Abstract
Over the past decade, it has been repeatedly demonstrated that homogeneity in electrochemical performance of lithium-ion cells plays a major role in determining the life and safety of lithium-ion battery modules or packs. Generally, the homogeneity of a battery pack is evaluated by characterizing the cells individually in terms of capacity, mass, impedance. Particularly, high quality electrochemical data heavily relies on the availability of high precision current source to minimize the discrepancy induced by the channel-to-channel variation. Here, a facile and precise measurement method is reported for screening cell-to-cell variations, in which voltage is the only indicator parameter independent of high precision current source. In detail, by connecting the cells in series (CiS), the measurement error of electrochemical data caused by stability and discrepancy of current sources among different charge/discharge equipment can be effectively avoided. The findings of this work showed that the cell-to-cell variations can be simply and sensitively detected with CiS configuration. For example, the relative standard deviation, which is the evaluation criterion of battery homogeneity, was 2.14% based on CiS while it was 0.43% based on individual measurements. The simple and precise CiS measurement is promising for evaluation of cell quality or module integration quality. In addition, this work can also provide a solid foundation for the development of detection algorithms for battery management systems to rapidly monitor battery homogeneity.
Similar content being viewed by others
Introduction
Lithium ion batteries (LIBs) have to be integrated into modules and packs for large-scale applications such as electric vehicles (EVs) and stationary energy storage systems 1,2,3,4,5,6,7. However, a reliable and long-lasting power system is determined mostly by the cell homogeneity rather than the performance of individual cells8,9. The cell-to-cell variations in terms of capacity and impedance will subject individual cells to different levels of state of charge (SOC), current, temperature and aging, which in turn would accelerate the degradation of electrochemical performance or even lead to safety performance of the power system2,10,11,12,13. For instance, a weak cell with a smaller capacity than the average in a series-connected module will be repeatedly overcharged (over-discharged) during charging (discharging) process if the charge/discharge cutoff condition is determined according to the total voltage14. The weaker cell will then have faster decay than others and will be susceptible to failure under harsh conditions. The available energy of the module will also be limited by the weakest cell15. Therefore, there is an urgent need to understand and minimize the cell-to-cell variations in a battery module.
There are different factors leading to variations among cells11, including manufacturing factors (manufacturing tolerances, quality control and process design), and environmental factors such as temperature gradient within the battery pack16. Rumpf et al.12 has evaluated the cell-to-cell variation due to manufacturing tolerances based on tests on 1100 commercial lithium-ion cells, and found relative variations of 0.28% and 0.72% for the cell capacity and impedance, respectively. Dubarry et al.17 has showed that cell-to-cell variations can drastically affect the performance and the reliability of both series and parallel battery packs. Baumhöfer et al.18 and Harris et al.19 showed that variation caused by production will result in a spread in battery degradation performance. Furthermore, theoretical analysis and industrial practice have showed that inhomogeneity of electrochemical characteristics in the unmatched cells inevitably resulted in voltage/SOC variations which can cause permanent internal damage in battery packs and energy storage systems13,20.
The energy loss and SOC estimation error can be minimized by reducing the cell-to-cell variation13,21. In order to achieve a stable configuration of a battery module or pack, a screening process is needed to ensure high homogeneity of the cells therein 11,22,23. Several screening processes have been previously proposed12,20,24,25,26. Multiple parameters, including capacity, mass, electrochemical impedance, direct current resistance and voltage, were employed to index the cell-to-cell variation. Rumpf et al.12 conducted a statistical analysis of parametric cell-to-cell variation of 15 different cell parameters and the correlations between parameters based on 1100 examined cells, and found it necessary to characterize variation in ohmic impedance when determining cell-to-cell variation. Dubarry et al.27,28 have conducted both statistical and electrochemical analyses to characterize cell-to-cell variations, and found that the cell capacity, resistance and rate capability can be regarded as three independent parameters for cell variations evaluation. Furthermore, as cell parameters show different evolution trends during aging, online algorithms are required to evaluate cell-to-cell variation during aging process15. Kum developed an overall cell selection framework with significant improvement in fabricating a module/pack with high homogeneity using second-life cells21. The proposed framework consisted of cell modeling, cell testing, parameter estimation technique, and a filtering algorithm. The screening result was effective, but multiple parameters were involved in this framework and the detection algorithm is complicated, making it challenging for online operation. Several approaches have also been proposed based on precise characterization of the electrochemical performance on individual cells. However, the measurement precision is a considerable technical barrier in these cell screening methods29,30,31,32,33,34,35,36,37. The major barrier for single cell approach is the difficulty in precisely measuring or estimating cell capacity, which involves the integration of measured current containing electronic noise over an extended period of charging or discharging. High precision equipment with a high-end current source is usually required for accurate cell-to-cell homogeneity evaluation32,38. An excellent implementation of this strategy is the development of high precision columbic efficiency (HPCE) measurement, which is only suitable for small scale deployment mainly due to its extremely high cost39,40. As a result, a facile, precise and less parameter involved cell-to-cell variation evaluation process would be ideal for estimation or fabrication of a reliable stationary or portable power system.
