Abstract
The norepinephrine-releasing neurons in the locus coeruleus (LC) are well known to regulate wakefulness/arousal. They display active firing during wakefulness and a decreased discharge rate during sleep. We have previously reported that LC neurons express large numbers of GABAB receptors (GABABRs) located at peri-/extrasynaptic sites and are subject to tonic inhibition due to the continuous activation of GABABRs by ambient GABA, which is significantly higher during sleep than during wakefulness. In this study, we further showed using western blot analysis that the activation of GABABRs with baclofen could increase the level of phosphorylated extracellular signal-regulated kinase 1 (ERK1) in LC tissue. Recordings from LC neurons in brain slices showed that the inhibition of ERK1/2 with U0126 and FR180204 accelerated the decay of whole-cell membrane current induced by prolonged baclofen application. In addition, the inhibition of ERK1/2 also increased spontaneous firing and reduced tonic inhibition of LC neurons after prolonged exposure to baclofen. These results suggest a new role of GABABRs in mediating ERK1-dependent autoregulation of the stability of GABABR-activated whole-cell current, in addition to its well-known effect on gated potassium channels, to cause a tonic current in LC neurons.
Similar content being viewed by others
Introduction
γ-Aminobutyric acid (GABA) is the principal inhibitory neurotransmitter in the forebrain. By acting at ionotropic GABAA receptors (GABAARs) located within the synaptic acting zone, GABA can rapidly increase the membrane permeability to Cl- in target neurons and produce fast phasic inhibitory transmission. This type of signaling is referred to as conventional synaptic transmission and features a specific method of communication between neurons with high temporal and spatial precision that enables the presynaptic neuron to shape the spiking pattern of the postsynaptic neuron. In addition to those located in the synaptic active zone, GABAARs containing specific subunits can also mediate a tonic form of inhibition that is not time-locked to presynaptic action potentials (APs) and is shown to profoundly modulate the input–output relationships of individual neurons. GABAAR-mediated tonic inhibition has been identified as an important player in both physiological and pathophysiological processes1,2.
In addition to GABAARs, GABA also acts on metabotropic GABAB receptors (GABABRs) to produce a much slower but very long-lasting inhibition at both presynaptic and postsynaptic sites3,4,5,6,7 compared with the fast phasic transmission mediated by GABAARs. At the presynaptic site, the activation of GABABRs reduces the release probability of synaptic vesicles through inhibiting N-type or P/Q-type voltage-dependent Ca2+channels; at the postsynaptic site, the activation of GABABRs produces hyperpolarization by increasing the potassium conductance of G protein-coupled inwardly rectifying K+(GIRK) or inwardly rectifying K+3 (Kir3) channels8,9,10. GABABRs were the first G protein-coupled receptor (GPCR) to be identified as an obligate heterodimer; a functional GABAB receptor is formed from the heterodimerization of the GABAB1 and GABAB2 receptor subunits, with the former constituting the GABA binding site and the latter being coupled to the Gproteins, comprising αi/o, β and γ subunits11,12,13. The binding of GABA to the GABAB1 receptor activates the coupled G protein to gate the pre- and postsynaptic ion channels described above via the β and γ subunits8,10. Despite the well-understood functional roles of the β and γ subunits, much remains to be learned about the role of receptor-induced lowering of cAMP levels by the αi/o subunit.
Electron microscopic studies have revealed that the subcellular distribution of GABABRs is mostly at peri-/extrasynaptic loci4,5,6,7, implying that, similar to GABAARs, these extrasynaptic GABABRs can mediate a tonic form of signaling by detecting ambient GABA. Indeed, it has been shown that ambient GABA can tonically induce a low level of presynaptic and postsynaptic GABABR activation to provide the control of transmitter release at the hippocampus and calyx of Held synapses and the control of the excitability of pyramidal neurons in the medial prefrontal cortex and noradrenergic (NAergic) neurons in the locus coeruleus (LC)7,9,14,15,16. The physiological roles of GABABR-mediated tonic inhibition have begun to emerge. Recently, it has been shown that tonic inhibition of LC NAergic neurons (hereafter referred to as LC neurons) could be an important player in the regulation of brain function states7,17. LC neurons have global NAergic projections to the forebrain and play important roles in the control of behaviors through the regulation of vigilance18,19. Furthermore, GABAergic transmission in the LC has been implied to be a mechanism underlying the effect of some anesthetics on consciousness17,20,21,22,23,24. It has been shown that LC neurons and NAergic A7 neurons in the pons express a large amount of GABABRs and are subject to GABABR-mediated tonic inhibition in brain slice preparations and in vivo7,9,17. Moreover, the suppression of the tonic inhibition of LC neurons could accelerate the regain of consciousness from isoflurane-induced deep anesthesia17. Tonic inhibition would require the activity of a substantial number of GABABRs on the membrane for a long period. Nevertheless, this would appear to conflict with the features of most GPCRs, including GABABRs, such that the receptor will undergo rapid desensitization upon activation by the ligand25. In this study, we report that, in LC neurons, the activation of GABABRs also activates extracellular signal-regulated kinase 1 (ERK1) signaling pathways, which is consistent with previous studies in the hippocampus and cerebellum26,27,28. We further show that the activation of ERK1 signaling pathways by GABABRs could prevent a rapid decline in the GABABR-activated whole-cell membrane current and help stabilize tonic inhibition.
Results
Activation of GABABRs increases pERK levels in the LC
We first examined whether the activation of GABABRs could also increase phosphorylated-ERK1/2 (pERK1/2) levels in the LC, as previously reported in hippocampal and cerebellar tissue26,27,28. We examined pERK1/2 levels in LC tissue punched from slices (Fig. 1A) bathed in 50 μM baclofen, a GABABR agonist, and the vehicle, artificial cerebrospinal fluid (aCSF) containing synaptic blockers (see Materials and Methods), using western blot analysis. As pERK1/2 levels were reported to peak at 10 min and start to decline at 20 min of baclofen stimulation in cultured cerebellar granule cells27, 15 min of baclofen stimulation was used in this study. In comparison to the tissues from the vehicle-bathed slices, the pERK1 level increased by 29.5 ± 8.2% in the LC tissues from the baclofen-bathed slices (Fig. 1B1) (p = 0.006; n = 9, Student’s paired t-test). There was no increase in the pERK2 level (p = 0.183; n = 9, Student’s paired t-test). We also compared pERK1/2 levels between LC tissues punched from slices bathed in baclofen and in baclofen plus 10 μM CGP54626, a GABABR antagonist. Compared to LC tissues from the baclofen-bathed slices, the inhibition of GABABRs with CGP54626 reduced the pERK1 level by 25.9 ± 4.1% (Fig. 1B2) (p = 0.003, n = 6, Student’s paired t-test), showing that the increase in the pERK1 level by baclofen stimulation was specific to GABABR activation. Interestingly, compared to LC tissues from the baclofen-bathed slices, the pERK2 level also significantly decreased by 31.3 ± 6.1% in CGP54626-bathed slices (p = 0.018, n = 6, Student’s paired t-test). As the ambient GABA in the pontine area can continuously activate GABABRs to exert tonic inhibition of LC neurons7,9, it could be that there was a basal pERK2 level produced by continuous GABABR activation. Accordingly, tonic GABABR activation left less room for a further increase in the pERK2 level by baclofen stimulation, and the inhibition of GABABRs could result in a significant reduction.
