctly by selecting a proper ROI size even when deformations are observed in biological tissue specimens. Thus, we carried out statistical analysis estimating the Nakagami shape parameter m and absolute values of ratio changes of m values, αmod., with a relatively large ROI set at 1.8 × 1.8 mm2 to prevent the effect of displacement caused by pulsation and body motion in this study, thereby creating156 ROIs for statistical analysis. The Nakagami shape parameter m was estimated by statistical analysis as follows. First, analytic signals were elicited by applying the Hilbert transformation to measured ultrasonic RF signals, and envelope signals were obtained. Then, the histograms of envelopes in each ROI were created by setting the ROI size at 1.8 × 1.8 mm2. The Nakagami distribution function is expressed by
where Γ(∙) and U(∙) are the gamma function and unit step function, respectively, r is the amplitude of envelopes of ultrasound scattered echoes, m is the Nakagami shape parameter, and Ω is a scaling parameter. Finally, the Nakagami shape parameter m for each ROI was estimated by fitting the Nakagami distribution function to the histogram of the envelope of the ultrasonic scattered echoes (see Fig. 3). The normalized mean squared error (NMSE) was calculated to evaluate the goodness of fitting of the Nakagami distribution function to the histograms. NMSE was calculated as
where M and h(ri) are the number of bins and the height of bin at each amplitude of envelopes of ultrasound scattered echoes. Mean values of NMSE of 156 ROIs at each reference temperature are listed in Table 1. The mean value of NMSE for the abdominal cavity is approximately 0.15. Meanwhile, the mean value of NMSE for the tumour tissue is approximately 0.04. The mean values of NMSE for the abdominal cavity and the tumour tissue are suitably small. In the study of Gambin and Kruglenko24, the goodness of fitting of the Nakagami distribution function to histograms of envelopes of ultrasonic RF signals scattered from biological tissue specimens was evaluated with NMSE. In the cited study, the values of NMSEs were from approximately 0.12 to 0.22. At the same time, in the present study, internal temperature changes in the biological tissue specimens were well detected using the Nakagami shape parameter. Therefore, the Nakagami distribution was considered to be a suitable approximation model.
Grey-scale B-mode images of the abdominal cavity at reference temperatures of T = 30.0 and 40.0 °C and representative histograms of envelopes obtained by setting the ROI size at 1.8 × 1.8 mm2 and fitting the Nakagami distribution function to the histogram. The red solid line indicates the Nakagami distribution function.
To express the temperature increase inside the abdominal cavity by the variation of brightness on a two-dimensional hot-scale image indicating absolute values of ratio changes of m values, the specific parameter αmod. was calculated using
where mTR and mT are the Nakagami shape parameters m at a baseline temperature and each temperature, respectively, and γ denotes the multiplying factor. In our previous ex vivo study23, it was clearly shown that the magnitude of the change in the Nakagami shape parameter (Δm) due to a temperature rise has a dependence on the initial m value at a baseline temperature; furthermore, Δm due to a temperature rise increases with increasing initial m value. Therefore, the ratio changes of m values were amplified with compensation considering the initial m values. In this study, the multiplying factor γ varying as a function of the initial m value was defined to be proportional to m−1 as
The hot-scale images indicating the absolute values of ratio changes of m values, αmod., for the abdominal cavity calculated using Eqs. (3) and (4) with the baseline temperature of TR = 30.0 °C are shown in Fig. 4. Note that the hot-scale images indicating the absolute values of ratio changes of m values, αmod., were constructed by comparing the Nakagami shape parameter m at a baseline temperature to m at each temperature estimated in each ROI with an overlap ratio of 0% between ROIs. In the hot-scale images, the overall increase in αmod. brightness with increasing reference temperature is clearly observed. The increase in αmod. brightness implies a temperature elevation in the abdominal cavity induced by RF current.
Temperature elevation inside tumour tissue detected in vivo by ultrasound scattered echoes
In real therapy treatments, the oncological hyperthermia device is applied to the tumour tissue. Therefore, an experiment to detect temperature increases inside tumour tissue should be conducted in our study. In the experiment on tumour tissue, heterotopic tumour tissue was grown around the right femoral region of a Slc:SD female rat. Ultrasound scattered echoes from the tumour tissue were measured while the tumour tissue was heated from 35.5 to 42.5 °C. The experimental setup, a close-up photo around the tumour tissue, and the schematic of the experimental setup for the tumour tissue experiment are shown in Fig. 5(a–c). In the tumour tissue experiment, we used temperatures measured at Point 5 as a reference temperature. Figure 6(a) shows the variations in the temperature inside the tumour tissue and the surface of the skin of the rat during heating. The temperature increase from the non-induced state ΔT at each point is plotted as a function of the reference temperature in Fig. 6(b). In Fig. 6(b), the ΔT distribution inside the tumour tissue is intricate, not systematic, as expected from the positional relationship between electrodes and the tumour tissue. ΔT at point 6 increases steeply in Fig. 6(b). Moreover, ΔT at point 6 is approximately one and half times larger than ΔT at points 4 and 5. A computer simulation study predicted that the temperature distribution inside muscle, tumour tissue, brain, and some organs induced non-locally by electrical energy under in vivo conditions is significantly complex, and hotspots occur28. It is presumed that non-uniform heating is caused by inhomogeneous dielectric and/or thermal properties of organs and muscle. Hence, it is thought that the tumour tissue was heated up non-uniformly by the RF current and that a few hotspots existed inside the tumour tissue in this study.