Therefore, a facile and precise evaluation method named as “Connection in Series” or “CiS”, was proposed to evaluate the cell-to-cell variation in this work. In the CiS method, cells are evaluated in series connection, and only the cell voltages are measured for cell consistency evaluation. CiS was demonstrated to be effective for cell-to-cell variation analysis for commercial grade batteries. The simplicity, reliability and availability of CiS process for cell selection with high homogeneity was verified for vehicular application and for evaluation of cell homogeneity from different cell makers. The CiS-based methodology research as shown in this work can provide important knowledge for the development of algorithms for battery management systems (BMS) to evaluate the homogeneity of battery packs and to forecast potential failures on the fly.
Results and Discussion
The index of cell-to-cell variation
The cell-to-cell performance variation in a battery pack is traditionally indexed by the capacity, mass, direct current resistance, impedance, etc.11. The most important concern is that cell-to-cell performance variation exists after assembling the cells in series or parallel into a module. This has triggered a major research effort for developing a low cost, effective, reliable method to evaluate the performance homogeneity of cells in a battery pack24. As the cell-to-cell variation in capacity, impedance, and other parameters will be intrinsically reflected by their SOC variations in CiS approach, the key focus of CiS approach has evolved into selecting the most representative SOC values for effective and reliable evaluation.
As is well known, the slopes of discharge curves are the most sensitive at the end of charging/discharging. For example, Fig. 1 shows the typical discharge curves of graphite/LiNi0.8Co0.15Al0.05O2(NCA) and graphite/LiCoO2(LCO) cells. It can be seen that a difference of 1% in practical capacity led to a significant difference in voltage at the end of discharge by 100 mV and 205 mV for NCA and LCO cells, respectively. A difference of 205 mV in voltage can be easily translated into 4.1% of practical range for typical battery measurement equipment rated as 5 V maximum range. Moreover, the variation in the instant voltage at the end of discharge is affected by both the capacity variation and resistance variation. As a result, the instant voltage at the end of discharge will be more sensitive for the detection of cell-to-cell variation than capacity and resistance for indexing the cell-to-cell variation. Furthermore, it is known that cell capacity is calculated by multiplying the discharge duration by the coulombic current, and strongly depends on the precision of the current passing through the cell. To eliminate the discrepancy resulting from the coulombic current measurement, a Connection-in-Series (CiS) approach was proposed here to precisely screen the cell-to-cell variation. In this method, the homogeneity of cells was effectively indexed by the voltage at the end of discharge, and additionally by the voltage at the end of charge and by the voltage after a period of rest after discharge24. In CiS configuration, the measurement was carried out with identical amount of charge delivered to the cells in series, and the voltage data were acquired almost at the same time. The screening index of cell-to-cell variation transformed from current capacities to voltages, achieving more precise and less expensive screening of cell homogeneity. Besides the significant advantage for high precision with low cost, the methodology research of CiS-based configuration can also provide seamless knowledge integration into the development of smart battery management system (BMS). This is because the CiS configuration has already been implemented into large battery packs, and the high precision voltage tracking for each cell is the base for the BMS.
Comparison of CiS and state-of-art test
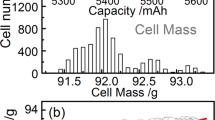
For this test, 80 cells (NCR18650BE) purchased from Panasonic, recognized as the top battery maker with high quality, were evaluated to compare the difference between CiS and state-of-the-art. For state-of-the-art test, both capacity (Q) and median voltage (Vmid, the voltages at the middle of capacity) of each cell were recorded as indexes for cell-to-cell variation. The results are shown in Fig. 2. The maximum and minimum values for median voltages (Vmax and Vmin) were 3.63 V and 3.57 V, respectively, with a difference (∆Vmid, ∆Vmid = Vmax − Vmin) of 0.057 V. The maximum and minimum capacities (Qmax and Qmin) were 3.156 mAh and 3.053 mAh, respectively, which differed from each other by only 0.103 mAh (∆Q, ∆Q = Qmax − Qmin). The relative standard deviation (δ) for Q was calculated to be 0.63%, and that for Vmid was 0.43%. They were all lower than 1%, indicating excellent cell homogeneity.
For CiS test, these 80 cells were connected in series for charge/discharge, and experienced the same current, same duration and same capacity. The cell voltage was the only measured parameter, as shown in Fig. 3. Cell voltages at three states were investigated, i.e. the instant voltage at the end of charge (Vc-end), the instant voltage at the end of discharge (Vd-end), and the voltage after the end of discharge for 30 min (Vd-30min). The results are shown in Fig. 3. ∆Vc-end, ∆Vd-end and ∆Vd-30min were 0.015 V, 0.294 V and 0.142 V, respectively. The relative standard deviations (δ) of Vc-end, Vd-end and Vd-30min were 0.09%, 2.14% and 0.93%, respectively. The SOCs of all cells were the same (0%) at the very beginning, and all the cells were charged the same capacities in series, reaching around 100%SOC. Therefore, a very small relative standard deviation (δ=0.09%) of Vc-end was observed. The difference in Vc-end was mainly caused by the precision of the instrument, uneven self-discharge and impedance variation. The 15 mV difference indicates only 0.3% relative error, which is within the instrument error for 5 V range measurement (0.5%). δ for Vd-30min was 0.93%, right between δ = 0.43% for Vmid and δ = 2.14% for Vd-end. This result will be applied in the next section.
