Abstract
Intensity-modulated radiotherapy with simultaneous integrated boost (IMRT-SIB) reduces overall treatment duration and results in less radiotherapy (RT)-induced dermatitis. However, the use of traditional sequential approach or IMRT-SIB is still under debate since there is not enough evidence of long-term clinical outcomes. The present study investigated 216 patients who underwent breast conserving surgery (BCS) between 2010 and 2013. The median age was 51 years (range, 21–81 years). All patients received IMRT-SIB, 50.4 Gy at 1.8 Gy per fraction to the whole breast and 60.2 Gy at 2.15 Gy per fraction to the tumor bed by integral boost. Among 216 patients, 175 patients received post-operative RT with forward IMRT and 41 patients had Tomotherapy. The median follow-up was 6.4 years. Forty patients (97.6%) in the Tomotherapy arm and 147 patients (84%) in the IMRT arm developed grade 0–1 skin toxicity (P = 0.021). For the entire cohort, the 5-year and 7-year overall survival (OS) rates were 94.4% and 93.1% respectively. The 7-year distant metastasis-free survival rates were 100% vs 89.1% in the Tomotherapy and IMRT arm respectively (P = 0.028). In conclusion, Tomotherapy improved acute skin toxicity compared with forward IMRT-SIB. Chronic skin complication was 1.9%. IMRT-SIB resulted in good long-term survival.
Similar content being viewed by others
Introduction
Breast cancer is the most commonly diagnosed cancer and the leading cause of cancer death in women globally1. The long-term survival of women with early breast cancer who were treated with breast-conserving surgery (BCS) and postoperative radiotherapy (RT) was the same when matched with the rate among women who underwent radical mastectomy2,3. The 20-year overall (OS) and breast-cancer-specific survival (CSS) rates were similar in the two groups3. Furthermore, additional RT boost to the surgical bed is found to improve 10-year local tumor control4. The most common adjuvant RT after BCS is conventionally administered in a 7–8 week period, with doses of 1.8–2.0 Gy per fraction to a total dose of approximately 50 Gy followed by a sequential boost irradiation of 10–16 Gy to the tumor bed.
Since the last decade, there has been an emerging role of hypofractionated RT for patients with breast cancer after BCS5,6. Hypofractionation uses a lower total dose and reduces acute toxicity compared with conventional schedules7,8,9. With technology advancement, intensity-modulated radiotherapy (IMRT) integrates the boost concept in the daily radiation sessions by increasing the dose per fraction within the boost volume10. This is the so-called IMRT with simultaneous integrated boost (SIB). SIB with more homogeneous dose distributions has been implemented in clinical routines nowadays11. The rationale of IMRT-SIB is the reduction of overall treatment duration in view of less RT-induced side effect; nonetheless, the use of sequential or SIB in patients treated with hypofractionated RT is still under debate since there is not enough evidence of long-term survival.
To the best of our knowledge, no evaluation of long-term survival between forward and inverse IMRT-SIB has been reported. We previously demonstrated that conventional RT with sequential boost for post-operative treatment of breast cancer resulted in more severe acute dermatological toxicity compared to IMRT-SIB12. The endpoints of this study are the long-term survival rates after forward IMRT-SIB or inverse IMRT-SIB performed by Tomotherapy.
Methods
Patients
This retrospective study comprised 216 consecutive female patients who were diagnosed with pathologically-proven breast cancer between March 2010 and June 2013. The exclusion criteria included excluding patients with synchronous bilateral breast cancer, or a history of previous irradiation to the thorax, or neo-adjuvant chemotherapy. We staged all patients by the 2010 TNM classification system (AJCC 7)13 and collected the data regarding post-RT acute and chronic skin reaction, date of diagnosis, adjuvant chemotherapy, hormonal treatment, RT treatment planning, pathological reports including primary surgery, hormonal receptor status and Human epidermal growth factor receptor 2 (Her2) over-expression status. The study was approved by the Ethical and Research Committee in the university hospital (KMUHIRB-E(I)- 20190053) and it was conducted under compliance of the Institutional Review Board regulations in accordance with the Helsinki Declaration of 1975 as revised in 1983. All the patients provided written informed consent for treatment prior to surgery and RT. All data approved by the Ethical committee were anonymized and de-identified for analysis.