Grey-scale B-mode images obtained from the tumour tissue at reference temperatures of 35.5 and 42.5 °C and histograms of envelopes with the Nakagami distribution function fit (red solid line) at different ROIs are shown in Fig. 7. The shallow region below approximately 5 mm in the B-mode images for the tumour tissue experiment is indistinct. In the tumour tissue experiment, we conducted a statistical analysis of ultrasound scattered echoes on the area enclosed with the dotted red line in Fig. 7. The hot-scale m-parameter images for the tumour tissue drawn by setting the baseline temperature at 35.5 °C are shown in Fig. 8. In the hot-scale images, the overall temperature elevation inside the tumour tissue with increasing reference temperature was observed as an increase in the αmod. brightness as well as the results of the healthy rat experiment.
Grey-scale B-mode images of tumour tissue at reference temperatures of T = 35.5 and 42.5 °C and representative histograms of envelopes obtained by setting the ROI size at 1.8 × 1.8 mm2 and fitting the Nakagami distribution function to the histogram. The red solid line indicates the Nakagami distribution function.
Discussion
The hot-scale images indicating the absolute values of ratio changes of m values, αmod., show the overall increases in temperature inside the abdominal cavity and the tumour tissue of the living rats. To evaluate the increase in αmod. with increasing temperature inside the abdominal cavity and the tumour tissue quantitatively, the mean value of αmod. on the analysis area of the ultrasonic RF signal was calculated at each reference temperature. Figure 9(a) shows the mean values of αmod. for the abdominal cavity of the healthy rat and the tumour tissue plotted as a function of the reference temperature. The error bar indicates the standard error of the mean of αmod. in Fig. 9(a,b). The mean value of αmod. for both the abdominal cavity and the tumour tissue monotonically rises with an increase in the reference temperature. Figure 9(b) shows the ΔT dependence of the mean value of αmod. for the abdominal cavity and the tumour tissue. We calculated the Pearson correlation coefficient p between the mean value of αmod. and ΔT. The coefficient of correlation p shows impressively large positive values, which are 0.99 for the abdominal cavity and 0.93 for the tumour tissue. An increase in αmod. indicated a temperature elevation inside the abdominal cavity, and the tumour tissue heated by RF current was confirmed quantitatively by the strong correlation between αmod. and ΔT in this study.
Finally, since it was reported that a thermal lesion in soft tissue could be detected by changes in the Nakagami shape parameter29, it should be clarified that the increase in αmod. was not caused by heat-induced denaturation inside the tumour tissue due to the RF current being passed in this study. To observe the cross-section of the tumour tissue, the tissue was cut along the plane of ultrasound scanning after we completed the experiment. Figure 10(a) shows a close-up photo of the tumour tissue that was separated from the rat body. The cross-section of the tumour tissue is shown in Fig. 10(b). No visible transmutation was observed on the cross-section of the tumour tissue. Therefore, it can be concluded that the temperature elevation inside the tumour tissue of a living rat induced by RF current heating was detected by the hot-scale images, indicating the absolute values of ratio changes of m values.
In this study, we propose that our acoustic method is useful for detecting temperature elevations in tumour tissue heated by RF current in vivo. In our future study, the quantitative relationship between αmod. and ΔT should be investigated using a wide variety of tumour tissues to establish a method to measure the absolute value of ΔT in vivo.