The parameter analysis of 80 cells from Panasonic by CiS. (a) discharge/charge curves; (b) The statistics of the voltages at the end of charge (Vc-end), at the end of discharge (Vd-end) and at the point of 30 min rest after discharge (Vd-30min), respectively. Their relative standard deviations (δ) were 0.09%, 0.93% and 2.14%, respectively. The instrument error was 0.5% for 5 V range measurement.
As shown in Fig. 4(a–c), Vd-end and Vd-30min exhibited good correlations with cell capacity, with the correlation coefficients reaching 0.6635 and 0.6434, respectively. However, Vc-end showed weak correlation with cell capacity. Moreover, correlations between cell resistance and Vd-end, Vd-30min and Vc-end were also presented in Fig. 4(d–f), where the resistance was calculated by dividing the difference between Vd-end and Vd-60s by the discharge current. Vd-60s was the cell voltage after the end of discharge for 60 s. Vd-end and Vd-30min also showed strong correlations with cell resistance. Therefore, we can conclude that Vd-end and Vd-30min measured by the proposed CiS method do have good correlations with cell capacity and resistance, and thus can be applied to evaluate cell-to-cell variations. Moreover, we can find that ∆Vd-end and its δ were very large, being 294 mV and 2.14% respectively. Compared with state-of-the-art test results (δ = 0.63% for Q and δ = 0.43% for Vmid), Vd-end was more sensitive to the cell-to-cell variation for the same group of cells. This verified the effectiveness of CiS and validity of Vd-end as an ideal index for cell-to-cell variation.
Correlations between cell capacity and the three voltage indexes measured by CiS method. (a–c) Correlations between cell capacity and Vd-end, Vd-30min and Vc-end; (d–f) Correlations between cell resistance and Vd-end, Vd-30min and Vc-end; Cell resistance was calculated by dividing the difference between Vd-end and Vd-60s by the discharge current. Vd-60s was the cell voltage after the end of discharge for 60 s.
CiS application I. Time dependence of cell-to-cell variation
Due to the high sensitivity of homogeneity evaluation by CiS, the proposed CiS method was used to evaluate the possible aging differences between high quality cells shelved for 2.5 years. For this test, 80 cells from Panasonic were initially evaluated in December of 2015 (as shown in Figs. 2 and 3), and evaluated again in June of 2018. During the period between two evaluations, the cells were stored in individual packages at fully charged state at 25 °C, with the environment humidity controlled below 30%. The results are presented in Fig. 5. The same cells were tested under the same conditions, and the only change was that the two tests were 2.5 years apart. Interestingly, all the indexes for homogeneity increased. The δ value for Vc-end increased slightly from 0.10% to 0.12%. δ for Vd-30min increased considerably from 0.07% to 0.23%, but was still very small. δ for Vd-end nearly doubled from 0.57% to 0.99%. Moreover, ∆Vd-end also increased from 85 mV to 163 mV, equivalent to the capacity difference increase from 0.13% to 0.26% when calculated by the principle shown in Fig. 1. Therefore, the CiS method can also applied to track the evolution of cell variations, and provides the basic information for cell equalization algorithm41.
The homogeneity screening parameters of 80 cells after rest for 2.5 years. (a,c) charge(0.5 C)/discharge(1 C) curves of 80 cells which were connected in series; (b,d) The statistics of Vc-end, Vd-30min and Vd-end, respectively; (a,b) the parameters collected in December of 2015; (c,d) the parameters collected in June of 2018. When discharge/charge was at 2 C, the cells showed relative standard deviations (δ) of 0.09%, 0.93% and 2.14% for Vc-end, Vd-30min and Vd-end, respectively.
Considering the correlation between aging speed and internal chemistry, it is reasonable that cells with similar chemical/physical states at certain time point will experience different aging speeds under the same environmental stress (such as electric stress, temperature stress, mechanical stress) and present varied chemical/physical states in the following time points18,42,43. In this study, cell degradation under room temperature storage was mainly caused by the SEI film formation side reaction, which is influenced by the surface area of the anode active material, the thickness of the anode and the original SEI film formed during the preparation process44,45. Therefore, although the cells with similar chemical/physical states were degraded under the same operation conditions, they will still experience different aging speeds over a long time period. In this sense, time is effective to amplify the cell-to cell variation. The test presented in this section indicated that a slight change in cell parameters can result in subtle differences that cannot be detected at fresh state. Moreover, this test can be applied for evaluation of battery quality from different battery makers. This may lead to a standard test protocol for battery quality evaluation, which is still a challenge in vehicular application.