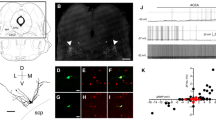
The activation of GABABRs increases pERK1 levels in LC tissue. (A) The images show two sagittal brainstem slices from an animal. The LC in the left slice was punched (A1) for western blot analysis, and the right slice was used for comparison (A2). IHC with anti-TH antibody was performed for the two slices, as shown in the insets showing merged fluorescence images of anti-TH (red) and DAPI (blue) staining of the dashed rectangular areas at high magnification. A comparison of the two slices shows that the punched area contained mostly TH-ir tissue. (B) Images show representative western blot analysis results for pERK1/2 in LC tissue punched from slices bathed in vehicle or baclofen (B1) and from slices bathed in baclofen or baclofen plus CGP54626 (B2). The plot in the right panels summarizes the results. Each paired circle and line indicates the result of a single experiment; bars and capped lines denote the mean and SEM, respectively. The asterisks denote significant differences compared to the control at p < 0.05 (*) and p < 0.01 (**); ns denotes no significance compared to the control.
Characterization of IGABABR in LC neurons
To confirm that the increase in pERK1 levels upon GABABR activation occurred in LC neurons and to explore the possible physiological role of the elevated ERK activity, we performed whole-cell patch recording from LC neurons and tested the effects of ERK blockers on the whole-cell current induced by GABABR activation. All recordings described hereafter were performed with the addition of synaptic blockers to the bath medium to avoid secondary effects via fast synaptic transmissions. We adapted previously described criteria for identifying NAergic neurons in the dorsal pontine area7,29,30 to validate that the recorded neurons were LC neurons. The criteria were as follows: (1) the recorded neuron should be immunoreactive to anti-tyrosine hydroxylase (TH) antibody (Fig. 2A); (2) the recorded neuron should be able to spontaneously fire APs followed by prominent afterhyperpolarization; and (3) the recorded neuron should display a delay in AP generation upon the injection of depolarizing current pulses, with Vm held at ~ −70 mV (Fig. 2B). A very interesting observation from the whole-cell recordings of LC neurons was the appearance of spontaneous oscillation at ~ 0.2 Hz of the membrane voltage in current-clamp recordings (Fig. 2C) or of the membrane current in voltage-clamp recordings (Fig. 2D). The oscillating events displayed some similar features to those of spontaneous APs, such as being biphasic and generated at a rate similar to spontaneous APs. Since LC neurons are electrically coupled to gap junctions31,32, these events could be due to flow through the gap junctions of currents underlying the APs generated from other LC neurons in the proximity and electrically coupled to the recorded neuron7. This argument is further supported by the results showing that these events were blocked by CBX, a gap junction blocker (Fig. 2D). We refer to the events recorded using the voltage clamp as IOsc.
Recordings from LC neurons (A) Images showing the identification of LC neurons with post hoc IHC using the anti-TH antibody. A1 and A3 show online phase contrast images of a sagittal brainstem slice at low (A1) and high magnification (A3). A2 and A4 show fluorescence images of anti-TH staining of the same field and magnification as shown in A1 and A3, respectively. A5 shows a fluorescence image of the same field and magnification as in A4 showing a recorded neuron filled with biocytin. This neuron also displayed TH-ir, as indicated by the asterisk. Abbreviations: Me5, mesencephalic trigeminal nucleus; scp, superior cerebellar peduncle. (B) Representative current-clamp recording from the TH-ir (LC) neuron shown in A, showing Vm responses (top traces) to current injection (bottom traces). Note the delay in the onset of AP (see arrow) elicited from Vm held at −70 mV. (C) A representative V-clamp (left bottom half) and I-clamp (right upper half) recording from an LC neuron. The arrow indicates switching of the recording from V-clamp to I-clamp mode. Note the biphasic IOsc in the V-clamp recording and the spontaneous APs and voltage oscillation in the I-clamp recording. The inserted green trace shows activity marked by the dashed rectangle on a faster and larger scale. Asterisks mark the voltage oscillations. The vertical bar to the left of the trace shows the amplitude scale for V-clamp (50 pA) or I-clamp (50 mV) recordings; the one to the right of the inserted trace shows the amplitude scale for the inserted trace; the bottom horizontal bar shows the time scale for the whole trace (120 s) and the inserted trace (20 s). (D) A representative experiment with V-clamp recordings from an LC neuron showing that IOsc are blocked by the application of 100 μM CBX, a gap junction blocker; top and bottom traces show recordings before and after CBX application, respectively.
Bath application of 100 μM baclofen induced an outward current that was blocked by subsequent application of 10 μM CGP54626 (Fig. 3A), showing that the current was mediated by GABABRs. This observation is consistent with our previous reports7,9, further demonstrating that the current was generated by the opening of GIRK channels downstream of the activation of GABABRs by baclofen. Hereafter, we refer to the current as the GABABR-mediated current (IGABABR). Interestingly, the induction of IGABABR was associated with the suppression of IOsc activity, and the activity reappeared upon subsequent application of CGP54626 to counteract the effect of baclofen (Fig. 3A–C).
The inhibition of ERK1/2 decreases the τ50 of IGABABR in LC neurons. (A-C) Representative recording of IGABABR from LC neurons in the control slice (A), U0126-treated slice (B), and FR180204-treated slice (C). The red double-headed line marks τ50, and the long and short green dashed lines mark the means of the membrane current recorded at baseline (before baclofen) and upon CGP54626 application. Note that the difference is measured as ITonic, as indicated by the asterisk. The activity marked with the green square bracket is enlarged and shown at the bottom (traces a-c). Note the increased frequency of IOsc with CGP54626 application compared with baseline. (D-G) Plots show summarized results of the amplitude (D) and τ50 (E) of IGABABR, ITonic (F) and the rate of IOsc (G). Each circle (D-E) or dashed line (F-G) shows the result of an individual experiment; bars (D-E) or circles (F-G) denote the mean, and capped lines denote the SEM. The asterisks indicate a significant difference in τ50 (E) or in the increment of IOsc frequency (G) compared to the control at p < 0.01 (**) or at p < 0.005 (***). p denotes a significant increase in IOsc frequency after CGP54626 application (G); ns denotes no significant difference compared to the control.
