Abstract
The adenosine A2B receptor is a critical protein in intestinal water secretion. In the present study, we screened compound libraries to identify inhibitors of the A2B receptor and evaluated their effect on adenosine-induced intestinal fluid secretion. The screening identified the dihydropyridine calcium antagonists nifedipine and nisoldipine. Their respective affinities for the A2B receptor (Ki value) were 886 and 1,399 nM. Nifedipine and nisoldipine, but not amlodipine or nitrendipine, inhibited both calcium mobilization and adenosine-induced cAMP accumulation in cell lines. Moreover, adenosine injection into the lumen significantly increased fluid volume in the colonic loop of wild-type mice but not A2B receptor-deficient mice. PSB-1115, a selective A2B receptor antagonist, and nifedipine prevented elevated adenosine-stimulated fluid secretion in mice. Our results may provide useful insights into the structure–activity relationship of dihydropyridines for A2B receptor. As colonic fluid secretion by adenosine seems to rely predominantly on the A2B receptor, nifedipine could be a therapeutic candidate for diarrhoea-related diseases.
Similar content being viewed by others
Introduction
Adenosine is an important mediator of multiple functions, such as intestinal secretion, contraction, inflammation, and sensation in the gastrointestinal tract1,2,3. Among adenosine receptors, the A2B receptor is highly expressed in the colon and has critical roles in pathological conditions4. In general, adenosine levels and A2B receptor expression are low under normal conditions, but increase in response to ischemia, inflammation, and tissue injuries. The activated A2B receptor stimulates intestinal water secretion and sensation or modulates inflammatory response and colonic motility5,6. As a result, it is associated with the development of gastrointestinal diseases, such as irritable bowel syndrome (IBS), secretary diarrhoea, and inflammatory bowel disease7,8. Therefore, this receptor has attracted substantial attention as a therapeutic target. Although pharmacological and molecular tools, including genetically modified mouse models, have revealed multiple functions of the A2B receptor, such as immunomodulation, relaxation of smooth muscle, and intestinal secretion5,9, our understanding of its biology remains unclear.
Diarrhoea is caused by bacterial and viral infections, inflammatory processes, drugs, genetic disorders, and abnormal intestinal secretion or electrolyte absorption10,11. However, the pathophysiology of diarrhoea is not fully understood. Chloride secretion into the lumen following activation of the cystic fibrosis transmembrane conductance regulator (CFTR) channel in intestinal epithelial cells plays a crucial role in secretory diarrhoea1,12. Several studies suggest that A2B receptor activation causes fluid secretion into the lumen. In vitro studies using colonic epithelial cells have demonstrated that adenosine increases luminal water volume through apical or basolateral A2B receptors13,14. Although both cAMP activation via Gs proteins and the intracellular calcium pathway contribute to chloride or water secretion in epithelial cells, chloride release is most likely due to cAMP elevation following A2B receptor activation1,15. A recent report has shown that A2B receptor-mediated direct activation of the CFTR channel is associated with water secretion in epithelial cells12. Nevertheless, evidence that the A2B receptor modulates fluid secretion has remained limited, in part because of a lack of appropriate pharmacological agents or genetically modified mice.
Recently, we reported that blockade of the A2B receptor could be beneficial for diarrhoea-predominant IBS8. Inhibition of the receptor by pharmacological agents ameliorated colonic hypersensitivity and stress-induced defecation in rodent models of IBS. In the present study, we screened a library of approved medicinal compounds for candidate drugs that could suppress adenosine A2B receptor activation. We also determined the role of various adenosine receptor subtypes involved in colonic fluid secretion induced by luminal adenosine and examined the effect of the candidate drug on fluid secretion in mice.