CiS application II. Rate dependence of cell-to-cell variation
Both thermodynamic and kinetic factors contribute to the cell-to-cell variations2,10,11,12,27,46,47, and kinetic factors are generally presented as impedances in battery. In this sense, the rate dependence of cell-to-cell variations will help to identify the influencing factors for battery kinetics introduced during manufacturing11. However, the cell-to-cell variation dependence on C-rate is unclear. On one hand, the subtle variation in impedance can be magnified in numerical value at high C-rate. On the other hand, high C-rate operation may cause temperature rise inside the battery, which is known to help decrease the battery impedance. That is, high C-rate may lead to impedance reduction in numerical value. Furthermore, high rate charge/discharge implies high measurement error for normal charge/discharge instrument, which affects the analysis of cell-to-cell variation. Here, the preferable C-rate for CiS was determined, and the possible application of CiS at different C-rates was also presented. CiS approach was employed to investigate the rate dependence of cell-to-cell variations. The evaluation of cells from different manufacturers was further discussed.
The cells produced by Panasonic (NCR18650BE, 3.0 Ah), LG Chem (18650 B4, 2.6 Ah) and Lishen (LR1865SK, 2.6 Ah) were evaluated respectively. Firstly, 20 cells with good consistency were selected among 80 cells by CiS at 0.1 C-rate according to δ. Then, cell-to-cell variation was further checked at 0.5 and 1 C-rate. The results are shown in Fig. 6. All the tests confirmed that Vd-end was the most sensitive parameter for cell-to-cell variation. The charge tests were stopped when any cell reached 4.2 V. It was found that each Vc-end was very close to 4.2 V, indicating that the uniformity was good for charging process. The 20 cells of any brand selected according to Vd-end can ensure high homogeneity of Vc-end, proving the effectiveness of CiS. It is known that high homogeneity of Vc-end is important to avoid overcharging in a module or pack configuration.
The statistics of the Vc-end (3 lines at the top), Vd-end (3 lines at the bottom) and Vd-30min (3 lines in the middle) of 20 cell-series modules operated at various C-rates with cells produced by different battery makers. (a) 20 cells from Panasonic, NCR18650BE, 3.0Ah; (b) 20 cells from LG Chem, 18650 B4, 2.6Ah; (c) 20 cells from Lishen, LR1865SK, 2.6Ah. The tests were carried out at 0.1 (black), 0.5 (red) and 1.0 (blue) C-rate, respectively. The tested 20 cells were the most uniform ones screened by CiS from stochastic 80 cells, respectively.
For Vd-30min, cell homogeneity at different C-rates can be roughly determined by the fluctuation range of the statistical curves. Then, it was found that cells from Panasonic exhibited good homogeneity at both 0.5 and 1.0 C-rate, as shown in the middle of Fig. 6(a). Cells from LG Chem showed good consistency only at 1.0 C-rate, as shown in Fig. 6(b). However, the homogeneity of cells from Lishen was worse compared to the other two. In this sense, the ranking order of homogeneity was Panasonic, LG Chem and Lishen.
Interestingly, the sequence of cell number changed in homogeneity screening by different C-rates, according to voltage value. For example, in Fig. 6(a), the Vd-end of cell No.6 was higher than that of the two adjacent cells at 1 C rate, but it was lower at 0.1 C rate. That might be induced by variations in cell rate capability. Cell No.6 had a smaller capacity than the two adjacent cells and thus showed a lower Vd-end at 0.1 C. Inversely, cell No.6 exhibited a larger capacity when C-rate increased to 1 C, indicating that cell No.6 might has better rate capability than the adjacent cells27,28. The cells from LG Chem and Lishen also showed similar behavior, as presented in Fig. 6(b,c). This indicates that the rate dependence of cell-to-cell variation may provide more information about the battery performance, such as variations in rate capability, which is an important index for cell-to-cell variations27,28.
Quantitatively, relative standard deviation (δ) is proposed as an appropriate index for homogeneity. In statistics, δ is also known as the coefficient of variation, a standardized measure of dispersion for a probability distribution48,49. It was observed that the evolution trend of δ for Vd-end was similar to that for Vd-30min, as shown in Fig. 7(a,b). However, the former was larger (more sensitive) than the latter. The relative standard deviation also confirmed once again that Vd-end is a suitable index for cell-to-cell variation.
The cells from Panasonic exhibited the lowest δ values at all C-rates, and the δ values of Vd-end and Vd-30min were both less than 1.5%. The cells from LG Chem showed larger variations with the δ of Vd-end and Vd-30min increasing to more than 2.0% at 0.1 C. The cells from Lishen showed the worst homogeneity with largest δ at all C-rates. Figure 7 indicates quantitatively that the ranking order of cell homogeneity was Panasonic, LG Chem and Lishen. It is well known that the cells from Panasonic have top quality, featuring homogeneous slurry, uniform coating of electrodes, etc. This is consistent with the above results.