The IGABABR underwent partial decline upon prolonged (15 minutes) exposure to baclofen in the control condition. As seen in Fig. 3A, upon the application of baclofen, IGABABR was quickly induced and peaked with a mean amplitude of 98.1 ± 20.4 pA (n = 11; Fig. 3D), followed by a gradual decline to approximately half the peak amplitude. We quantified the decline in IGABABR by measuring τ50, defined as the time required for the IGABABR to decline from its peak amplitude to half that value, and it was 356 ± 49 s in the control condition (Fig. 3E). The subsequent coapplication of CGP54626 15 min after baclofen application suppressed IGABABR to a level below baseline (the membrane current before baclofen application; see green dotted lines and asterisk in Fig. 3A), showing a basal tone of GABABR activation in LC neurons. The membrane current underlying the basal tone of GABABR activation is referred to as the tonic current (ITonic), measured as the difference between the membrane currents at baseline and upon CGP54626 application (see asterisks in Fig. 3A). The ITonic in the control was −9.4 ± 2.4 pA (n = 11) (Fig. 3F). Consistent with the observation of the basal tone of GABABR activation, we also observed a significantly higher frequency of IOsc during CGP54626 application than at baseline.The frequency of IOsc at baseline and during the CGP54626 application was 0.15 ± 0.05 Hz and 0.38 ± 0.03 Hz, respectively (n = 11; p = 0.001, post hoc Bonferroni test after two-way repeated-measures ANOVA) (Fig. 3Aa, Fig. 3G).
Inhibition of ERK1/2 accelerates the decline of IGABABR LC neurons
We next tested the effects of two selective ERK blockers, U0126 and FR180204, on IGABABR in LC neurons. We first examined whether there were possible effects of the two ERK1/2 inhibitors on LC neurons by recording the basal spontaneous firing rate (SFR). The cell-attached configuration of the patch recording was used to avoid interference in the ion composition of the cytoplasm by the pipette solution. We found that the application of 20 μM U0126 for 30 min significantly decreased the SFR from 0.51 ± 0.13 to 0.33 ± 0.09 Hz (Fig. 4A,B) (n = 6 cells, p = 0.028, paired Wilcoxon-sign rank test). In contrast, the application of 20 μΜ FR180204 for 30 min significantly increased the SFR from 0.47 ± 0.09 to 0.73 ± 0.08 Hz (Fig. 4A,C) (n = 6 cells, p = 0.006, Student’s paired t-test), and the application of the vehicle (0.1% DMSO) did not have an effect on the SFR (Fig. 4A,D) (baseline: 0.54 ± 0.14 Hz, DMSO: 0.55 ± 0.14 Hz, n = 8 cells, p = 0.8, Student’s paired t-test). The differential effects of the two drugs on the SFR might be ascribed to the fact that FR180204 directly targets ERK1/2, while U0126 targets the mitogen-activated protein kinase that acts upstream of ERK1/2. The results also suggest that LC neurons might have basal ERK1/2 activity, which could regulate various types of ion channels involved in the regulation of the membrane potential of LC neurons. To minimize the nonspecific effects, we pretreated the slices for 2 hrs and continuously perfused them throughout the recording with U0126 or FR180204 so that a stable baseline could be obtained before the application of baclofen and CGP54626.In slices pretreated and perfused with U0126 or FR180204, the peak amplitude of IGABABR showed no difference in U0126- (n = 9 cells) and FR180204- (n = 10 cells) treated slices compared to the peak amplitude of IGABABR measured in control slices (Kruskal-Wallis test, p = 0.644 among the comparisons between control and ERK blocker groups) (Fig. 3A–D). In contrast, both drugs significantly accelerated the decline in IGABABR compared with the control condition. The τ50 measured from IGABABR recorded in the U0126-treated and FR180204-treated slices was 163 ± 21 s and 150 ± 21 s, respectively; both were significantly shorter than the measurements obtained using the control slice (Kruskal-Wallis test, p = 0.001 among the comparison between control and ERK blocker groups; p = 0.009 for control vs. U0126 and p = 0.002 for control vs. FR180204 using post hoc Dunn’s multiple comparisons test) (Fig. 3A–C,E). The ITonic revealed by the subsequent application of CGP54626 in slices pretreated with U0126 was −7.6 ± 4.0 pA; it was 0.9 ± 7.0 pA in slices pretreated with FR180204; no difference was observed in either case compared with the control (two-way repeated-measures ANOVA, sphericity assumed, F(1, 27) = 3.672, p = 0.066 for CGP effects; sphericity assumed, F (2, 27) = 1.337, p = 0.279 for the comparison among ERK blockers) (Fig. 3A−C,F).
Effects of ERK1/2 inhibitors on the firing rate of LC neurons. (A) Representative episodes of firing rate recording from LC neurons before (baseline) (left panel) and after 30 mins of drug applications (right panel). The top, middle and bottom traces show the application of U0126, FR180204 and the vehicle (DMSO), respectively. (B-D) Summarized results show the change in the SFR upon U0126 (B), FR180204 (C) and DMSO (D) application. The asterisks indicate a significant difference compared to the control at p < 0.05 (*) or at p < 0.01 (**).
Compared with baseline, the application of CGP54626 also increased the frequency of IOsc in U0126- and FR180204-treated slices, with the extent of the increase being significantly less in FR180204-treated slices than that in the control slices (two-way repeated-measures ANOVA, sphericity assumed, F(1, 26) = 55.106, p = 0.000 for CGP effects; sphericity assumed, F (2, 26) = 6.255, p = 0.006 for the comparison among ERK blockers). Upon CGP54626 application, the frequency of IOsc significantly increased from 0.24 ± 0.02 to 0.33 ± 0.3 Hz in U0126-treated slices (n = 9 cells, p = 0.025, post hoc Bonferroni test); however, the extent of the increase showed no difference compared to the control (p = 1, post hoc Bonferroni test). In FR180204-tretaed slices, the frequency of IOsc significantly increased from 0.48 ± 0.06 to 0.57 ± 0.08 Hz (n = 9 cells, p = 0.032, post hoc Bonferroni test)and the extent of the increase was significantly less than that in the control (p = 0.001, post hoc Bonferroni test) (Fig. 3G). Again, the significant reduction in the extent of the increase in SFR upon CGP54626 application observed in FR180204-tretaed slices but not in U0126-treatred slices might be ascribed to the fact that FR180204 specifically targets ERK1/2, while U0126 targets the mitogen-activated protein kinase that acts upstream of ERK1/2.