Methods
In this study, we conducted in vivo experiments using two Slc:SD female rats, which weighed approximately 0.3 kg. One was arranged for an experiment measuring ultrasound scattered echoes from the abdominal cavity (healthy rat experiment), and the other was arranged for an experiment with malignant tumour tissue (tumour tissue experiment). In the tumour tissue experiment, we prepared 9 L (glioma) cell line-derived heterotopic tumour tissue grown around the right femoral region of the Slc:SD female rat. The rats were anaesthetized by an inhalation anaesthesia system using isoflurane during the heat treatment in both experiments. In the healthy rat experiment, the abdominal cavity was heated from 30.0 to 41.0 °C by the energy of a capacitive-coupled RF current at a frequency f = 13.56 MHz between two flexible round-shaped electrodes holding both sides of that rat’s body. In the tumour tissue experiment, the tumour tissue was heated from 35.5 to 42.5 °C by passing an RF current at the frequency f = 13.56 MHz between the two electrodes holding the rat’s right hind limb including the tumour tissue. The electrodes were connected to a custom-built RF generator unit through an impedance matching circuit. The electrodes were water cooled to prevent surface overheating. Reference temperatures inside the abdominal cavity and the tumour tissue were measured by inserting fibre optic temperature sensor probes (m3300; LumaSense Technologies, Santa Clara, CA, USA). Ultrasonic echoes scattered from the heated specimens were measured at 1.0 °C intervals using an ultrasonic measurement system (RSYS0002; Microsonic, Kokubunji, Tokyo, Japan) with a linear array transducer (UST-5412; Hitachi-Aloka Medical, Mitaka, Tokyo, Japan). The two dimensional size of the scanning area in the specimens was approximately 30 mm in depth and 25 mm in width. The parameters in the ultrasonic measurement are listed in Table 2. The RF generator emits substantial electromagnetic waves, and it is strong enough to affect the piezoelectric elements of the transducer. The RF generator was paused emitting electromagnetic waves during acquisitions of ultrasonic echoes at the respective temperatures. The experimental setups for the healthy rat experiment and for the tumour tissue experiment are shown in Figs. 1(a) and 5(a). A close-up photo around the tumour tissue is shown in Fig. 5(b). Figures 1(b) and 5(c) show the schematic of the experimental setups for the healthy rat experiment and tumour tissue experiment. Points 1–7 in Figs. 1(b) and 5(c) indicate the positions of the tip of each sensor probe in the experimental setups for the healthy rat and tumour tissue experiments. In this study, we used temperatures measured at Points 2 and 5 as a reference temperature for the healthy rat experiments and tumour tissue experiments. It should be noted that we measured ultrasound scattered echoes at 1.0 °C intervals by referring to the reference temperatures. The application of RF current was stopped manually every 1.0 °C during acquisition of ultrasonic echo signals. After in vivo experiments, hot-scale images of temperature elevation inside specimens were processed by conducting statistical analysis of ultrasonic scattered echoes with custom-made software based on MATLAB R2018b (The MathWorks, Natick, MA, USA, https://www.mathworks.com/) and Python 3.6.0 (Python Software Foundation, DE, USA, https://www.python.org/).
Ethical approval
All experiments and procedures involving animals were conducted in accordance with the Institutional Animal Experiment Handling Rules as approved by the Institutional Animal Care and Use Committee at University of Toyama (A2017OPR-2).
Data availability
The datasets during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Kuroda, K. et al. Non-invasive MR temperature imaging-work in progress. Med. Imaging Technol 21, 201–207 (2003).
Wyatt, C. et al. Hyperthermia MRI temperature measurement: evaluation of measurement stabilisation strategies for extremity and breast tumours. Int. J. Hyperthermia 25, 422–433 (2009).
Hynynen, K., McDannold, N., Mulkern, R. V. & Jolesz, F. A. Temperature monitoring in fat with MRI. Magn. Reson. Med. 43, 901–904 (2000).
Solovchuk, M. A., Hwang, S. C., Chang, H., Thiriet, M. & Sheu, T. W. Temperature elevation by HIFU in ex vivo porcine muscle: MRI measurement and simulation study. Med. Phys. 41, 052903 (2014).
Ehses, P. et al. MRI thermometry: fast mapping of RF-induced heating along conductive wires. Magn. Reson. Med. 60, 457–461 (2008).
Mulder, H. T., Curto, S., Paulides, M. M., Franckena, M. & Van Rhoon, G. C. Systematic quality assurance of the BSD2000-3D MR-compatible hyperthermia applicator performance using MR temperature imaging. Int. J. Hyperthermia 35, 305–313 (2018).
Varghese, T. et al. Ultrasound monitoring of temperature change during radiofrequency ablation: preliminary in-vivo results. Ultrasound Med. Biol. 28, 321–329 (2002).
Liu, Y.-D. et al. Adaptive ultrasound temperature imaging for monitoring radiofrequency ablation. PLoS One 12, e0182457 (2017).
Fuzesi, K. et al. Temperature dependence of speed of sound and attenuation of porcine left ventricular myocardium. Ultrasonics 82, 246–251 (2018).
Fujii, M. & Zhang, X. Noncontact measurement of internal temperature distribution in a solid material using ultrasonic computed tomography. Exp. Therm. Fluid Sci. 24, 107–116 (2001).
Daniels, M. J., Varghese, T., Madsen, E. L. & Zagzebski, J. A. Non-invasive ultrasound-based temperature imaging for monitoring radiofrequency heating-phantom results. Phys. Med. Biol. 52, 4827–4843 (2007).