Furthermore, the cells from the three manufacturers all exhibited increasing δ values with decrease in C-rate. The changes in δ with C-rates can be explained by the different slopes of the voltage curves at different C-rates, as presented in Fig. 8. The discharge voltage curves exhibited small slope with the increase in C-rate due to the influence of polarization. Thus, Vd-end became less sensitive to cell-to-cell variation as C-rate increased. Moreover, the changes of δ with C-rates can help to evaluate the rate capabilities of the cells from different manufacturers. According to Fig. 8, a larger decrease in the δ of Vd-end with the increase of C-rates indicated a larger increase of polarization and thus worse rate capability. Form the results in Fig. 7(a), we can observe that the Panasonic cells have the best rate capabilities among the three manufacturers. A index named RC was also defined as the ratio between the capacity at 1 C and 0.1 C to evaluate rate capability referring to ref. 17. The Panasonic cell exhibited the best rate capability with a RC ratio of 83.7%, while the RC ratios of LG Chem and Lishen cells were 79.6% and 80.4%, respectively, consistent with results from the CiS method. This finding also enriches the understanding of rate dependence of cell-to-cell variations11.
Conclusions
Cell homogeneity is critical for performance and durability of energy storage systems. The state-of-the-art evaluation of cell homogeneity based on individual cell performance involves intertwined parameters (such as capacity and impedance) and a large number of measurements. Based on the charge/discharge characteristics of lithium-ion batteries, a CiS method that indexes cell-to-cell variations only by a state voltage was proposed in this paper. In detail, the voltage at the end of discharge Vd-end was demonstrated to be a sensitive index for cell-to-cell variation, and the relative standard deviation (δ) was proposed to be an appropriate index for cell homogeneity in parameter processing. Low C-rate was preferred for CiS, as the cell homogeneity decreased with decrease in C-rate. Compared with the state-of-the-art evaluation method11, the proposed CiS approach can save equipment cost, manpower, analysis test and data processing. Investigations on the variation of cell homogeneity with storage duration showed that cell homogeneity became worse during aging even though the cells started from quite similar physical and chemical states. Besides, the dependence of cell homogeneity on C-rate can be used as an index for quality control or quality evaluation. The information may help battery makers to improve cell design, manufacturing and quality control. CiS was shown to be a facile and highly precise approach for evaluating and tracking the consistency of lithium-ion batteries. It can not only be developed as a universal standard test protocol of cell-to-cell variation for vehicular and stationary energy storage application, but also have possible applications in cell screening.
Materials and Methods
Materials
Cylinder cells (18650 type) were purchased from market and were not balanced before. The cell manufacturers were Panasonic (NCR18650BE, 3.0 Ah), LG Chem (18650 B4, 2.6 Ah) and Lishen (LR1865SK, 2.6 Ah), respectively. The cathode material was LiNi0.8Co0.15Al0.05O2(NCA) for Panasonic cell, and was LiCoO2 (LCO) for LG Chem and Lishen cells. The anode material of the three kinds of cells was artificial graphite. The separators were all polypropylene. Neware test system was used to perform charge-discharge tests, and Arbin EVTS battery testing system was used to record cell voltage during charge-discharge.
State-of-the-art test method
The capacities and median voltages of Panasonic cells were measured individually before the CiS tests at 25 °C according to the product specification. The charge protocol was CC-CV with 1/5 C reaching an upper voltage limit of 4.2 V, and the cutting-off current was 0.05 C. After a rest period of 30 min, the cells were discharged with a constant current 1/3 C to 2.5 V.
CiS test method
All cells with 100%SOC were connected in series. The voltage of every single cell was recorded by Arbin EVTS battery testing system (accuracy of voltage control was ±0.1% FSR). The homogeneity of 80 Panasonic cells at 2.5 year intervals was investigated by CiS. As shown in Fig. 9, 80 cells were connected and tested in series. During the tests, the same amount of charge/discharge capacity passed through the 80 cells, and the voltage of each cell was measured simultaneously. Cells were firstly discharged at 0.2C-rate until any single voltage reached 2.95 V. Then, the cells were rested 10 min and were charged at 0.5C-rate when any single voltage reached 4.2 V. The discharging process consisted of discharging at 1C-rate until any single voltage reached 3.0 V, and then a 30 min rest. The C-rate was determined by nominal capacity of the tested cell. X C represents the current that can fully discharge the cell from 100%SOC to 0%SOC in 1/X hour. 1 C was 3.0 A for Panasonic NCR18650BE cells (3.0Ah), and 2.6 A for both LG Chem 18650B4 cells (2.6Ah) and Lishen LR1865SK cells (2.6Ah). Three instant voltages, i.e. the end of charging (Vc-end), discharging (Vd-end) and discharging for 30 min (Vd-30min), in the process were recorded and their discrete factors were calculated as the criteria for homogeneity evaluation.