ERK1/2 activated by GABABRs is essential to sustain tonic inhibition of LC neurons
An interpretation of the above observations could be that the activation of GABABRs in LC neurons not only opened GIRK channels but also triggered ERK-dependent autoregulation of receptors to prevent the quick desensitization of GABABRs upon prolonged exposure to the agonist. To test this possibility, we examined the effects on tonic inhibition of LC neurons7 with prolonged exposure of GABABRs to baclofen at a saturating concentration with the inhibition of ERK activity. If the above interpretation is correct, the inhibition of ERK-dependent autoregulation would result in a reduced functionality of GABABRs, as indexed by a reduction in the tonic inhibition of LC neurons after prolonged agonist exposure. We found that a precise assessment of tonic inhibition by directly measuring GABABR-mediated ITonic was difficult due to the high noise level imposed by the high IOsc activity. This phenomenon might account for the lack of a significant difference in ITonic in the U0126- or FR180204-treated slices compared to the control (Fig. 3F).Accordingly, we examined the SFR of LC neurons to assess GABABR-mediated tonic inhibition.
To examine the effects on tonic inhibition of LC neurons with prolonged exposure of GABABRs to baclofen with the inhibition of ERK activity, we perfused the slices with 100 μM baclofen, the minimum dosage for producing the saturation of GABABR functionality in LC neurons7, for 15 min after obtaining a stable cell-attached recording from an LC neuron. The slices were then washed with 20 μM baclofen for an additional 15 min, followed by the co-administration of 20 μM baclofen and CGP54626 (Fig. 5A). Based on the dose-dependent relationship of IGABABR induced by baclofen7, we estimated that 20 μM baclofen would produce 70% of the maximum GABABR activation in LC neurons. Therefore, bathing the slices in 20 μM ambient baclofen could largely amplify tonic inhibition for easier observation. As seen (Fig. 5A,B), after a 15-minute period of pre-exposure to the agonist at a saturating concentration, a significant increase in the SFR upon CGP54626 application was observed in LC neurons bathed in 20 μM baclofen in the control slices (n = 10 cells, p = 0.000, Student’s paired t-test), the U0126-treated slices (n = 7 cells, p = 0.012, Student’s paired t-test) and in the FR180204-treated slices (n = 7 cells, p = 0.001, Student’s paired t-test). The GABABR-mediated tonic inhibition under the condition was defined as:
where SFRBac and SFRBac+CGP54626 are the SFRs recorded in 20 μM baclofen and 20 μM baclofen plus CGP54626, respectively. The calculated tonic inhibition was 147.3 ± 39.4% in the control (n = 10), which was significantly stronger than that in U0126-treated slices of 34.8 ± 8.9% (n = 7) and in FR180204-treated slices of 16.6 ± 3.2% (n = 7) (One-way ANOVA, p = 0.008 among the comparison between control and ERK blocker groups; p = 0.045 for control vs. U0126 and p = 0.013 for control vs. FR180204 using post hoc Bonferroni test) (Fig. 5C).
The inhibition of ERK1/2 attenuates GABABR-mediated tonic inhibition of LC neurons. (A) The top bar shows the experimental protocol, and the bottom traces show representative episodes of recordings with baclofen (left panel) and CGP54626 (right panel) from an LC neuron in control slices (upper raw), U0126-treated (middle), and FR180204-treated slices (bottom raw). The recordings were performed using the cell-attached configuration to record spontaneous APs. (B & C) Summarized results show the change in the spontaneous firing rate upon CGP54626 application (B) and GABABR-mediated tonic inhibition (C). Each dashed line (B) or cross (C) shows the results of an individual experiment; the circles (B) or bars (C) denote the mean, and capped lines denote the SEM. The asterisks indicate a significant difference compared to the control at p < 0.05 (*).
ERK1/2 activated by GABABR does not have an effect on pGABAB2R
Finally, we examined the possible regulatory site of ERK1/2 regulation of GABABR functionality upon receptor activation. To this end, we compared the level of phosphorylated GABAB2 receptor subunit at the serine 783 (S783) site between LC tissue treated with baclofen and that treated with baclofen plus FR180204 using western blot analysis, as this regulatory site is shown to enhance GABABR activation of GIRK33. If ERK1/2 regulated GABABR functionality by phosphorylating the S783 site of the GABAB2 receptor subunit, the pGABAB2 receptor level should be significantly higher in baclofen-treated LC tissue than in the tissue treated with baclofen plus FR180204. However, we did not find a difference between the two groups in the level of phosphorylation of the GABAB2 receptor at the S783 site. Compared to the control (LC tissues from the baclofen-bathed slices), the pGABAB2R level was 96.3 ± 6.9% of the control in LC tissues from the slice bathed in baclofen plus FR180204 (Fig. 6) (n = 6, p = 0.651, Student’s paired t-test).
ERK1/2 activated by GABABR does not have an effect on pGABAB2R. Left images show representative western blot analysis results for pGABAB2R in LC tissue punched from slices bathed in baclofen or in baclofen plus FR180204. The plot in the right panel summarizes the results. Each paired circle and line indicates the result of a single experiment; bars and vertical lines denote the mean and SEM, respectively. ns denotes no significance compared to the control.
Discussion
In this study, we provide biochemical and electrophysiological evidence showing that GABABRs can mediate the autoregulation of GABABR-activated whole-cell current through the activation of ERK1 in LC neurons. Since ambient GABA in the LC is significantly higher during rapid eyemovement (REM)/non-REM sleep than during wakefulness20,22, ERK1-dependent autoregulation of GABABR functionality could be a mechanism enabling the receptors to be continuously activated by the ambient GABA without undergoing severe desensitization, thereby providing a stable, tonic inhibition of LC neurons.
In many brain regions, including the LC, electron microscopic studies have shown that GABABRs are located predominantly close to neurotransmitter release sites at presynaptic terminals; in contrast, they are mainly located at peri-/extrasynaptic but not at synaptic active zones in postsynaptic neurons4,5,6,7. These locations of GABABRs imply that most of the receptors are not directly activated by synaptically released GABA in the synaptic cleft but by ambient GABA in peri-/extrasynaptic spaces. The concentration of ambient GABA critically depends on GABA spillover from the synaptic cleft, and the amount of GABA spillover is determined by the frequency and pattern of AP arrival at axonal terminals. It is also determined by the operation of GABA transporters located in the neuron and glia cell membrane34,35,36,37,38,39. GABA transporters not only serve in the reuptake of GABA in the synaptic cleft but also operate in a reverse mode that actually causes release but not reuptake of the neurotransmitter, a so-called nonvesicular release process, which would occur when high-frequency and repeated APs arrive at axonal terminals40,41,42. Accumulating reports have shown that ambient GABA generated by the abovementioned neuronal and glial activity could sufficiently and tonically activate peri-/extrasynaptic GABABRs at both presynaptic and postsynaptic sites, thereby exerting tonic inhibition for the control of transmitter release from presynaptic terminals and the spiking activity in postsynaptic neurons7,9,14,15,16,34,35,36,39. The regulation of ambient GABA by AP-dependent vesicular and nonvesicular release could modulate tonic inhibition of neurons that express peri-/extrasynaptic GABAARs and/or GABABRs, and the processes could be linked to the long-term regulation of brain function. LC neurons are well known to be involved in the regulation of the wakefulness–sleep cycle; they fire APs in a brain state-dependent manner, displaying active firing during wakefulness, a decreased discharge rate during non-REM sleep and silence during REM sleep18,43. Obviously, a mechanism that provides prolonged and stable inhibition of LC neurons could promote REM/non-REM sleep.
