Abstract
Neonicotinoids selectively modulate insect nicotinic acetylcholine receptors (insect nAChRs). Studies have shown that serine with ability to form a hydrogen bond in loop C of some insect nAChR α subunits and glutamate with a negative charge at the corresponding position in vertebrate nAChRs may contribute to enhancing and reducing the neonicotinoid actions, respectively. However, there is no clear evidence what loop C properties underpin the target site actions of neonicotinoids. Thus, we have investigated the effects of S221A and S221Q mutations in loop C of the Drosophila melanogaster Dα1 subunit on the agonist activity of imidacloprid and thiacloprid for Dα1/chicken β2 nAChRs expressed in Xenopus laevis oocytes. The S221A mutation hardly affected either the affinity or efficacy for ACh and imidacloprid, whereas it only slightly reduced the efficacy for thiacloprid on the nAChRs with a higher composition ratio of β2 to Dα1 subunits. The S221Q mutation markedly reduced the efficacy of the neonicotinoids for the nAChRs with a higher composition of the β2 subunit lacking basic residues critical for binding neonicotinoids. Hence, we predict the possibility of enhanced neonicotinoid resistance in pest insect species by a mutation of the serine when it occurs in the R81T resistant populations lacking the basic residue in loop D of the β1 subunit.
Similar content being viewed by others
Introduction
Neonicotinoids are insecticides that modulate competitively insect nAChRs1,2,3. They represent high selective toxicity to insect over vertebrate nAChRs and show high selective toxicity to insects with diverse actions1,2,3,4,5,6. They show high systemicity in crop plants, enabling seed treatments and now make up >25% of global pesticide sales7. Their potential risks to non-target pests, including pollinators, has been demonstrated8. The use of some neonicotinoids in the field is now restricted in the EU9,10. It remains of interest to understand the mechanism of target-site actions of neonicotinoids in order to assist in the design of new, more eco-friendly pesticides.
Studies of target site actions show that basic residues in loop D of the nAChRs binding site play a key role in electronic interactions with the nitro or cyano group of neonicotinoids1,2,5,11,12,13. Indeed, the R81T mutation in loop D of aphids was first predicted13 then shown14 to reduce the affinity of neonicotinoids, thus resulting in resistance. We found earlier that a mutation of serine at position 221 to glutamate in the YXCC motif in loop C of the fruitfly (D. melanogaster) Dα1 subunit also markedly reduced the agonist action of imidacloprid and thiacloprid on Dα1/chicken β2 hybrid nAChR expressed in Xenopus laevis oocytes, pointing to a contribution of the serine221 to the selective action of the neonicotinoids tested15. A plausible explanation of this result is that repulsion of the electrostatically negative nitro group of imidacloprid and cyano group of thiacloprid by electronegative glutamate residue led to the reduced agonist actions. However, this mutation also changes the size of the residue.
In this study, the Ser221 of the Dα1 subunit was mutated to alanine or glutamine and then imidacloprid and thiacloprid as representatives of neonicotinoids carrying a nitro and cyano group, respectively (Fig. 1), were tested on the wild type and mutant Dα1β2 AChRs with two different subunit composition ratios to clarify the role for Ser221 in the selective interactions with neonicotinoids.
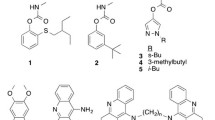
Imidacloprid and thiacloprid tested in this study, Figures are illustrated as ball and sticks where carbons, hydrogens, nitrogens, chlorine and sulfur are colored white, sky blue, blue, light green and yellow, respectively. Delocalized double bonds are shown as broken lines. Each chemical structure was drawn using Chem 3D combined with Chem Draw (PerkinElmer, Waltham, MA, USA).
Methods
Mutations of cDNA and preparation of cRNAs
All experimental protocols for preparing recombinant DNAs were approved by Kindai University. cDNAs of the fruitfly Dα1 (Accession number NM_079757) and chicken β2 subunit (Accession number NM_204813) were cloned into the pcDNA3.1 (+) vector (Thermo Fisher Scientific, Waltham, MA, USA). The S221A mutation was introduced with forward primer 5′- TTCTACGCATGCTGCGAGGAGCCG-3′, reverse primer 5′- GCAGCATGCGTAGAACTTCTCGTT-3′, whereas the S221Q mutations was introduced with forward primer 5′- TGCTGCGAGGAGCCGTATCTGGACA-3′, reverse primer 5′- CTGGTAGAACTTCTCGTTCCGCACC-3′. The entire nucleotide sequence of the S221A mutant cDNA was confirmed by automated sequencing using a 3130xl genetic analyzer (Thermo Fisher Scientific).