To investigate the dependence of consistency to rate, 20 cells with the best homogeneity were selected among 80 cells for the three kinds of cells respectively. In this process, the charge/discharge rate was 0.1 C. Then the consistency of each kind of 20 cells was measured using CiS method at 0.5 and 1 C-rate for both charge and discharge at 25 °C. The test protocol was as follows:
- (1)
all cells were charged to 100% SOC. (the charge protocol refers to the battery specifications provided by manufacturer: charging to 4.2 V with 0.3 C constant current (CC) and then constant voltage (CV) charging with cut-off current of 0.05 C);
- (2)
the 20 cells were connected in series to form a module, and the voltages of individual cells were recorded;
- (3)
the module was discharged at 0.1 C to 3.0 V of any single cell;
- (4)
rest for 30 min;
- (5)
the module was then charged at 0.1 C up to 4.2 V of any single cell; then steps 3, 4, and 5 were successively repeated at 0.5 C and 1.0 C, respectively.
References
Schoenleber, M., Uhlmann, C., Braun, P., Weber, A. & Ivers-Tiffee, E. A Consistent Derivation of the Impedance of a Lithium-Ion Battery Electrode and its Dependency on the State-of-Charge. Electrochim. Acta 243, 250–259, https://doi.org/10.1016/j.electacta.2017.05.009 (2017).
Schuster, S. F., Brand, M. J., Berg, P., Gleissenberger, M. & Jossen, A. Lithium-ion cell-to-cell variation during battery electric vehicle operation. J. Power Sources 297, 242–251, https://doi.org/10.1016/j.jpowsour.2015.08.001 (2015).
Wang, H. W. et al. In Proceedings of the 3rd 2017 International Conference on Sustainable Development Vol. 111 AER-Advances in Engineering Research (eds L. W. Haur et al.) 84–87 (2017).
Wen, F., Lincheng, Jiang, J. C., Wang, Z. G. & Ieee. In 2012 Asia-Pacific Power and Energy Engineering Conference Asia-Pacific Power and Energy Engineering Conference (2012).
Zhang, C. P. et al. Study on battery pack consistency evolutions and equilibrium diagnosis for serial- connected lithium-ion batteries. Appl. Energy 207, 510–519, https://doi.org/10.1016/j.apenergy.2017.05.176 (2017).
Zhong, L., Zhang, C. B., He, Y. & Chen, Z. H. A method for the estimation of the battery pack state of charge based on in-pack cells uniformity analysis. Appl. Energy 113, 558–564, https://doi.org/10.1016/j.apenergy.2013.08.008 (2014).
Zhou, L., Zheng, Y. J., Ouyang, M. G. & Lu, L. G. A study on parameter variation effects on battery packs for electric vehicles. J. Power Sources 364, 242–252, https://doi.org/10.1016/j.jpowsour.2017.08.033 (2017).
Tanim, T. R. et al. Advanced diagnostics to evaluate heterogeneity in lithium-ion battery modules. eTransportation 3, 100045, https://doi.org/10.1016/j.etran.2020.100045 (2020).
Han, X. et al. A review on the key issues of the lithium ion battery degradation among the whole life cycle. eTransportation 1, 100005, https://doi.org/10.1016/j.etran.2019.100005 (2019).
Devie, A. & Dubarry, M. Durability and Reliability of Electric Vehicle Batteries under Electric Utility Grid Operations. Part 1: Cell-to-Cell Variations and Preliminary Testing. Batteries-Basel 2, https://doi.org/10.3390/batteries2030028 (2016).
An, F., Chen, L., Huang, J., Zhang, J. & Li, P. Rate dependence of cell-to-cell variations of lithium-ion cells. Sci Rep 6, 35051, https://doi.org/10.1038/srep35051 (2016).
Rumpf, K., Naumann, M. & Jossen, A. Experimental investigation of parametric cell-to-cell variation and correlation based on 1100 commercial lithium-ion cells. J Energy Storage 14, 224–243, https://doi.org/10.1016/j.est.2017.09.010 (2017).
Rumpf, K. et al. Influence of Cell-to-Cell Variations on the Inhomogeneity of Lithium-Ion Battery Modules. J. Electrochem. Soc. 165, A2587–A2607, https://doi.org/10.1149/2.0111811jes (2018).
Liu, K., Li, K., Peng, Q. & Zhang, C. A brief review on key technologies in the battery management system of electric vehicles. Frontiers of Mechanical Engineering 14, 47–64, https://doi.org/10.1007/s11465-018-0516-8 (2018).
Hu, X. et al. State estimation for advanced battery management: Key challenges and future trends. Renewable and Sustainable Energy Reviews 114, https://doi.org/10.1016/j.rser.2019.109334 (2019).
Tomaszewska, A. et al. Lithium-ion battery fast charging: A review. eTransportation 1, 100011, https://doi.org/10.1016/j.etran.2019.100011 (2019).
Dubarry, M. et al. Battery energy storage system modeling: Investigation of intrinsic cell-to-cell variations. Journal of Energy Storage 23, 19–28, https://doi.org/10.1016/j.est.2019.02.016 (2019).