In addition to ambient GABA, the operation of GABABR-mediated tonic inhibition would require a substantial and steady amount of GABABRs on the membrane. Nevertheless, many GPCRs undergo desensitization, a process in which a receptor reduces its response after prolonged exposure to the agonist, which helps to attenuate or terminate signal transduction to protect the target cells from overstimulation25. It is now well known that desensitization involves the phosphorylation of GPCRs by G protein-coupled receptor kinases (GRKs), which lead to the uncoupling of the receptors from G proteins. This process is followed by the internalization of GPCRs through the recruitment of arrestins that trigger clathrin-mediated endocytosis of the phosphorylated receptor44. Interestingly, a growing amount of evidence suggests that GABABRs, unlike many other GPCRs, are not the substrate for GRKs other than GRK4/5 and do not conform to the above-described agonist-induced internalization45. Nevertheless, GABABRs exhibit significant rates of constitutive endocytosis via clathrin-dependent and dynamin-dependent mechanisms under basal conditions, followed by the recycling of large numbers of GABABRs back to the plasma membrane to maintain steady-state cell surface numbers45,46,47,48. These processes perhaps reflect the long cell surface half-lives of GABABRs and make them suitable for mediating a stable and persistent effect such as tonic inhibition. For example, unlike neurokinin receptors, which undergo almost complete desensitization after a few seconds of exposure to substance-P in NAergic A7 neurons29, GABABR desensitization progresses at a much slower rate in NAergic A7 neurons9 as well as in LC neurons. The regulation of the surface stability of the GABABR number usually involves the phosphorylation of the receptor molecule per se. It has been shown that the phosphorylation of S892 of the GABAB2 receptor by protein kinase-A promotes cell surface stability of the GABABR number46; in cultured hippocampal neurons, S867 in GABAB1 receptors is phosphorylated by Ca2+/calmodulin-dependent protein kinases downstream of NMDA receptor activation, which induces the internalization of GABABRs. The phosphorylation of the receptor molecule also regulates GABABR signaling by altering the efficacy of receptor–effector coupling. The phosphorylation of S783 of theGABAB2 receptor by 5′AMP-dependent protein kinase (AMPK) has been shown to enhance GABABR activation of GIRKs33. In addition to the promotion of cell surface stability of the receptor number, the phosphorylation of S892 of the GABAB2receptor by protein kinase-A facilitates receptor–effector coupling49 and slows KCTD12-induced desensitization of the GIRK current50. In addition to the receptor molecules, the phosphorylation of effectors, such as N-type Ca2+ channels, by tyrosine kinase can interact with regulators of G protein signaling (RGS) to form a regulatory complex that provides additional mechanism for regulation in the GABABR-mediated currents51. Another mechanism reported to be involved in the regulation of GABABR desensitization includesthe agonist-induced association of the receptors with GRK4/5, which results in GABABR desensitization in a phosphorylation-independent manner. The interaction of GABAB1 and GABAB2 receptor subunits with NEM-sensitive fusion protein (NSF) primes the receptors for phosphorylation by protein kinase C (PKC) to uncouple them from G proteins. The association of GABABRs with various types of auxiliary proteins, such as potassium channel tetramerization domain-containing proteins (KCTDs), via the C-terminus of GABAB2 receptors can render the receptor complex competent for desensitization.
Our western blot analysis showed that baclofen could increase pERK1 levels in LC tissue, and this effect was not observed in LC tissue treated with CGP54626 to block GABABRs. These observations show that the baclofen-induced increase in pERK1 levels in the LC was GABABR-dependent, consistent with the results of previous reports in the hippocampus and cerebellum26,27,28. In addition to the well-known effect on gating GIRKs, the results of these previous and present studies demonstrate an additional role of GABABR activation in activating ERK in hippocampal CA1 pyramidal neurons, cerebellar granule neurons and LC neurons. By performing whole-cell recordings directly from LC neurons, we further observed that the inhibition of ERK1/2 with two selective blockers produced consistent effects on the activity induced by baclofen application in LC neurons. These results confirmed that the elevation of pERK1 levels by GABABR activation occurred in LC neurons, although we could not completely rule out the possibility that the effects on LC neuron activity were secondary. We did not validate the link between GABABR activation and the increased pERK1 level. However, it has been reported that the increase in pERK1/2 levels by GABABR activation relies on a pertussis-toxin-sensitive Gi/o heterotrimeric protein-dependent pathway by releasing Gβγ in cultured cerebellar granule neurons27. Interestingly, this signaling pathway for ERK1/2 phosphorylation seems to be unique to GABABRs, as the activation of Gi/o-coupled α2-adrenoceptors or Gi/o-coupled 5HT1A receptors in the adult mouse CA1 region did not result in ERK1/2 phosphorylation26. As shown in our electrophysiological experiments, the inhibition of ERK1/2 activity with two selective blockers accelerated the decay of IGABABR, and it is likely that the signaling mechanisms of GABABR trafficking and/or desensitization may involve an ERK-dependent intermediate step that has not yet been identified. Therefore, the inhibition of the ERK-dependent intermediate step accelerated the decline of IGABABR. Our western blot analysis showed that the activation of ERK1 downstream of GABABR activation did not have an effect on the level of phosphorylation of the GABAB2receptor at the serine 783 site. Activated ERK1 could regulate GABABR functionality at other serine sites in GABAB1 and/orGABAB2 receptors, as previously described. Furthermore, it could be involved in the interaction between RGS and phosphorylated GIRKs by tyrosine kinase51,52, as it has been recently suggested that the GABABR activates tyrosine kinase and downstream ERK1/253. Apparently, the mechanisms underlying the regulation of GABABR desensitization by ERK1 remain to be clarified.
In conclusion, here, we report that the activation of GABABRs with baclofen increases the phosphorylation of ERK1 in LC tissue and that the blockade of ERK1/2 activity with two selective blockers, U0126 and FR180204, accelerates the decay of IGABABR induced by prolonged baclofen application in LC neurons. Furthermore, an increase in the spontaneous firing rate and a decrease in the tonic inhibition of LC neurons occurs after prolonged GABABR activation by baclofen at a saturating concentration. These results suggest a new functional role of Gαi/ο upon GABABR activation in mediating an ERK1-dependent autoregulation of the stability of GABABR-activated whole-cell current in LC neurons, in addition to the well-known action of gating GIRKs with Gβ,γ subunits. We argue that activated ERK1 signaling might help maintain a dynamic balance between the desensitization and recycling of receptors back to the membrane to sustain GABABR-mediated tonic inhibition of LC neurons. However, it should be noted that postnatal day 8–10 rats were used in this study, and there is a possible weakness that the same phenomena may not be fully present in adulthood.