Each cDNA construct was linearized by digesting with XhoI. cRNAs encoding wild type and mutant Dα1 subunits as well as chicken β2 subunit was prepared from the cDNA construct using a mMESSAGE mMACHINE T7 ULTRA Kit (Thermo Fischer Scientific). cRNA was dissolved in RNase free water at a concentration of 1 μg/μL. To express (Dα1)3(β2)2 and (Dα1)2(β2)3 nAChRs in Xenopus oocytes, cRNAs of Dα1 and β2 subunits were mixed at ratios of 5:1 and 1:5, respectively15,16.
Female frogs (X. laevis) were anesthetised and oocytes were obtained according to the U.K. Animals (Scientific Procedures) Act, 1986. After treatment with collagenase and the follicle cell layers were removed from oocytes6,13,17,18. Each oocyte was injected with 50 nL of the cRNA solution and incubated for 4 or 5 days at 16 °C in the standard oocyte saline (SOS, pH 7.6) supplemented with penicillin (100 units/mL), streptomycin (100 μg/mL), gentamycin (20 μg/mL) and sodium pyruvate (2.5 mM) at 16 °C15,16. Each data was obtained using oocytes from at least two frogs.
Voltage-clamp electrophysiology
Agonist activity of ACh and neonicotinoids were evaluated by voltage-clamp electrophysiology as previously described. Oocytes were secured in a chamber and perfused with a SOS containing 0.5 μM atropine at a flow rate of 5–10 mL/min6,13,17. Two glass electrodes containing 2 M KCl were impaled into oocytes and oocyte membrane currents in response to bath applied agonists were recorded using an Axoclamp 900 A amplifier with Clampex 10 (Molecular Devices, San Jose, CA, USA) at a membrane potential of −100 mV. The membrane current data were digitized by a Digidata 1550B A/D converter (Molecular Devices) and analyzed by Clampfit 10 (Molecular Devices).
The compound was bath-applied in SOS for 3–5 s at an interval of 3–5 min with increasing agonist concentrations15,16. When recording the peak amplitude of the responses to imidacloprid and thiacloprid at concentrations higher than 1 μM, one oocyte was used for recording one response and abandoned to prevent underestimation of agonist responses resulting from irreversible nAChR desensitization.
Data fitting
Peak current amplitude of each response to all the agonists were normalized to that of the response to 100 μM ACh and fitted by non-linear regression using a Prism 6 (GraphPad software, San Diego, CA, USA) according to the following equation:
where Imax is the maximum normalized response, EC50 is the half maximal response, X is the log [agonist concentration (M)] and nH is the Hill coefficient. Experiments were repeated (n = 5). Imax and pEC50 (= −logEC50) values were obtained as mean ± standard error of the mean. Concentration-response curve illustration and statistical analyses were performed using Prism 6.
Homology modeling
The homology models of wild type and S221A mutant of the orthosteric agonist binding domain of (Dα1)3(β2)2 nAChR complexed with thiacloprid were built using Modeller19 with the structure coordinate of acetylcholine binding protein from Lymnaea stagnalis complexed with thiacloprid (PDB id 3WTK)20. Amino acid sequences of Dα1 and β2 subunits were aligned with the sequence of AChBP, and the models in complex with thiacloprid were built using automodel algorism of Modeller by taking into considerations water molecules observed frequently in the AChBP-neonicotinoid complexes. Models are illustrated using PyMOL 2.3 (Schrödinger, New York, NY, USA).
Results and Discussion
Effects of S221A and S221Q mutations on the agonist activity of ACh
First, we investigated the agonist action of ACh on the wild type, S221A mutant and S221Q mutant Dα1β2 nAChRs. ACh activated wild type (Dα1)3(β2)2 and (Dα1)2(β2)3 nAChRs concentration-dependently with pEC50 values of 6.81 ± 0.05 and 6.79 ± 0.04 (Fig. 2, Table 1). The S221A and S221Q had a minimal effect on the pEC50 value for the (Dα1)3(β2)2 and (Dα1)2(β2)3 nAChRs (Fig. 2, Table 1). Hence, the effects of these mutations on the neonicotinoid actions, if any, can be interpreted as the result of changes in the selective interactions with neonicotinoids.
Agonist actions of ACh on wild type, S221A mutant and S221Q mutant of (Dα1)3(β2)2 and (Dα1)2(β2)3 nAChRs expressed in X. laevis oocytes. Current responses to ACh for the nAChRs tested are shown above concentration-response data. Horizontal lines indicate bath applications of ACh. Each data plotted represents mean ± standard error of the mean (n = 5).