Baumhöfer, T., Brühl, M., Rothgang, S. & Sauer, D. U. Production caused variation in capacity aging trend and correlation to initial cell performance. Journal of Power Sources 247, 332–338, https://doi.org/10.1016/j.jpowsour.2013.08.108 (2014).
Harris, S. J., Harris, D. J. & Li, C. Failure statistics for commercial lithium ion batteries: A study of 24 pouch cells. J. Power Sources 342, 589–597, https://doi.org/10.1016/j.jpowsour.2016.12.083 (2017).
Kim, J. & Cho, B. H. Screening process-based modeling of the multi-cell battery string in series and parallel connections for high accuracy state-of-charge estimation. Energy 57, 581–599, https://doi.org/10.1016/j.energy.2013.04.050 (2013).
Lee, K. & Kum, D. Development of cell selection framework for second-life cells with homogeneous properties. International Journal of Electrical Power & Energy Systems 105, 429–439, https://doi.org/10.1016/j.ijepes.2018.08.015 (2019).
Gallardo-Lozano, J., Romero-Cadaval, E., Milanes-Montero, M. I. & Guerrero-Martinez, M. A. Battery equalization active methods. J. Power Sources 246, 934–949, https://doi.org/10.1016/j.jpowsour.2013.08.026 (2014).
Gogoana, R., Pinson, M. B., Bazant, M. Z. & Sarma, S. E. Internal resistance matching for parallel-connected lithium-ion cells and impacts on battery pack cycle life. J. Power Sources 252, 8–13, https://doi.org/10.1016/j.jpowsour.2013.11.101 (2014).
He, X. M. et al. A Facile Consistency Screening Approach to Select Cells with Better Performance Consistency for Commercial 18650 Lithium Ion Cells. Int. J. Electrochem. Sci. 12, 10239–10258, https://doi.org/10.20964/2017.11.01 (2017).
Kim, J., Shin, J., Chun, C. & Cho, B. H. Stable Configuration of a Li-Ion Series Battery Pack Based on a Screening Process for Improved Voltage/SOC Balancing. Ieee Transactions on Power Electronics 27, 411–424, https://doi.org/10.1109/tpel.2011.2158553 (2012).
Kim, J., Shin, J., Jeon, C., Cho, B. & Ieee. In 2011 Twenty-Sixth Annual Ieee Applied Power Electronics Conference and Exposition Annual IEEE Conference on Applied Power Electronics Conference and Exposition (APEC) 1984–1991 (2011).
Dubarry, M., Vuillaume, N. & Liaw, B. Y. Origins and accommodation of cell variations in Li-ion battery pack modeling. Int J Energ Res 34, 216–231, https://doi.org/10.1002/er.1668 (2010).
Dubarry, M. & Baure, G. Perspective on Commercial Li-ion Battery Testing, Best Practices for Simple and Effective Protocols. Electronics 9, https://doi.org/10.3390/electronics9010152 (2020).
Wang, Y., Fang, H., Wang, B. & Liu, H. Highly precise determination of the state of charge of vehicular battery. Instrumentation Science & Technology 44, 445–457, https://doi.org/10.1080/10739149.2016.1143836 (2016).
Stevens, D. A. et al. Using High Precision Coulometry Measurements to Compare the Degradation Mechanisms of NMC/LMO and NMC-Only Automotive Scale Pouch Cells. J. Electrochem. Soc. 161, A1364–A1370, https://doi.org/10.1149/2.0971409jes (2014).
Smith, A. J., Burns, J. C., Zhao, X. M., Xiong, D. J. & Dahn, J. R. A High Precision Coulometry Study of the SEI Growth in Li/Graphite Cells. J. Electrochem. Soc. 158, A447–A452, https://doi.org/10.1149/1.3557892 (2011).
Smith, A. J., Burns, J. C., Xiong, D. & Dahn, J. R. Interpreting High Precision Coulometry Results on Li-ion Cells. J. Electrochem. Soc. 158, A1136–A1142, https://doi.org/10.1149/1.3625232 (2011).
Rowe, A. W., Camardese, J., McCalla, E. & Dahn, J. R. High Precision Coulometry Studies of Single-Phase Layered Compositions in the Li-Mn-Ni-O System. J. Electrochem. Soc. 161, A1189–A1193, https://doi.org/10.1149/2.112406jes (2014).
Ramadan, H. S., Becherif, M. & Claude, F. Extended kalman filter for accurate state of charge estimation of lithium-based batteries: a comparative analysis. Int. J. Hydrog. Energy 42, 29033–29046, https://doi.org/10.1016/j.ijhydene.2017.07.219 (2017).
Pop, V. et al. Modeling battery behavior for accurate state-of-charge indication. J. Electrochem. Soc. 153, A2013–A2022, https://doi.org/10.1149/1.2335951 (2006).
Li, S. R., Chen, C. H., Camardese, J. & Dahn, J. R. High Precision Coulometry Study of LiNi0.5Mn1.5O4/Li Coin Cells. J. Electrochem. Soc. 160, A1517–A1523, https://doi.org/10.1149/2.087309jes (2013).