Materials and Methods
Animals
The use of animals in this study was approved by the Institutional Animal Care and Use Committee for National Taiwan University (permission #NTU103-EL-00076) and the Institutional Animal Care and Use Committee for Chung Shan Medical University(permission #1423), the guidelines of which comply with the “Codes for Experimental Use of Animals” of the Council of Agriculture of Taiwan based on the Animal Protection Law of Taiwan. Every effort was made to minimize the number of animals used and their suffering. Sprague-Dawley rat pups of both sexes were used and sacrificed for slice preparation on postnatal days 8–10 for the electrophysiological experiments and postnatal days 14–16 for the western blot analysis.
Preparation of brainstem slices and electrophysiology
The animals were anesthetized with 5% isoflurane in O2 and decapitated, followed by rapid exposure of the brain and chilling with ice-cold aCSF consisting of the following (in mM): 119 NaCl, 2.5 KCl, 1.3 MgSO4, 26.2 NaHCO3, 1 NaH2PO4, 2.5 CaCl2, and 11 glucose, oxygenated with 95% O2 and 5% CO2, pH 7.4. Sagittal brainstem slices (300 μm) containing the LC were prepared using a vibroslicer (D.S.K. Super Microslicer Zero 1, Dosaka EM, Kyoto, Japan), maintained in a moist air-liquid (aCSF) interface chamber and allowed to recover for at least 90 minutes before being transferred to an immersion-type chamber mounted on an upright microscope (BX51WI, Olympus Optical Co., Ltd., Tokyo, Japan) for recording. Throughout the recording period, they were perfused at 2–3 ml/min with oxygenated aCSF containing synaptic blockers. The synaptic blockers contained 5 mM kynurenic acid, 100 μM picrotoxin and 1 μM strychnine to block glutamatergic, GABAergic and glycinergic synaptic transmission, respectively. The baseline described in the control slices refers to the recordings made under these conditions before administering baclofen and CGP54626. For recordings made in the U0126-treated slices and FR180204-treated slices, baseline refers to recording in the aCSF containing synaptic blockers, 0.1% DMSO and U0126 or FR180204.
Neurons were viewed using Nomarski optics. Patch pipettes were pulled from borosilicate glass tubing (1.5 mm outer diameter, 0.32 mm wall thickness; Warner Instruments Corp., Hamden, CT, USA) and had a resistance of approximately 3–5 MΩ when filled with the pipette solutions. To record the GABABR-mediated current, the pipette solution consisted of the following (in mM): 131 K-gluconate, 20 KCl, 10 HEPES, 2 EGTA, 8 NaCl, 2 ATP, and 0.3 GTP; pH adjusted to 7.2 with KOH. Recordings were performed at 29–31 °C in the whole-cell or cell-attached configuration with a patch amplifier (Multiclamp 700 B; Axon Instruments Inc., Union City, CA, USA). For current-clamp recordings of whole-cell configuration, the bridge was balanced, and the recordings were accepted only if the recorded neuron had a membrane potential (Vm) of at least −45 mV without applying a holding current and if the APs were able to overshoot 0 mV. For voltage-clamp recordings, the Vm was clamped to −70 mV unless specified. The serial resistance was monitored throughout the recording, and the data were discarded if the values varied by more than 20% of the original value, which was usually less than 20 MΩ. In a series of experiments, the cell-attached configuration was used. In the recording, the patch amplifier was set in voltage-clamp mode with the pipette voltage set to 0 mV (holding current = 0 pA) so that the recorded neurons were at their resting membrane potential. All signals were low-pass filtered at a corner frequency of 2 kHz and digitized at 10 kHz using the Micro 1401 interface running Signal software for episode-based capture and Spike2 software for continuous recording (Cambridge Electronic Design, Cambridge, UK). All data are presented as the mean±the standard error of the mean (SEM). For statistical comparisons, the normality of the data was first tested using the Shapiro-Wilk test. Student’s paired t-test and the nonparametric paired Wilcoxon-sign rank test were used for the comparison of data collected before and after drug application (Fig. 4, Fig.5B). One-way ANOVA was used for the comparison of IGABABR parameters (Fig. 3D,E) and tonic inhibition (Fig. 5C) among the control, U0126 and FR180204 groups. Two-way repeated-measures ANOVA was used for the comparison of ITonic and oscillation rates (Fig. 3F,G) between baseline and CGP54626 application among the control, U0126 and FR180204 groups. The criterion for significance was p < 0.05.
All chemicals used to prepare the aCSF and pipette solution were from Merck (Frankfurt, Germany); baclofen, biocytin, carbenoxolone (CBX), kynurenic acid, picrotoxin, and strychnine were from Sigma (St. Louis, USA); and CGP54626 hydrochloride (CGP), U0126, and FR180204 were from Tocris-Cookson (Bristol, UK).
Biocytin histochemistry and immunohistochemistry
In some experiments, 6.7 mM biocytin was included in the internal solution to fill the recorded neurons. The detailed procedures for viewing the biocytin-filled neurons and post hoc immunohistochemistry (IHC) for cell type identification of the recorded neurons have been described previously29. Briefly, after recording, the slices were fixed overnight at 4 °C in 4% paraformaldehyde (Merck) in 0.1 M phosphate buffer (PB), pH 7.4, and then subjected to biocytin histochemistry and IHC procedures without further sectioning. The slices were incubated for 1 hr at room temperature in phosphate-buffered saline (PBS) containing 0.03% Triton X-100 (PBST), 2% bovine serum albumin (BSA), and 10% normal goat serum (NGS), followed by incubation overnight at 4 °C in PBST containing a 1/2000 dilution of rabbit antibodies against TH (Merck Millipore, Darmstadt, Germany) and a 1/200 dilution of streptavidin Alexa Fluor 488 (Jackson ImmunoResearch, West Grove, PA, USA). After rinsing with PBST, they were incubated for 2 hrs with a 1/200 dilution goat anti-rabbit IgG antibodies conjugated to Alexa Fluor 594 (Jackson ImmunoResearch, West Grove, PA, USA) in PBST and then observed under a fluorescence microscope (Axioplan 2, Zeiss, Oberkochen, Germany) or a confocal microscope (LSM780, Zeiss Microsystems, Jena, Germany).