Pouch, A. M., Cary, T. W., Schultz, S. M. & Sehgal, C. M. In vivo noninvasive temperature measurement by B-mode ultrasound imaging. J. Ultrasound Med 29, 1595–1606 (2010).
Mori, S., Ohashi, M., Hirata, S. & Hachiya, H. Stability evaluation of parameter estimation of multi-rayleigh model for ultrasound B-mode image of liver fibrosis. Japanese Journal of Applied Physics 55, 07KF09 (2016).
Omura, M. et al. Tissue characterization of skin ulcer for bacterial infection by multiple statistical analysis of echo amplitude envelope. Japanese Journal of Applied Physics 55, 07KF14 (2016).
Takuma, O. et al. Estimation of scatterer size and acoustic concentration in sound field produced by linear phased array transducer. Japanese Journal of Applied Physics 54, 07HF14 (2015).
Isono, H., Hirata, S. & Hachiya, H. Quantitative analysis of ultrasonic images of fibrotic liver using co-occurrence matrix based on multi-rayleigh model. Japanese Journal of Applied Physics 54, 07HF15 (2015).
Oelze, M. L. & Mamou, J. Review of quantitative ultrasound: envelope statistics and backscatter coefficient imaging and contributions to diagnostic ultrasound. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 63, 336–351 (2016).
Wirtzfeld, L. A. et al. Quantitative ultrasound comparison of MAT and 4T1 mammary tumors in mice and rats across multiple imaging systems. J. Ultrasound Med 34, 1373–1383 (2015).
Sannachi, L. et al. Non-invasive evaluation of breast cancer response to chemotherapy using quantitative ultrasonic backscatter parameters. Med. Image Anal. 20, 224–236 (2015).
Byra, M., Nowicki, A., Wroblewska-Piotrzkowska, H. & Dobruch-Sobczak, K. Classification of breast lesions using segmented quantitative ultrasound maps of homodyned K distribution parameters. Med. Phys. 43, 5561 (2016).
Trop, I. et al. The added value of statistical modeling of backscatter properties in the management of breast lesions at US. Radiology 275, 666–674 (2015).
Takeuchi, M. et al. Measurement of internal temperature in biological tissue specimen with deformation by statistical analysis of ultrasonic scattered echoes. Japanese Journal of Applied Physics 57, 07LB17 (2018).
Takeuchi, M. et al. Investigation of initial value dependence in the statistical analysis of ultrasonic scattered echoes for the non-invasive estimation of temperature distribution in biological tissue. Japanese Journal of Applied Physics 58, SGGE09 (2019).
Gambin, B. & Kruglenko, E. Temperature measurement by statistical parameters of ultrasound signal backscattered from tissue samples. Acta Phys. Pol. A 128, A72–A78 (2015).
Byra, M., Kruglenko, E., Gambin, B. & Nowicki, A. Temperature monitoring during focused ultrasound treatment by means of the homodyned K distribution. Acta Phys. Pol. A 131, 1525–1528 (2017).
Tsui, P. H. et al. Ultrasound temperature estimation based on probability variation of backscatter data. Med. Phys. 39, 2369–2385 (2012).
Tsui, P. H. & Chang, C. C. Imaging local scatterer concentrations by the Nakagami statistical model. Ultrasound Med. Biol. 33, 608–619 (2007).
Kok, H. P. et al. Current state of the art of regional hyperthermia treatment planning: a review. Radiat. Oncol 10, 196–196 (2015).
Zhou, Z. et al. Monitoring radiofrequency ablation using real-time ultrasound Nakagami imaging combined with frequency and temporal compounding techniques. PLoS One 10, e0118030 (2015).
Acknowledgements
We would like to express our heartfelt gratitude to Yuta Matsui of Tateyama Kagaku Device Technology Co., Ltd. and Yoshitaka Omoto of University of Toyama for their technical support.
Author information
Authors and Affiliations
Contributions
M.T. and H.H. conceived the idea and designed the experiment. M.T. analysed the data and prepared the first draft of the manuscript. K.T. prepared the animal experiment protocol for the experiment. G.A. grew the tumour tissue inside the rat and fabricated the hyperthermia system. M.T., G.A., K.T., R.N. and H.H. carried out the experiment. T.S., G.A., R.N. and H.H. contributed to the analysis. All authors were involved in the revision of the draft manuscript and discussions. All authors approved the final content.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Takeuchi, M., Sakai, T., Andocs, G. et al. Temperature elevation in tissue detected in vivo based on statistical analysis of ultrasonic scattered echoes. Sci Rep 10, 9030 (2020). https://doi.org/10.1038/s41598-020-65562-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-65562-4
- Springer Nature Limited
This article is cited by
-
Basic concept and clinical applications of quantitative ultrasound (QUS) technologies
Journal of Medical Ultrasonics (2021)