Effects of S221A and S221Q mutations on the agonist activity of neonicotinoids
Given the evidence that the S221A mutation in the Dα1 subunit had only a minor impact on the agonist action of ACh on the Dα1β2 nAChRs, we tested imidacloprid and thiacloprid on the wild type and S221A mutant nAChRs. Imidacloprid increased inward currents concentration-dependently in oocytes expressing the (Dα1)3(β2)2 and (Dα1)2(β2)3 nAChRs (Fig. 3a). Thus, the peak amplitude was normalized by the amplitude of the 10 μM ACh response and the concentration-response data were fitted to obtain the pEC50 and Imax values. In contrast with the effects of the S221E mutation that markedly reduced the affinity in terms of the pEC50 value of imidacloprid15, the S221A mutation hardly affected the agonist activity of the compound, irrespective of the subunit composition ratio (Fig. 3a, Table 2).
Agonist actions of imidacloprid and thiacloprid on wild type and S221A mutant Dα1β2 nAChRs expressed in X. laevis oocytes. In (A) and (B), inward current oocytes expressing the nAChRs in response to imidacloprid are shown above the concentration-response curves. Horizontal lines indicate bath applications of imidacloprid and thiacloprid. (a) Concentration-normalized response relationships of imidacloprid for the wild type and mutant (Dα1)3(β2)2 and (Dα1)2(β2)3 nAChRs. (b) Concentration-normalized response relationships of thiacloprid for the wild type and mutant (Dα1)3(β2)2 and (Dα1)2(β2)3 nAChRs. Each data plotted represents mean ± standard error of the mean (n = 5).
Thiacloprid evoked lower amplitude response than imidacloprid in oocytes expressing the nAChRs tested. The S221A mutation only slightly reduced the Imax value of thiacloprid for the nAChRs with increased β2 subunit composition ratio (Fig. 3b, Table 2, P > 0.05 by one-way ANOVA (Bonferroni-test), but P < 0.05 by one-tailed t-test). The result suggests that thiacloprid does not rely mainly on the hydrogen bond with Ser221 when it binds to the Dα1/Dα1 subunit interface containing basic residues in loop E and loop G1,15,16, but does when it binds to the Dα1/β2 subunit interface lacking basic residues necessary for the interactions with neonicotinoids1,2,5,11,13,20.
We previously hypothesized that the reduced affinity of the neonicotinoids by the S221E mutation in the Dα1/β2 nAChRs stems from electrostatic repulsion of the neonicotinoids containing a negative charge by the glutamate residue containing a negative charge15, as explained for the reduced agonist actions of imidacloprid on the Dα2/β2 nAChR by the P242E mutation in loop C21. To test this hypothesis, we investigated a mutation of Ser221 to glutamine having a similar size to glutamate but lacking a negative charge on the agonist activity of imidacloprid and thiacloprid on the hybrid nAChRs. The mutation was ineffective in changing the affinity but reduced the efficacy of imidacloprid in the (Dα1)3(β2)2 nAChR (P < 0.05, one-way ANOVA (Bonferroni-test)). Such effect on the agonist actions not only of imidacloprid, but also of thiacloprid, became more evident by increasing the β2 subunit composition ratio (Fig. 4a,b, Table 2, P < 0.05, one-way ANOVA (Bonferroni-test)). Hence, the reduced neonicotinoid affinity by the S221E mutation is mainly due to electrostatic repulsion by the glutamate residue, and that the reduced neonicotinoid efficacy in the (Dα1)2(β2)3 nAChR by Gln221 is due to its interference with neonicotinoid access to the Dα1/β2 interface by either steric interactions, or omission of the hydrogen bond or both.
Agonist actions of imidacloprid and thiacloprid on wild type and S221Q mutant Dα1β2 nAChRs expressed in Xenopus laevis oocytes. Current responses to imidacloprid are shown above the concentration-response curves. Horizontal lines indicate bath applications of imidacloprid and thiacloprid. (a) Concentration-normalized response relationships of imidacloprid for the wild type and mutant (Dα1)3(β2)2 and (Dα1)2(β2)3 nAChRs. (b) Concentration-normalized response relationships of thiacloprid for the wild type and mutant (Dα1)3(β2)2 and (Dα1)2(β2)3 nAChRs. Each data plotted represents mean ± standard error of the mean (n = 5).