Results
Identification of nifedipine through screening for adenosine A2B receptor binding compounds
Screening of a compound library identified 12 candidate drugs with binding affinity for the A2B receptor (Supplementary Table 1). Of these, three were already known, four had a xanthine-like structure, and two were dihydropyridine calcium channel blockers. We focused on the latter, nifedipine and nisoldipine, because dihydropyridine is similar to the structure of BAY60-6583, a known adenosine A2B receptor agonist (Fig. 1). Nifedipine inhibited [3H]-DPCPX binding to the A2B receptor membrane in a dose-dependent manner and its Ki value was 886 ± 514 nM (Fig. 2a). We also examined the binding activity of other dihydropyridine calcium channel blockers and Fig. 1 summarizes the results. Nisoldipine but not nitrendipine and nilvadipine significantly suppressed [3H]-DPCPX binding, whereas isradipine and amlodipine exhibited only weak binding to the A2B receptor (Fig. 2a). The Ki values of nisoldipine and isradipine were 1.40 ± 0.46 µM and 11.47 ± 4.39 µM, respectively. We confirmed the effect of nifedipine on adenosine-induced calcium mobilization in CHO cells stably expressing the A2B receptor. Dose-dependent calcium mobilization by adenosine was observed, with fluorescence being nearly maximal with 10 µM of adenosine (data not shown). As shown in Fig. 2b,c, adenosine caused immediate calcium mobilization while nifedipine dose-dependently blocked it but did not stimulate it. Nisoldipine had a similar effect, whereas amlodipine and nitrendipine did not. The IC50 values of nifedipine and nisoldipine were 42.4 µM and 34.0 µM, respectively.
Summary of the compounds. ‘Ki (A2B)’ values are the [3H]-DPCPX binding assay results and ‘cAMP’ values are the adenosine-induced cAMP assay results. Pharmacophore analysis of the compounds was performed. Green, blue, and orange circles represent the hydrogen donor site binding His2807.43, a hydrogen donor with side-chain binding to Tyr101.35, and the common –OCH3 group close to Ser2797.42, respectively. Green cross ‘X’ represents the mismatch between the ligand NO2 group and the pharmacophore. #, the values of BAY60-6583 were adopted from van der Hoeven et al.44.
Binding affinity of dihydropyridine calcium blockers for the A2B receptor and effect on calcium mobilization. (a) Curves generated from competition displacement experiments in HEK293 cells expressing human adenosine A2B receptor in the presence of [3H]-DPCPX and various calcium antagonists (nifedipine, nisoldipine, amlodipine, isradipine, nitrendipine, and nilvadipine). Data represent the average of three independent experiments. (b) Calcium mobilization assay in CHO cells stably expressing both human adenosine A2B receptor and Gα15 protein and incubated with various concentrations of nifedipine. Cells were stimulated by injection with 1 µM adenosine (Ado) solution or HBSS. Calcium mobilization (∆F/F) was calculated based on fluorescence intensity readings. (c) Calcium mobilization assay in the presence of various concentrations of nisoldipine, amlodipine, and nitrendipine. All data are expressed as mean ± SEM. [3H]-DPCPX, 8-cyclopentyl-1,3-dipropylxanthine, [dipropyl-2,3-3H(N)].
Effect of dihydropyridine calcium blockers on adenosine-induced cAMP production in intestinal epithelial cells
Next, we examined the effect of nifedipine on intracellular cAMP levels in colonic epithelial T84 cells. Fluid secretion and dominant A2B receptor expression have already been reported in this cell line13. First, we characterized the cells’ cAMP response to adenosine agonists. Adenosine or BAY60-6583 (a specific A2B receptor agonist) induced cAMP accumulation in a significant dose-dependent manner, whereas a selective A2B antagonist, PSB-1115, suppressed adenosine-produced cAMP elevation (IC50 = 84.0 nM) (Fig. 3a,b). Pharmacological analysis using specific agonists showed that CPA (A1 receptor) increased cAMP levels in T84 cells and both BAY60-6583 (A2B receptor) and IB-MECA (A3 receptor) induced modest stimulation, whereas CGS21680 (A2A receptor) had only a minor effect (Fig. 3b). cAMP elevation by CPA, CGS21680, and IB-MECA was completely blocked by PSB-1115 (Fig. 3c), suggesting that the observed effect was mediated by their non-specific binding to the A2B receptor. Thus, cAMP accumulation induced by adenosine in T84 cells likely involves the A2B receptor.