Radiotherapy
All patients in this study were treated with a plan that integrated both breast and boost beams individually designed for herself. The fractionation schemes were 60.2 Gy to the tumor bed and 50.4 Gy to the whole breast. Such scheme was biologically equivalent to the traditional sequential boost-technique consisting of 50 Gy to the whole breast followed by a boost irradiation of 12 Gy in 6 fractions, using an alpha/beta ratio of 4 Gy for tumor response14. We recorded acute and chronic skin reactions during routine follow-up in accordance with the Common Terminology Criteria of Adverse Events version 4.03 (CTCAE v4.03).
After all organs at risks (OAR) and region of interest were contoured manually from axial-computed tomography (CT) images12, we utilized the Hi-Art helical Tomotherapy, version 2.2.4.1 (TomoTherapy, Inc., Madison, WI) unit or Eclipse, version 8.6 (Varian medical Systems Inc., Palo Alto, USA) to make IMRT-SIB treatment plans. IMRT were planned forwardly or inversely. We covered the PTV with the 95% iso-dose line, and minimized the volumes receiving higher than 110% of the dose prescribed to the PTV. Dose volume constraints for OAR were: whole lung V20Gy <20% and heart V25Gy <10%. Tomotherapy combines a rotational inverse IMRT with a translational movement of the couch15,16. Volumetric arc planning was not used.
Systemic therapy
The patients with either node-positive disease or high risk node-negative tumors received adjuvant chemotherapy after BCS. Based on tumor size, grading, hormonal receptor status and age, the risk was determined individually at the discretion of the physician. The chemotherapy regimen, adjuvant hormonal therapy and the use of Trastuzumab were detailed in our previous report12.
Statistical analysis
Firstly, we used Pearson’s chi-square test for categorical variables or Student’s t-test for continuous variables to compare the demographic characteristics and clinical variables between Tomotherapy and IMRT. Then we performed multiple logistic regressions to compute the adjusted ORs and 95% CIs with SPSS software package, version 20.0 for Windows (SPSS, Chicago, IL, USA). P <0.05 was considered statistically significant.
Results
The median age of this retrospective cohort was 51 years (range, 21–81 years). Table 1 summarizes the clinical characteristics of the 216 patients, divided by planning method into IMRT and Tomotherapy. The median follow-up was 6.4 years (range: 476 days – 2868 days). Forty-one patients (19%) received IMRT-SIB via Tomotherapy and 175 patients (81%) underwent IMRT-SIB. No significant difference was observed in terms of age, laterality, pathological tumor or nodal classification, pathological stage, hormonal receptors, the addition of chemotherapy or hormonal therapy, Her2 over-expression, surgical margin, V20 for whole lung, or chronic dermatological complications. Ductal carcinoma was found in 70.7% and 85.1% of Tomotherapy and IMRT arms respectively (P = 0.04). Both arms had acceptable V25 to the heart, yet the median V25 to the heart was smaller in the IMRT arm (P = 0.004).
Acute and chronic skin toxicity
For the entire cohort, 187 patients (86.6%) had grade 0–1 acute RT-induced dermatitis. Twenty-three patients (13.1%) in the IMRT-SIB arm and 10 patients (24.4%) in the Tomotherapy arm had grade 0 dermatitis. Most of the patients who developed RT-induced dermatitis had acute grade 1 erythema during RT. In the majority of cases, 124 patients (70.9%) in the IMRT-SIB arm and 30 patients (73.2%) in the Tomotherapy arm had grade 1 dermatitis. Among 216 patients, only one patient (0.6%) had grade 3 acute toxicity. This patient was in the IMRT group. There was no grade 4 toxicity. All patients in the Tomotherapy group experienced grade 0–2 toxicity, with no cases ≥ grade 3. Forty patients (97.6%) in the Tomotherapy arm and 147 patients (84%) in the IMRT arm developed grade 0–1 skin toxicity (P = 0.021). Less patients suffered from grade 2–3 RT-induced dermatitis (P = 0.021) in the Tomotherapy arm. Twenty-seven patients (15.4%) in the IMRT-SIB arm and 1 patient (2.4%) in the Tomotherapy arm developed grade 2 dermatitis.