Protein purification and western blot analysis
Brain slices were prepared using procedures described above and were maintained in the interface chamber and allowed to recover for at least 90 minutes. In each experiment, 5 or 6 rats were used for brain slice preparation. The slices from the left hemispheres were used as controls, and those from the right hemispheres were treated with a tested drug for comparison. After 15 min of drug or vehicle incubation at 30–32 °C, the LC was punched out using an 18 G needle with the tip blunted (Fig. 1A). The LC from the left hemispheres was pooled together in a test tube, as well as those from the right hemispheres in another tube. Then, the two tubes of LC tissue were simultaneously subjected to protein purification and western blotting under the same conditions. The above-described experiment was repeated 9 times in Fig. 1B1 (n = 9), 6 times in Fig. 1B2 (n = 6), and 6 times in Fig. 5 (n = 6). For protein purification, the samples were lysed in extraction buffer containing cell lysis buffer (Cell Signaling Technology), 1 μg/ml leupeptin, 1 μg/ml aprotinin, 2 mM PMSF, 0.5 mM Na3VO4, and 5 mM NaF and centrifuged at 16,100 × g for 30 min at 4 °C. The supernatant was then collected, and the protein concentration was determined using a BCA Protein Assay Kit (Pierce) with bovine serum albumin (BSA) as the standard. The samples (30 μg of protein) were separated by 8~10% SDS-PAGE and transferred to a nitrocellulose membrane (Bio-Rad Laboratories). The membrane was incubated with 5% BSA and 5% nonfat milk in Tris-Tween-buffered saline (TTBS) buffer containing 50 mM Tris-HCl (pH 7.5), 0.15 M NaCl, and 0.1% Tween 20 for 1 hr at room temperature, followed by an overnight incubation at 4 °C with primary antibodies in TTBS buffer. In Fig. 1B, the primary antibodies were rabbit polyclonal antibodies against p44/42 MAPK (ERK1/2) (1/1000, Cell Signaling Technology) or rabbit monoclonal antibodies against phospho-p44/42 MAPK (ERK1/2) (1/750, Cell Signaling Technology); in Fig. 5, the primary antibodies were rabbit monoclonal antibodies against the GABAB2 receptor (1/1000, Cell Signaling Technology) and rabbit polyclonal antibodies against phospho-S783 of the GABAB2 receptor (1/1000, Rockland Immunochemicals). The membrane was sequentially incubated for 1 hr at room temperature with biotinylated goat anti-rabbit IgG antibodies (Vector Laboratories), followed by avidin-biotinylated horseradish peroxidase (HRP) complex (Vector Laboratories) in TTBS. The bound antibodies were detected using an enhanced chemiluminescence (ECL) detection system (Fujifilm), and the intensities of the bands were quantified using the ImageGauge program (Fujifilm Software). For statistical comparisons, Student’s paired t-test was used, as the data all passed the normality test using the Shapiro-Wilk test (Fig. 1 and 6).
References
Belelli, D. et al. Extrasynaptic GABAA receptors: form, pharmacology, and function. J. Neurosci. 29, 12757–12763 (2009).
Walker, M.C.&Kullmann, D.M. Tonic GABAA receptor-mediated signaling in epilepsy. In:Jasper’s Basic Mechanisms of the Epilepsies [Internet]. (eds Noebels, J. L., Avoli, M., Rogawski, M. A., Olsen, R. W. & Delgado-Escueta, A. V.) 4th edition. (Bethesda (MD): National Center for Biotechnology Information(US) (2012).
Ulrich, D. & Bettler, B. GABAB receptors: synaptic functions and mechanisms of diversity. Curr. Opin. Neurobiol. 17, 298–303 (2007).
Kulik, A. et al. Subcellular localization of metabotropic GABAB receptor subunits GABAB1a/b and GABAB2 in the rat hippocampus. J. Neurosci. 23, 11026–11035 (2003).
Chen, L., Boyes, J., Yung, W. H. & Bolam, J. P. Subcellular localization of GABAB receptor subunits in rat globus pallidus. J. Comp. Neurol. 474, 340–352 (2004).
Luján, R., Shigemoto, R., Kulik, A. & Juiz, J. M. Localization of the GABAB receptor 1a/b subunit relative to glutamatergic synapses in the dorsal cochlear nucleus of the rat. J. Comp. Neurol. 475, 36–46 (2004).
Wang, H.-Y. et al. GABAB receptor-mediated tonic Inhibition regulates the spontaneous firing of locus coeruleus neurons in developing rats and citalopram-treated rats. J. Physiol. (London). 593, 161–180 (2015).
Bettler, B., Kaupmann, K., Mosbacher, J. & Gassmann, M. Molecular structure and physiological functions of GABAB receptor. Physiol. Rev. 84, 835–867 (2004).
Wu, Y. et al. GABAB receptor-mediated tonic inhibition of noradrenergic A7 neurons in the rat. J. Neurophysiol. 105, 2715–2728 (2011).
Gassmann, M. & Bettler, B. Regulation of neuronal GABAB receptor functions by subunit composition. Nat. Rev. Neurosci. 13, 380–394 (2012).
Jones, K. A. et al. GABAB receptors function as a heteromeric assembly of the subunits GABABR1 and GABABR2. Nature 396, 674–679 (1998).
Kaupmann, K. et al. GABAB-receptor subtypes assemble into functional heteromeric complexes. Nature 396, 683–687 (1998).
White, J. H. et al. Heterodimerization is required for the formation of a functional GABAB receptor. Nature 396, 679–682 (1998).
Laviv, T. et al. Basal GABA regulates GABABR conformation and release probability at single hippocampal synapses. Neuron 67(2), 253–267 (2010).
Wang, Y., Neubauer, F. B., Luscher, H. R. & Thurley, K. GABAB receptor-dependent modulation of network activity in the rat prefrontal cortex in vitro. Eur.J.Neurosci. 31, 1582–1594 (2010).
Wang, T. et al. Modulation of synaptic depression of the calyx of Held synapse by GABAB receptors and spontaneous activity. J. Physiol. 591, 4877–4894 (2013).
Hung, W.-C. et al. GABAB receptor-mediated tonic inhibition of locus coeruleus neurons plays a role in deep anesthesia induced by isoflurane. Neuroreport 31 (7), 557–564 (2020).
Aston-Jones, G. & Cohen, J. D. An Integrative Theory of Locus Coeruleus- Norepinephrine Function: Adaptive Gain and Optimal Performance. Annu. Rev. Neurosci. 28, 403–450 (2005).
Aston-Jones, G. & Waterhouse, B. Locus coeruleus: From global projection system to adaptive regulation of behavior. Brain Res. 1645, 75–78 (2016).
Nitz, D. & Siegel, J. M. GABA release in the locus coeruleus as a function of sleep/wake state. Neuroscience 78, 795–801 (1997).
Moore, J. T. et al. Direct Activation of Sleep-Promoting VLPO Neurons by Volatile Anesthetics Contributes to Anesthetic Hypnosis. Curr. Biol. 22, 2008–2016 (2012).
Nelson, L. E. et al. The sedative component of anesthesia is mediated by GABAA receptors in an endogenous sleep pathway. Nat. Neurosci. 5, 979–984 (2002).