Models of Dα1/β2 nAChRs complexed with thiacloprid
To further understand the role for Ser221 for the interaction with neonicotinoids, we modeled the Dα1/Dα1 subunit interface of the wild type and S221A mutant (Dα1)3(β2)2 nAChRs in complex with thiacloprid (Fig. 5), since the effects of the mutations on thiacloprid were greater than those on imidacloprid (Figs. 3 and 4). Thiazolidine ring of thiacloprid stacked with the aromatic ring of Tyr220, while the CN group interacted electrostatically with Arg57 in loop G and Lys140 in loop E and formed a hydrogen bond with the main chain NH group of Ser221 (Fig. 5). The hydroxy group of Ser221 formed hydrogen bond networks with the cyano group of thiacloprid as well as Arg57 and Lys140 (Fig. 5a). Thiacloprid can bind to the Dα1/Dα1 subunit interface in the S221A mutant because the basic residues in loop E and loop G support the binding (Fig. 5b), explaining the low impact of the mutation on the neonicotinoid action on the nAChRs with a higher composition ratio of Dα1 to β2. It is also suggested that when the mutations tested reduce the neonicotinoid sensitivity of the nAChRs with a higher composition of β2 to Dα1 because the β2 subunit does not possess any basic residue interacting with the nitro or cyano group of neonicotinoids and this would also be the case for imidacloprid13. The serine in loop C of the α1 subunit is encoded by AGC and AGT in Anopheles gambiae and Locusta migratoria, respectively, while being encoded by TCG and TCA in Myzus persicae and Nilaparvata lugens, respectively. We therefore predict that the serine to alanine mutation by a mutation of the first nucleotide T to G in M. persicae and N. lugens may occur and influence the neonicotinoid sensitivity of pests carrying the R81T mutation in loop D of the β1 subunit, and that the serine to glutamine mutation would occur less frequently than the serine to alanine mutation since two nucleotide mutations are needed. Further, we presume that a mutation of the serine to glycine may also occur by a mutation of the first nucleotide A to G, not only in the genome, but also in the mRNA22, and reduce the neonicotinoid sensitivity of A. gambiae and L. migratoria. As such, it is important to investigate whether these and related nucleotide mutations occur and affect the neonicotinoid sensitivity of pest insect species carrying the R81T mutation.
Models of Dα1/Dα1 subunit interface complexed with thiacloprid in (a) wild type and (b) S221A mutant (Dα1)3(β2)2 nAChRs. In the models, principal and complementary Dα1 subunits are illustrated as cartoon and colored pale cyan and yellow, respectively. Nitrogens, oxygen and sulfur atoms are colored blue, red and sand yellow, respectively, whereas carbons of thiacloprid are colored white. Electrostatic or hydrogen bond interactions are indicated by broken lines.
In conclusion, we have investigated the effects of S221A and S221Q mutations in loop C of the Drosophila Dα1 subunit on the agonist actions of ACh, imidacloprid and thiacloprid on the (Dα1)3(β2)2 and (Dα1)2(β2)3 nAChRs. Both mutations hardly lowered the affinity of the neonicotinoids. Hence, hydrogen bond capability of the serine residue has a minor contribution to the interactions with neonicotinoids in the insect/vertebrate hybrid nAChRs. However, the effects of these mutations on the efficacy of the neonicotinoids were evident in the (Dα1)2(β2)3 nAChR, pointing to a role for the serine in determining the neonicotinoid actions at the Dα1/β2 interface lacking the basic residues involved in the interactions with neonicotinoids. Although studies are needed to confirm this using nAChRs that are composed completely of insect nAChR subunits, the present results provided a new insight in the mechanism of selective actions of neonicotinoids and predicted resistance which may arise from the mutations tested in pest populations, notably with the R81T mutation.
Data availability
All data and material used in this study are available when requested.
References
Ihara, M. & Matsuda, K. Neonicotinoids: molecular mechanisms of action, insights into resistance and impact on pollinators. Curr Opin Insect Sci 30, 86–92 (2018).
Ihara, M., Buckingham, S. D., Matsuda, K. & Sattelle, D. B. Modes of action, resistance and toxicity of insecticides targeting nicotinic acetylcholine receptors. Curr Med Chem 24, 2925–2934 (2017).
Casida, J. E. Neonicotinoids and other insect nicotinic receptor competitive modulators: Progress and prospects. Annu Rev Entomol 63, 125–144 (2018).
Matsuda, K. et al. Neonicotinoids: insecticides acting on insect nicotinic acetylcholine receptors. Trends Pharmacol Sci 22, 573–580 (2001).
Matsuda, Ihara, M. & Sattelle, D. B. Neonicotinoid insecticides: Molecular targets, resistance, and toxicity. Annu Rev Pharmacol Toxicol 60, 241–255 (2020).