Chronic grade 1 skin toxicity was recorded in four patients (1.9%). They had grade 1 late effect such as induration or fibrosis without telangiectasia. One patients in the Tomotherapy arm and 3 patients in the IMRT arm developed late grade 1 skin toxicity (P = 0.572).
Survival
Table 2 shows the survival rates and the comparison between IMRT and Tomotherapy. For the entire cohort, the 5-year and 7-year OS rates were 94.4% and 93.1% respectively. Figures 1A–C are the Kaplan-Meier curves of OS, cancer specific survival (CSS) and distant metastasis-free survival (DMFS) divided by T classification (T0–1 versus T2–4). In Table 3, univariate analysis suggested that pT0–1, pN0–1, pathological stage 0–1, ER (+), PR (+), and the use of hormone therapy were favorable prognostic factors for longer OS. After controlling for significant covariables in a multivariable model, pathological stage and ER (+) were associated with improved OS.
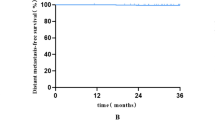
(A) Kaplan-Meier curves of overall survival according to tumor size (Log Rank test, P = 0.008). (B) Kaplan-Meier curves of distant metastasis-free survival according to tumor size (Log Rank test, P < 0.001). (C) Kaplan-Meier curves of cancer-specific survival according to tumor size (Log Rank test, P < 0.001). (D) Kaplan-Meier curves of distant metastasis-free survival according to radiotherapy modality (Log Rank test, P = 0.028).
Since the 7-year (100% vs 89.1%, P = 0.028, Log rank; Fig. 1D) DMFS in Tomotherapy was significantly longer than that in the IMRT arm, we performed Cox regression as shown in Table 4, illustrating that the pathological stage 0–1 (hazard ratio [HR], 6.974; 95% CI, 2.471 to 19.681; P < 0.001) was an independent favorable prognostic factor for DMFS in multivariate analysis. Tomotherapy did not confer a significant DMFS benefit in multivariate analysis (P = 0.971).
Discussion
To the best of our knowledge, the present study is the first to evaluate the differences of survival rates longer than five years between forward IMRT-SIB and inverse IMRT-SIB via Tomotherapy. Medical physicists specified beam parameters and manually optimized them in forward-planned IMRT which involves multi-leaf collimators to create a nonuniform fluence. On the other hand, inverse-planned IMRT uses optimization algorithms to create fluence maps and shape dose distributions17. Many researchers have discovered the merits of IMRT, SIB or Tomotherapy for lessening skin reaction and have reported safe short-term toxicity profiles9,18,19,20. More than a decade ago, Pignol et al. had documented that breast IMRT significantly reduced the occurrence of moist desquamation compared with the traditional wedged technique21. More recent methods to optimize the delivery of ionizing radiation have included three-dimensional conventional RT (3D-CRT) incorporating SIB, IMRT-SIB, VMAT-SIB and Tomotherapy22,23. IMRT plans reduce the unwanted excessive dose to the breast compared with the conventional photon boost plan, especially for the patient with a deep-seated tumor24,25,26. Increasing relevant evidence has been generated to consider SIB as an alternative to traditional sequential techniques27.
Hammer et al., reported that when 3D-CRT incorporated SIB, chronic grade 2 fibrosis was observed in 13.4% of 546 patients28. De rose et al. reported a phase II trial of 787 patients that used VMAT-SIB technique to the whole breast and tumor bed in 15 fractions, for a total dose of 40.5 and 48 Gy29. At the end of RT in their study, acute skin toxicity was grade 1 in 51.1% of all patients, and grade 2 in 9.7%. In the present study of IMRT-SIB, 71.3% of all patients had acute grade 1 and 13% had grade 2. At two years of follow-up, De rose et al. noted chronic grade 1 in 13.5% of patients. The chronic skin complication rate in our study after a median follow-up of 6.4 years was 1.9%, with no cases ≥grade 2. Milder acute dermatitis was observed in the Tomotherapy arm (P = 0.021).