Responsiveness of cAMP production to adenosine receptor agonists in T84 cells. (a) Intracellular cAMP levels in confluent epithelial T84 cells incubated with various concentrations of either adenosine (Ado) or the selective A2B receptor antagonist PSB-1115 for 15 min. (b) Intracellular cAMP levels in confluent T84 cells incubated with various concentrations of the selective receptor agonists CPA (A1R), CGS21680 (A2AR), BAY60-6583 (A2BR), or IB-MECA (A3R). (c) Intracellular cAMP levels in confluent T84 cells incubated with various concentrations of selective receptor agonists (100 μM) and the A2B receptor antagonist PSB-1115 (100 nM). All data are expressed as mean ± SEM.
We then examined the effect of dihydropyridine calcium blockers on cAMP response in T84 cells. In the absence of adenosine, both nifedipine and nisoldipine slightly decreased intracellular cAMP levels, nitrendipine caused an increase, and amlodipine had no significant effect (Fig. 4a). In addition, nifedipine dose-dependently inhibited cAMP accumulation (Fig. 4b) induced by either adenosine (IC50 = 10.8 µM) or BAY60-6583 (IC50 = 21.5 µM). Nisoldipine, but not amlodipine or nitrendipine, exhibited similar dose-dependent inhibition of adenosine-induced cAMP levels (IC50 = 8.7 µM) (Fig. 4c). The results are summarized in Fig. 1. To determine whether nifedipine decreases intracellular cAMP levels independent of its A2B receptor blockade, we examined the effect of nifedipine on an adenylate cyclase activator, forskolin, which induced increases in cAMP levels. We observed no influence by nifedipine on elevated cAMP by the compounds, suggesting that adenosine-stimulated cAMP elevation is dependent on the A2B receptor (data not shown).
Effect of calcium blockers on adenosine-induced cAMP accumulation in T84 cells. (a) Intracellular cAMP levels in confluent T84 cells incubated with various concentrations of nifedipine, nisoldipine, amlodipine, or nitrendipine. (b) Intracellular cAMP levels in the presence of varying concentrations of adenosine (0–100 μM) or 10 µM BAY60-6583 for 15 min. (c) Intracellular cAMP levels in the presence of 100 µM adenosine (Ado) and various concentrations of nisoldipine, amlodipine, or nitrendipine. All data are expressed as mean ± SEM.
Effect of nifedipine on adenosine-induced colonic fluid secretion in mice
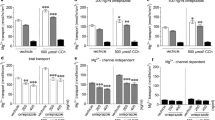
Finally, we investigated whether the A2B receptor stimulated intestinal fluid secretion and whether nifedipine suppressed it in vivo. First, we characterized fluid secretion induced by luminal adenosine in C57BL/6 mice. As shown in Fig. 5a, adenosine injection into the colonic lumen increased fluid content in a dose-dependent manner. Analysis of subtype-specific agonists revealed that only the adenosine A2B receptor agonist BAY60-6583 significantly increased luminal fluid content (Fig. 5b). Confirming this point, increased fluid production by luminal adenosine was absent from A2B receptor-knockout mice (Fig. 5c). These results suggest that stimulation of fluid secretion by luminal adenosine is dependent on the A2B receptor, and it was suppressed by nifedipine and PSB-1115 (Fig. 5d).
Involvement of adenosine A2B receptor and effectiveness of nifedipine in colonic fluid secretion in mice. (a) Representative image of colonic loop treated with vehicle (saline containing 2.5% DMSO) or 1 mM adenosine (Ado), and colonic fluid volume following injection of various doses of adenosine. (b) Effect of the selective adenosine receptor agonists CPA (A1R), CGS21680 (A2AR), BAY60-6583 (A2BR), and IB-MECA (A3R) on colonic fluid volume in C57/BL6 mice. (c) Adenosine-induced colonic fluid secretion in C57/BL6 and A2B receptor-deficient mice. (d) Effect of nifedipine or PSB-1115 on adenosine (1 mM)-induced colonic fluid secretion in mice. All data are expressed as mean ± SEM. *P < 0.05 or **P < 0.01 (vs. vehicle). #P < 0.05 or ##P < 0.01 (vs Ado in wild-type).