Adams, B. D., Zheng, J. M., Ren, X. D., Xu, W. & Zhang, J. G. Accurate Determination of Coulombic Efficiency for Lithium Metal Anodes and Lithium Metal Batteries. Advanced Energy Materials 8, https://doi.org/10.1002/aenm.201702097 (2018).
Harlow, J. E., Stevens, D. A., Burns, J. C., Reimers, J. N. & Dahn, J. R. Ultra High Precision Study on High Capacity Cells for Large Scale Automotive Application. J. Electrochem. Soc. 160, A2306–A2310, https://doi.org/10.1149/2.096311jes (2013).
Smith, A. J., Burns, J. C., Trussler, S. & Dahn, J. R. Precision Measurements of the Coulombic Efficiency of Lithium-Ion Batteries and of Electrode Materials for Lithium-Ion Batteries. J. Electrochem. Soc. 157, A196–A202, https://doi.org/10.1149/1.3268129 (2010).
Bond, T. M., Burns, J. C., Stevens, D. A., Dahn, H. M. & Dahn, J. R. Improving Precision and Accuracy in Coulombic Efficiency Measurements of Li-Ion Batteries. J. Electrochem. Soc. 160, A521–A527, https://doi.org/10.1149/2.014304jes (2013).
Feng, F., Hu, X., Liu, J., Lin, X. & Liu, B. A review of equalization strategies for series battery packs: variables, objectives, and algorithms. Renewable and Sustainable Energy Reviews 116, https://doi.org/10.1016/j.rser.2019.109464 (2019).
Feng, X. et al. A reliable approach of differentiating discrete sampled-data for battery diagnosis. eTransportation 3, 100051, https://doi.org/10.1016/j.etran.2020.100051 (2020).
Ren, D. et al. A comparative investigation of aging effects on thermal runaway behavior of lithium-ion batteries. eTransportation 2, 100034, https://doi.org/10.1016/j.etran.2019.100034 (2019).
Liu, K., Li, Y., Hu, X., Lucu, M. & Widanage, W. D. Gaussian Process Regression With Automatic Relevance Determination Kernel for Calendar Aging Prediction of Lithium-Ion Batteries. IEEE Transactions on Industrial Informatics 16, 3767–3777, https://doi.org/10.1109/tii.2019.2941747 (2020).
Tang, X. et al. Real-time aging trajectory prediction using a base model-oriented gradient-correction particle filter for Lithium-ion batteries. Journal of Power Sources 440, https://doi.org/10.1016/j.jpowsour.2019.227118 (2019).
Feng, X. et al. Mechanisms for the evolution of cell variations within a LiNixCoyMnzO2/graphite lithium-ion battery pack caused by temperature non-uniformity. Journal of Cleaner Production 205, 447–462, https://doi.org/10.1016/j.jclepro.2018.09.003 (2018).
Chun, C. Y., Cho, B. H. & Kim, J. Implementation of discharging/charging current sensorless state-of-charge estimator reflecting cell-to-cell variations in lithium-ion series battery packs. International Journal of Automotive Technology 17, 909–916, https://doi.org/10.1007/s12239-016-0088-8 (2016).
Kleiman, A. S. Statistical estimation of standard deviation of observations in measurements. Meas Tech 20, 184–186, https://doi.org/10.1007/BF00824477 (1977).
Hürlimann, W. A uniform approximation to the sampling distribution of the coefficient of variation. Stat Probabil Lett 24, 263–268, https://doi.org/10.1016/0167-7152(94)00182-8 (1995).
Acknowledgements
This work was supported by Ministry of Science and Technology of China (No. 2019YFE010200 and 2019YFA0705703) and the National Natural Science Foundation of China (No. U1564205). Research at Argonne National Laboratory was supported by U.S. Department of Energy (DOE), Vehicle Technologies Office. Argonne National Laboratory is operated for the US Department of Energy by U Chicago Argonne, LLC, under contract DE-AC02-06CH11357. The authors thank Joint Work Plan for Research Projects under the Clean Vehicles Consortium at U.S. and China – Clean Energy Research Center (CERC-CVC2.0, 2016-2020).
Author information
Authors and Affiliations
Contributions
L.W. conceived the idea and led the project. L.X. performed the experiments and analyzed the data; Z.C. and D.R. mined and analyzed the data; Prof. G.T., Dr. K.A. and Prof. X.H. gave the suggestions and fully supported during the whole process. All authors together prepared and reviewed the manuscript. We would also like to thank the anonymous reviewers for their valuable comments and suggestions.
Corresponding authors
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Xie, L., Ren, D., Wang, L. et al. A Facile Approach to High Precision Detection of Cell-to-Cell Variation for Li-ion Batteries. Sci Rep 10, 7182 (2020). https://doi.org/10.1038/s41598-020-64174-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-64174-2
- Springer Nature Limited
This article is cited by
-
Mg–Al hydroxide intercalated ionic liquids for quasi-solid-state lithium batteries
Journal of Solid State Electrochemistry (2023)