In the aspect of survival, McDonald et al. compared IMRT with conventional 3D-CRT. They reported no statistically significant difference in OS, CSS, or recurrence, DMFS, late toxicity, or second malignancies after a median follow-up of 6.3 years30. Furthermore, the same team utilized IMRT-SIB, delivering 1.8 Gy to surrounding breast tissue and 2.14 Gy to the surgical bed simultaneously, yielding a breast dose of 45 Gy in 25 fractions and cavity dose of 59.92 Gy in 28 fractions31. This is similar to our SIB regimen of 60.2 Gy and 50.4 Gy in 28 fractions.
Until recently, there was no long-term result from prospective randomized trials regarding IMRT-SIB32. The present study demonstrated 5-year and 7-year OS, DMFS and CSS from forward IMRT-SIB and inverse IMRT-SIB via Tomotherapy. In the present study, positive ER (HR, 0.259; 95% CI, 0.093 to 0.725; P = 0.010) was an independent favorable prognostic factor and having pathological stage 2–4 (HR, 3.223; 95% CI, 1.131 to 9.182; P = 0.028) was an independent unfavorable prognostic factor. The mechanism for the significantly longer 7-year DMFS in Tomotherapy arm is unclear (100% vs 89.1%, P = 0.028), since there were more infiltrating ductal carcinomas (IDC) in the IMRT arm (85.1% vs 70.7%, P = 0.04). Other pathological types included infiltrating lobular carcinoma (ILC) and mucinous carcinomas were more common in the Tomotherapy arm. Chen et al. reported that the prognosis of ILC is poorer than that of IDC33. They found higher percentages of metastatic lymphadenopathy and distant metastases in ILC. Some studies have reached different conclusions and documented that ILC had better prognosis than IDC34,35. One study suggested that ILC had higher risk of metastatic disease36. Our multivariable analysis revealed that only pathological stages 0–1 (HR, 6.974; 95% CI, 2.471 to 19.681; P < 0.001) was an independent favorable prognostic factor for DMFS (Table 4).
SIB delivers different doses to different target volumes within a single RT fraction15. It reduces the overall treatment time and lowers the expense for patients37. We believe SIB is more economically efficient in terms of time and money. Tomotherapy appears to improve target coverage while sparing OAR because of its high conformity; when paired with SIB, it maintains the ability to deliver adequate dose coverage38. Studies have reported that helical Tomotherapy avoided unnecessary breast overdose while improving ipsilateral lung dosimetry16,38; furthermore, static ports of Tomotherapy in TomoDirect were proven to prevent unwanted dosages to the surrounding normal tissues39. Tomotherapy significantly reduced cardiac doses and slightly increases in dosage to other tissues in left-sided breast cancer patients with poor cardiac anatomy17,20,40,41,42. Mean heart dosage is a good prognosticator to monitor the heart sequelae43. In the present study, mean dosage to the heart was 4.9 Gy in the entire cohort, and the median V25 Gy to the heart was 5.3% and 3.1% in Tomotherapy and IMRT respectively (P = 0.04) Such difference did not affect OS, DMFS or CSS as shown in Table 2. We will continue to follow this cohort.
The present study has the inter-observer variability, since the physicians involved in the toxicity scoring were not blinded; besides, helical Tomotherapy rather than TomoDirect was utilized due to institutional facility restrictions. Most of all, obvious limitation is its retrospective nature.
Conclusions
In the setting of IMRT-SIB, Tomotherapy improved acute skin toxicity compared with forward IMRT-SIB. Chronic skin complications reached 1.9%. Both forward IMRT-SIB and inverse IMRT-SIB via Tomotherapy resulted in good 5-year and 7-year survival. Longer follow-up is intended.
Data availability
The data used to support the findings of this study are included within the article.
References
Bray, F. et al. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians 68, 394–424, https://doi.org/10.3322/caac.21492 (2018).
Fisher, B. et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. The New England journal of medicine 347, 1233–1241, https://doi.org/10.1056/NEJMoa022152 (2002).
Veronesi, U. et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. The New England journal of medicine 347, 1227–1232, https://doi.org/10.1056/NEJMoa020989 (2002).