Discussion
Increased intestinal fluid secretion plays an important role in diarrhoea-related diseases such as IBS, and infection- or drug-induced diarrhoea10,11. Several studies have reported an association between adenosine A2B receptor and intestinal fluid secretion. Inhibition of the A2B receptor has been shown to be effective against IBS with diarrhoea in rodents8. To identify candidate A2B receptor inhibitors, we screened a library of medicines in clinical use. As their pharmacokinetics, pharmacodynamics, metabolism, tolerability, and toxicity are already known in humans, they can be relatively easily included in a pilot study16. Using a competitive binding assay, we identified four drugs with xanthine-like structures and three known A2B receptor binding compounds, including aminophylline, confirming the specificity of the screening (Supplementary Table 1). Considering that A2B receptor antagonists without a xanthine backbone had not been reported previously, we focused on two dihydropyridine calcium antagonists, nifedipine and nisoldipine, whose structure is similar to BAY60-6583, a selective A2B receptor agonist (Fig. 1). The inhibitory action of nifedipine and nisoldipine could reveal important structure–activity information.
Unlike other calcium antagonists (e.g., nitrendipine, amlodipine, nilvadipine, and isradipine), nifedipine and nisoldipine exhibited significant binding affinity for the A2B receptor. This property may be explained by the presence of an ortho-nitric oxide substitute on the benzene ring of nifedipine and nisoldipine that is absent from other compounds (Fig. 1). This nitric oxide group might be critical in preventing the binding of adenosine to the A2B receptor. To confirm the competition assay results, we performed a calcium mobilization test in CHO cells stably expressing the A2B receptor. Both nifedipine and nisoldipine inhibited but did not stimulate this calcium response, with the former exhibiting a stronger inhibitory action, in line with the competitive binding assay results. This result also suggested that both calcium blockers have antagonistic activity for A2B receptor.
As a mechanism of colonic fluid secretion by A2B receptor activation, intracellular cAMP elevation by Gs protein and mobilisation of intracellular calcium by Gq protein have been suggested1. Nichols et al. recently reported that cAMP elevation but not calcium mobilisation mediated by Gq-protein in T84 colonic epithelial cells predominantly contributes to secretary activity into the lumen through CFTR channel activation17. Therefore, we confirmed the antagonistic activities of nifedipine and nisoldipine on adenosine- or BAY60-6583-dependent activation in T84 cells using a cAMP accumulation assay. Again, amlodipine and nitrendipine showed no effect, consistent with the calcium assay results. We also determined a weak inhibition of intracellular cAMP levels by nifedipine and nisoldipine in the absence of adenosine stimulation. Amlodipine and nitrendipine did not inhibit adenosine-induced cAMP levels and showed a weak increase in cAMP without adenosine stimulation.
While the expression profiles of adenosine receptors and intestinal fluid secretion have been extensively characterized in T84 cells18, responsiveness to specific agonists for each adenosine receptor has not been examined. Here, we investigated the cAMP response to activation of each adenosine receptor. We speculated that cAMP production by adenosine receptor agonists originated predominantly from A2B receptor activation in the cells. In Fig. 3b, CPA, CGS21680, and IB-MECA caused cAMP elevation at a concentration of nearly 100 µM. However, we consider that these results are derived from non-specific A2B receptor binding of these agonists, because the Ki values of CPA, CGS21680, and IB-MECA for A2B receptors are 18,600 nM,>10,000 nM, and 11,000 nM, respectively19. In addition, elevated intracellular cAMP levels by these agonists were blocked by a selective A2B antagonist, PSB-1115 (Fig. 3c). Furthermore, a previous study showed low or undetectable expression of A1 and A3 receptors in T84 cells18. Thus, adenosine- or BAY60-6583-induced cAMP accumulation may be predominantly mediated by A2B receptor. Although we do not evaluate this in normal intestinal epithelial cells and cannot fully exclude the association with A2A receptor, activation of A2B receptor by binding of extracellular adenosine might cause an increase in intracellular cAMP levels through Gs protein, followed by CFTR channel activation in intestinal epithelial cells, leading to fluid secretion.