Ihara, M. et al. Diverse actions of neonicotinoids on chicken α7, α4β2 and Drosophila-chicken SADβ2 and ALSβ2 hybrid nicotinic acetylcholine receptors expressed in Xenopus laevis oocytes. Neuropharmacology 45, 133–144 (2003).
Jeschke, P., Nauen, R. & Beck, M. E. Nicotinic acetylcholine receptor agonists: a milestone for modern crop protection. Angew Chem Int Ed Engl 52, 9464–9485 (2013).
Hladik, M. L., Main, A. R. & Goulson, D. Environmental risks and challenges associated with neonicotinoid insecticides. Environ Sci Technol 52, 3329–3335 (2018).
Goulson, D. signatories. Call to restrict neonicotinoids. Science 360, 973 (2018).
Cressey, D. The bitter battle over the world’s most popular insecticides. Nature 551, 156–158 (2017).
Matsuda, K., Kanaoka, S., Akamatsu, M. & Sattelle, D. B. Diverse actions and target-site selectivity of neonicotinoids: structural insights. Mol Pharmacol 76, 1–10 (2009).
Shimomura, M. et al. Effects of mutations of a glutamine residue in loop D of the α7 nicotinic acetylcholine receptor on agonist profiles for neonicotinoid insecticides and related ligands. Br J Pharmacol 137, 162–169 (2002).
Shimomura, M. et al. Role in the selectivity of neonicotinoids of insect-specific basic residues in loop D of the nicotinic acetylcholine receptor agonist binding site. Mol Pharmacol 70, 1255–1263 (2006).
Bass, C. et al. Mutation of a nicotinic acetylcholine receptor β subunit is associated with resistance to neonicotinoid insecticides in the aphid Myzus persicae. BMC Neurosci 12, 51 (2011).
Hikida, M. et al. Combined effects of mutations in loop C and the loop D-E-G triangle on neonicotinoid interactions with Drosophila Dα1/chicken β2 hybrid nAChRs. Pestic Biochem Physiol 151, 47–52 (2018).
Ihara, M. et al. Loops D, E and G in the Drosophila Dα1 subunit contribute to high neonicotinoid sensitivity of Dα1-chicken β2 nicotinic acetylcholine receptor. Br J Pharmacol 175, 1999–2012 (2018).
Matsuda, K. et al. Effects of the α subunit on imidacloprid sensitivity of recombinant nicotinic acetylcholine receptors. Br J Pharmacol 123, 518–524 (1998).
Okuhara, D., Furutani, S., Ito, K., Ihara, M. & Matsuda, K. Splice variants of pH-sensitive chloride channel identify a key determinant of ivermectin sensitivity in the larvae of the silkworm Bombyx mori. Mol Pharmacol 92, 491–499 (2017).
Fiser, A. & Sali, A. Modeller: generation and refinement of homology-based protein structure models. Methods Enzymol 374, 461–491 (2003).
Ihara, M. et al. Studies on an acetylcholine binding protein identify a basic residue in loop G on the α1-strand as a new structural determinant of neonicotinoid actions. Mol Pharmacol (2014).
Shimomura, M., Yokota, M., Matsuda, K., Sattelle, D. B. & Komai, K. Roles of loop C and the loop B-C interval of the nicotinic receptor α subunit in its selective interactions with imidacloprid in insects. Neurosci Lett 363, 195–198 (2004).
Grauso, M., Reenan, R. A., Culetto, E. & Sattelle, D. B. Novel putative nicotinic acetylcholine receptor subunit genes, Dα5, Dα6 and Dα7, in Drosophila melanogaster identify a new and highly conserved target of adenosine deaminase acting on RNA-mediated A-to-I pre-mRNA editing. Genetics 160, 1519–1533 (2002).
Acknowledgements
This study was supported by KAKENHI (Grant number 17H01472) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Contributions
S. Shimada, M.K., S. Shigetou, K.T., Y.K., L.M. and M.I. measured the neonicotinoid actions on the expressed nicotinic receptors. S. Shimada, M.K., S. Shigetou, K.T., Y.K., L.M., M.I. and K.M. analyzed data. S. Shimada, M.I. and K.M. illustrated the figures and wrote the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Shimada, S., Kamiya, M., Shigetou, S. et al. The mechanism of loop C-neonicotinoid interactions at insect nicotinic acetylcholine receptor α1 subunit predicts resistance emergence in pests. Sci Rep 10, 7529 (2020). https://doi.org/10.1038/s41598-020-64258-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-64258-z
- Springer Nature Limited