Bartelink, H. et al. Impact of a Higher Radiation Dose on Local Control and Survival in Breast-Conserving Therapy of Early Breast Cancer: 10-Year Results of the Randomized Boost Versus No Boost EORTC 22881-10882 Trial. Journal of Clinical Oncology 25, 3259–3265, https://doi.org/10.1200/jco.2007.11.4991 (2007).
Whelan, T. J. et al. Long-Term Results of Hypofractionated Radiation Therapy for Breast Cancer. New England Journal of Medicine 362, 513–520, https://doi.org/10.1056/NEJMoa0906260 (2010).
Haviland, J. S. et al. The UK Standardisation of Breast Radiotherapy (START) trials of radiotherapy hypofractionation for treatment of early breast cancer: 10-year follow-up results of two randomised controlled trials. The Lancet Oncology 14, 1086–1094, https://doi.org/10.1016/S1470-2045(13)70386-3 (2013).
Lightowlers, S. V. et al. Preoperative breast radiation therapy: Indications and perspectives. European Journal of Cancer 82, 184–192, https://doi.org/10.1016/j.ejca.2017.06.014 (2017).
Mu, J., Xi, D., Ding, Y., Gu, W. & Li, Q. Chair Heterogeneity Index: Describing the dose heterogeneity inside the tumor volume where there is a boost volume. Scientific reports 8, 9763, https://doi.org/10.1038/s41598-018-28110-9 (2018).
Yee, C. et al. Radiation-induced Skin Toxicity in Breast Cancer Patients: A Systematic Review of Randomized Trials. Clin. Breast Cancer 18, e825–e840, https://doi.org/10.1016/j.clbc.2018.06.015 (2018).
Fiorentino, A. et al. Three-dimensional conformal versus intensity modulated radiotherapy in breast cancer treatment: is necessary a medical reversal? La Radiologia medica 122, 146–153, https://doi.org/10.1007/s11547-016-0700-z (2017).
Leonardi, M. C. et al. From technological advances to biological understanding: The main steps toward high-precision RT in breast cancer. The Breast 29, 213–222, https://doi.org/10.1016/j.breast.2016.07.010 (2016).
Lee, H. H. et al. Intensity modulated radiotherapy with simultaneous integrated boost vs. conventional radiotherapy with sequential boost for breast cancer - A preliminary result. Breast (Edinburgh, Scotland) 24, 656–660, https://doi.org/10.1016/j.breast.2015.08.002 (2015).
Edge, S. B. & Compton, C. C. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Annals of surgical oncology 17, 1471–1474, https://doi.org/10.1245/s10434-010-0985-4 (2010).
Joiner, M. & Bentzen, S. In Basic clinical radiobiolog (eds. M. C., Joiner & A. van der Kogel) Ch. 8, 102–118 (Hodder Arnold, 2009).
Van Parijs, H. et al. Breast Conserving Treatment for Breast Cancer: Dosimetric Comparison of Sequential versus Simultaneous Integrated Photon Boost. Bio. Med. Research International 2014, 8, https://doi.org/10.1155/2014/827475 (2014).
Hijal, T. et al. Simultaneous integrated boost in breast conserving treatment of breast cancer: A dosimetric comparison of helical tomotherapy and three-dimensional conformal radiotherapy. Radiotherapy and Oncology 94, 300–306, https://doi.org/10.1016/j.radonc.2009.12.043 (2010).
Michalski, A. et al. A dosimetric comparison of 3D-CRT, IMRT, and static tomotherapy with an SIB for large and small breast volumes. Med. Dosim 39, 163–168, https://doi.org/10.1016/j.meddos.2013.12.003 (2014).
Bautista Hernandez, M. Y., Lujan Castilla, P. J. & Quezada Bautista, A. A. Hypofractionation with concomitant boost using intensity-modulated radiation therapy in early-stage breast cancer in Mexico. Reports of practical oncology and radiotherapy: journal of Greatpoland Cancer Center in Poznan and Polish Society of Radiation Oncology 23, 276–283, https://doi.org/10.1016/j.rpor.2018.06.006 (2018).