Moreover, we observed that both adenosine- and BAY60-6583-stimulated cAMP increases were blocked by nifedipine. The latter may bind to an active site shared by both agonists. Site-directed mutagenesis suggests that Phe1732.52 and Trp2476.48 on the A2B receptor are important for BAY60-6583 and adenosine binding20,21. The present docking study supported the above mutagenesis analysis. Namely, our docking model shows that these amino acid residues can form ionic interactions with nifedipine (Fig. 6). Therefore, Phe1732.52 and Trp2476.48 may be required for the interaction between nifedipine and the A2B receptor. The present docking study also suggested the pharmacophore model. Figure 1 shows the pharmacophore analysis of the calcium antagonists based on the present assay results. The NO group should be essential in the A2B binding and bind the NH of His2807.43. The ester parts indicated by the blue circles are common structures binding the OH group of Tyr101.35and the variation of the side chains suggests that these parts should locate toward the outside of the A2B receptor. The –OCH3 groups are common structures among these compounds, and are located towards the inside of the receptor, with no additional space. The pharmacophore analysis of the assay results and the docking results are consistent with each other.
Docking model of the interaction between human adenosine A2B receptor and nifedipine. Nifedipine is shown as a stick model, with carbon coloured in yellow, nitrogen in blue, hydrogen in white, and oxygen in red. Broken lines connecting Tyr101.35, Ala642.61, His2807.42, and Ser2797.42 represent possible hydrogen bonds (or CH…O interactions) with nifedipine. The distances of nifedipine from Tyr101.35, Ala642.61, Phe173ECL2.52, His2807.42, and Ser2797.42 were calculated to be 3.38 Å, 3.42 Å, 3.35 Å, 2.86 Å, and 3.54 Å, respectively, without taking account of hydrogens. The dihydropyridine of nifedipine can form a π–π stacking interaction with Phe173ECL2.52.
Finally, we sought to confirm the in vitro results by examining the effect of nifedipine on luminal adenosine-induced intestinal fluid secretion in mice. Specifically, we investigated the effect of adenosine receptor agonists on fluid secretion in the colon. Only BAY60-6583, an A2B receptor agonist, significantly increased intestinal fluid content (Fig. 5b). This finding was confirmed using A2B receptor-deficient mice. These results agreed with those for cAMP accumulation in T84 cells. To our knowledge, this is the first evidence that the colonic A2B receptor is predominantly involved in water secretion into the lumen. We confirmed that adenosine-induced increases in fluid contents were blocked by the selective A2B antagonist PSB-1115 as well as nifedipine, suggesting that the latter’s inhibitory action relied on blocking the A2B receptor. However, A2B receptor in the colon is also expressed in various cell types, such as enteric neurons, vascular cells, and mast cells18, which can indirectly contribute to water secretion in vivo. Thus, we need to examine this possibility using epithelial cell-specific A2B receptor-deficient mice in our next study. The dose required to inhibit fluid secretion by adenosine was higher for nifedipine than PSB-1115. This is supported by the fact that the affinity of nifedipine for the receptor was lower than that of PSB-1115. However, nifedipine possesses antagonistic calcium activity at a nanomolar level22, which may also contribute to the suppression of fluid secretion, a possibility we could not exclude.
Nifedipine is commonly used in medication. In general, anti-diarrhoea drugs can cause constipation in humans. Nifedipine treatment also seems to be associated with constipation23. According to adverse events from prescribing information, constipation in nifedipine users has been reported approximately 1% of patients (versus 0% in placebo). Previous reports have showed that nifedipine slows small intestinal transit or suppresses colonic motility in humans24,25. Although these results suggest the potential of nifedipine as an anti-diarrhoea agent, its A2B receptor antagonistic activity for intestinal epithelial cells as well as its calcium inhibitory activity in intestinal smooth muscle cells can contribute to the effects.