Nelson, L. E. et al. The alpha2- adrenoceptor agonist dexmedetomidine converges on an endogenous sleep-promoting pathway to exert its sedative effects. Anesthesiology 98, 428–436 (2003).
Vazey, E. M. & Aston-Jones, G. Designer receptors manipulations reveal a role of the locus coeruleus noradrenergic system in isoflurane general anesthesia. Proc. Natl. Acad. Sci. USA 111, 3859–3864 (2014).
Rajagopal, S. & Shenoy, S. K. GPCR desensitization: Acute and prolonged phases. Cell Signal. 41, 9–16 (2018).
Vanhoose, A. M., Emery, M., Jimenez, L. & Winder, D. G. ERK activation by G-protein-coupled receptors in mouse brain is receptor identity-specific. J. Biol. Chem. 277, 9049–9053 (2002).
Tu, H. et al. Dominant role of GABAB2 and Gβγ for GABAB receptor-mediated-ERK1/2/CREB pathway in cerebellar neurons. Cell Signal. 19, 1996–2002 (2007).
Liu, L. et al. Baclofen mediates neuroprotection on hippocampal CA1 pyramidal cells through the regulation of autophagy under chronic cerebral hypoperfusion. Sci Rep. 5, 14474 (2015).
Min, M.-Y. et al. Physiological and morphological properties of, and effect of substance P on, neurons in the A7 catecholamine cell group in rats. Neuroscience 153, 1020–1033 (2008).
Min, M.-Y. et al. Roles of A-type potassium currents in tuning spike frequency and integrating synaptic transmission in noradrenergic neurons of the A7 catecholamine cell group in rats. Neuroscience 168, 633–645 (2010).
Ishimatsu, M. & Williams, J. T. Synchronous activity in locus coeruleus results from dendritic interactions in pericoerulear regions. J. Neurosci. 16, 5196–5204 (1996).
Ballantyne., D., Andrzejewski, M., Muckenhoff, K. & Scheid, P. Rhythms, synchrony and electrical coupling in the Locus coeruleus. Respir. Physiol. Neurobiol. 143, 199–214 (2004).
Kuramoto, N. et al. Phospho-Dependent Functional Modulation of GABAB Receptors by the Metabolic Sensor AMP-Dependent Protein Kinase. Neuron 53, 233–247 (2007).
Isaacson, J. S., Solís, J. M. & Nicoll, R. A. Local and diffuse synaptic actions of GABA in the hippocampus. Neuron 10, 165–175 (1993).
Zilberter, Y., Kaiser, K. M. & Sakemann, B. Dendritic GABA release depresses excitatory transmission between layer 2/3 pyramidal and bitufted neurons in rat neocortex. Neuron 24, 979–988 (1999).
Scanziani, M. GABA spillover activates postsynaptic GABA(B) receptors to control rhythmic hippocampal activity. Neuron 25, 673–681 (2000).
Smith, T. C. & Jahr, C. E. Self-inhibition of olfactory bulb neurons. Nat. Neurosci. 5, 760–766 (2002).
Alle, H. & Geiger, J. R. GABAergic spill-over transmission onto hippocampal mossy fiber boutons. J. Neurosci. 27, 942–960 (2007).
Beenhakker, M. P. & Huguenard, J. R. Astrocytes as gatekeepers of GABAB receptor function. J. Neurosci. 30, 15262–15276 (2010).
Rossi., D. J., Hamann, M. & Attwell, D. Multiple modes of GABAergic inhibition of rat cerebellar granule cells. J. Physiol. 548, 97–110 (2003).
Wu, Y., Yang, W., Díez-Sampedro, A. & Richerson, G. B. Nonvesicular inhibitory transmission via reversal of the GABA transporter GAT-1. Neuron 56, 851–865 (2007).
Koch, U. & Magnusson, A. K. Unconventional GABA release: mechanisms and function. Currr. Opin. Neurobiol. 19, 305–310 (2009).
Berridge, C. W. & Foote, S. L. Effects of locus coeruleus activation on electroencephalographic activity in neocortex and hippocampus. J Neurosci. 11, 3135–3145 (1991).
Krupnick, J. G. & Benovic, J. L. The role of receptor kinases and arrestins in G protein-coupled receptor regulation. Annu. Rev. Pharmaco. Toxicol. 38, 289–319 (1998).
Terunuma, M., Pangalos, M. N. & Moss, S. J. Functional Modulation of GABAB receptors by Protein Kinases and Receptor Trafficking. Adv. Pharmacol. 58, 113–122 (2010).
Fairfax, B. P. et al. Phosphorylation and chronic agonist treatment atypically modulate GABAB receptor cell surface stability. J. Biol. Chem. 279, 12565–12573 (2004).
Vagas, K. J. et al. The availability of surface GABAB receptors is independent of gamma-aminobutyric acid but controlled by glutamate in central neurons. J. Biol. Chem. 283, 24641–24648 (2008).
Benke, D., Zemoura, K. & Maier, P. J. Modulation of cell surface GABAB receptors by desensitization, trafficking and regulated degradation. World. J. Biol. Chem. 3, 61–72 (2012).
Gouve, A. et al. Cyclic AMP-dependent protein kinase phosphorylation facilitates GABAB receptor-effector coupling. Nat. Neurosci. 5, 415–424 (2002).
Adelfinger, L. et al. GABAB receptor phosphorylation regulates KDTC12-induced K+ current desensitization. Biochem. Pharmacol. 91, 369–379 (2014).
Schiff, M. L. et al. Tyrosine-kinase-dependent recruitment of RGS12 to the N-type calcium channel. Nature 408, 723–727 (2000).
Padgett, C. L. & Slesinger, P. A. GABAB receptor coupling to G-proteins and ion channels. Adv. Pharmacol. 58, 123–147 (2010).
Jiang, X. et al. GABAB receptor complex as a potential target for tumor therapy. J. Histochem. Cytochem. 60(4), 269–279 (2012).
Acknowledgements
This work was supported by grants MOST-104-2321-B-002-068 (M.-Y. Min) and MOST-103-2320-B-040-010-MY3 (H.-W. Yang) from the Ministry of Science and Technology, Taiwan.
Author information
Authors and Affiliations
Contributions
R.-N. Wu and C.-C. Kuo collected and analyzed the data. M.-Y. Min, R.-F. Chen and H.-W. Yang conceived and designed the experiments and wrote the first draft of the paper. All authors have contributed to the writing of this paper and have approved the final version.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wu, RN., Kuo, CC., Min, MY. et al. Extracellular Signal-Regulated Kinases Mediate an Autoregulation of GABAB-Receptor-Activated Whole-Cell Current in Locus Coeruleus Neurons. Sci Rep 10, 7869 (2020). https://doi.org/10.1038/s41598-020-64292-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-64292-x
- Springer Nature Limited