Yuen, C. Y. et al. Dosimetric comparison of simultaneous integrated boost versus concomitant electron boost in radiotherapy treatment of breast cancer. Journal of Radiotherapy in Practice 16, 334–341, https://doi.org/10.1017/S1460396917000127 (2017).
Wadasadawala, T. et al. First clinical report of helical tomotherapy with simultaneous integrated boost for synchronous bilateral breast cancer. The British journal of radiology 90, 20170152, https://doi.org/10.1259/bjr.20170152 (2017).
Pignol, J.-P. et al. A Multicenter Randomized Trial of Breast Intensity-Modulated Radiation Therapy to Reduce Acute Radiation Dermatitis. Journal of Clinical Oncology 26, 2085–2092, https://doi.org/10.1200/jco.2007.15.2488 (2008).
Huang, C.-J. & Lee, H.-H. Radiotherapy benefits outweigh risks for breast cancer in modern era. Translational Cancer Research; Vol 6, Supplement 6 (August 2017): Translational Cancer Research (2017).
Bantema-Joppe, E. J. et al. Five year outcomes of hypofractionated simultaneous integrated boost irradiation in breast conserving therapy; patterns of recurrence. Radiother Oncol 108, 269–272, https://doi.org/10.1016/j.radonc.2013.08.037 (2013).
Guerrero, M., Li, X. A., Earl, M. A., Sarfaraz, M. & Kiggundu, E. Simultaneous integrated boost for breast cancer using IMRT: a radiobiological and treatment planning study. Int J Radiat Oncol Biol Phys 59, 1513–1522, https://doi.org/10.1016/j.ijrobp.2004.04.007 (2004).
Harsolia, A. et al. Intensity-Modulated Radiotherapy Results in Significant Decrease in Clinical Toxicities Compared With Conventional Wedge-Based Breast Radiotherapy. International journal of radiation oncology, biology, physics 68, 1375–1380 (2007).
Hurkmans, C. W., Meijer, G. J., van Vliet-Vroegindeweij, C., van der Sangen, M. J. & Cassee, J. High-dose simultaneously integrated breast boost using intensity-modulated radiotherapy and inverse optimization. International journal of radiation oncology, biology, physics 66, 923–930 (2006).
Hamilton, D. G. et al. Impact of tumour bed boost integration on acute and late toxicity in patients with breast cancer: A systematic review. Breast (Edinburgh, Scotland) 27, 126–135, https://doi.org/10.1016/j.breast.2016.03.002 (2016).
Hammer, C. et al. Radiation-induced fibrosis in the boost area after three-dimensional conformal radiotherapy with a simultaneous integrated boost technique for early-stage breast cancer: A multivariable prediction model. Radiother Oncol 122, 45–49, https://doi.org/10.1016/j.radonc.2016.10.006 (2017).
De Rose, F. et al. Hypofractionation with simultaneous boost in breast cancer patients receiving adjuvant chemotherapy: A prospective evaluation of a case series and review of the literature. Breast (Edinburgh, Scotland) 42, 31–37, https://doi.org/10.1016/j.breast.2018.08.098 (2018).
McDonald, M. W., Godette, K. D., Butker, E. K., Davis, L. W. & Johnstone, P. A. Long-term outcomes of IMRT for breast cancer: a single-institution cohort analysis. Int. J. Radiat. Oncol. Biol. Phys. 72, 1031–1040, https://doi.org/10.1016/j.ijrobp.2008.02.053 (2008).
McDonald, M. W., Godette, K. D., Whitaker, D. J., Davis, L. W. & Johnstone, P. A. S. Three-Year Outcomes of Breast Intensity-Modulated Radiation Therapy With Simultaneous Integrated Boost. International journal of radiation oncology, biology, physics 77, 523–530 (2010).
Van Parijs, H. et al. Short course radiotherapy with simultaneous integrated boost for stage I-II breast cancer, early toxicities of a randomized clinical trial. Radiat Oncol 7, 80, https://doi.org/10.1186/1748-717x-7-80 (2012).
Chen, Z. et al. Invasive lobular carcinoma of the breast: A special histological type compared with invasive ductal carcinoma. PLoS One 12, e0182397, https://doi.org/10.1371/journal.pone.0182397 (2017).
Cristofanilli, M. et al. Invasive lobular carcinoma classic type: response to primary chemotherapy and survival outcomes. Journal of clinical oncology: official journal of the American Society of Clinical Oncology 23, 41–48, https://doi.org/10.1200/jco.2005.03.111 (2005).
DiCostanzo, D., Rosen, P. P., Gareen, I., Franklin, S. & Lesser, M. Prognosis in infiltrating lobular carcinoma. An analysis of “classical” and variant tumors. The American journal of surgical pathology 14, 12–23 (1990).
Rakha, E. A. et al. Invasive lobular carcinoma of the breast: response to hormonal therapy and outcomes. European journal of cancer (Oxford, England: 1990) 44, 73–83, https://doi.org/10.1016/j.ejca.2007.10.009 (2008).
Franco, P. et al. Tumor Bed Boost Integration during Whole Breast Radiotherapy: A Review of the Current Evidence. Breast Care (Basel) 10, 44–49, https://doi.org/10.1159/000369845 (2015).
Chira, C. et al. Helical tomotherapy for inoperable breast cancer: a new promising tool. Biomed Res Int 2013, 264306, https://doi.org/10.1155/2013/264306 (2013).
Franco, P. et al. Intensity-modulated and hypofractionated simultaneous integrated boost adjuvant breast radiation employing statics ports of tomotherapy (TomoDirect): a prospective phase II trial. J Cancer Res Clin Oncol 140, 167–177, https://doi.org/10.1007/s00432-013-1560-8 (2014).
Coon, A. B. et al. Tomotherapy and Multifield Intensity-Modulated Radiotherapy Planning Reduce Cardiac Doses in Left-Sided Breast Cancer Patients With Unfavorable Cardiac Anatomy. International Journal of Radiation Oncology*Biology*Physics 78, 104–110, https://doi.org/10.1016/j.ijrobp.2009.07.1705 (2010).
Liem, X. et al. Preliminary results of whole breast helical tomotherapy with simultaneous integrated boost in the adjuvant treatment of breast cancer. Cancer Radiother 18, 15–22, https://doi.org/10.1016/j.canrad.2013.07.149 (2014).
Wojcieszynski, A. P., Olson, A. K., Rong, Y., Kimple, R. J. & Yadav, P. Acute Toxicity From Breast Cancer Radiation Using Helical Tomotherapy With a Simultaneous Integrated Boost. Technol Cancer Res Treat https://doi.org/10.1177/1533034615574387 (2015).
Darby, S. C. et al. Risk of Ischemic Heart Disease in Women after Radiotherapy for Breast Cancer. New England Journal of Medicine 368, 987–998, https://doi.org/10.1056/NEJMoa1209825 (2013).
Acknowledgements
We wish to acknowledge our assistant Ms. Jin-Mei Pan for her help in artwork for this manuscript. This work was supported financially by the Center for Cancer Research, Kaohsiung Medical University (KMU-TC108A04), the Ministry of Science and Technology [MOST 108-2314-B-037-021-MY3], and Kaohsiung Medical University Hospital (KMUH108-8R66) in Taiwan. The funding source had no role in the study design; in the collection, analysis and interpretation of data; in the writing of the report and in the decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
H.H.L., C.H.C. and M.Y.H. participated in the design of the study, collection and interpretation of data. H.H.L. drafted and revised the manuscript. C.J.H., Y.K.C., F.C., H.H.L., M.Y.H. and C.H.C. are Radiation Oncologists who enrolled and treated the patients. C.H.C., K.H.L. and H.Y.C. analyzed and interpreted the data. S.H.K. is the Medical Physicist that ensures the quality of treatment planning for all cases. All authors critically reviewed and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lee, HH., Chen, CH., Luo, KH. et al. Five-year survival outcomes of intensity-modulated radiotherapy with simultaneous integrated boost (IMRT-SIB) using forward IMRT or Tomotherapy for breast cancer. Sci Rep 10, 4342 (2020). https://doi.org/10.1038/s41598-020-61403-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-61403-6
- Springer Nature Limited