Our study has also some limitations. First, we did not assess the binding affinity of other adenosine receptor subtypes. We have recently conducted preliminary experiments to determine whether nifedipine binds to A1 and A2A receptors. We have found a weak affinity of nifedipine for both receptors (the Ki values of A1 and A2A receptors were 4.3 µM and 4.1 µM, respectively). Thus, we could not fully exclude the involvement of other receptor subtypes in the present study. Although our pharmacological studies using selective agonists indicate the specific involvement of the A2B receptor, experiments using genetically modified cells (e.g., A2B receptor-knockout T84 cells) or mice (e.g., epithelial cell-specific A2B receptor-deficient mice) could be beneficial in future studies. Second, future studies should investigate normal intestinal cell lines, as their receptor expression profiles and responsiveness to adenosine may be different from those of cancer cells. In addition, other types of fluid secretion assays such as the Ussing chamber assay and 2D or 3D secretion assays are necessary to validate our idea. Third, in the present study we did not determine the influence of nifedipine or PSB-1115 on luminal fluid contents in the absence of adenosine treatment. Because A2B receptor can be mainly activated by extracellular adenosine in pathophysiological conditions, we focused on these conditions in this study. However, it is important to investigate whether nifedipine or PSB-1115 affect fluid secretory activity in normal conditions or modulate absorption processes in luminal fluid dynamics, as fluid absorption from epithelial cells occurs before secretion. Here, we observed colonic fluid contents 2 h after adenosine injection. Thus, we could not exclude the possibility that adenosine A2B receptor suppresses absorption.
In summary, the identification of nifedipine, an already approved drug, as an inhibitor of the A2B receptor could facilitate its application in clinical trials as a therapeutic candidate for diarrhoea-related diseases. Moreover, modelling of its interaction with the receptor’s active site could drive the design of specific modifications that ameliorate its function.
Methods
Chemicals
Nifedipine, nisoldipine, nitrendipine, nilvadipine, and isradipine were obtained from Tokyo Chemical Industry (Tokyo, Japan) and Wako Pure Chemical Industries (Osaka, Japan). PSB-1115 was purchased from Santa Cruz Biotechnology. Amlodipine besylate (amlodipine), adenosine deaminase (ADA), N6-cyclopentyladenosine (CPA), forskolin, and adenosine were purchased from Sigma (St. Louis, MO). CGS21680, IB-MECA, and EHNA hydrochloride (EHNA) were obtained from Abcam (Cambridge, UK). BAY60-6583 was purchased from Tocris Bioscience (Bristol, UK). The compound library consisting of 1,241 existing medicines was a kind gift from LTT Bio-Pharma Co., Ltd (Tokyo, Japan).
Animals
Male C57/BL6N mice (9–12 weeks old, 22–28 g) and male adenosine A2B receptor (A2BAR)-deficient mice were purchased from Charles River Laboratories Japan and Deltagen Inc26, respectively. Animals were housed in controlled temperature (22–24 °C) and light (12-h light/12-h dark cycle) conditions for more than 4 days before experiments. The heterozygous mice were backcrossed to C57BL/6 N strain mice for four generations to generate congenic C57BL/6 N strain ADORA2B gene mutant mice. Crossbreeding of C57BL/6 N strain ADORA2B mutant heterozygous mice generated the same background strain as wild-type (WT) or ADORA2B gene knockout homozygous mice. We have confirmed that both WT and C57BL/6 N mice showed similar colonic fluid secretion in normal conditions. The phenotypic properties of the genetically modified mice have also been characterized in previous studies27,28,29. All animal studies were performed in accordance with the ARRIVE30,31 and National Institutes of Health guidelines, and were approved by the Animal Care Committees of St. Marianna University (approval numbers: TG180529-5 and 1804015).
Competitive receptor binding assay
The ligand binding assay was performed using 8-cyclopentyl-1,3-dipropylxanthine,[dipropyl-2,3-3H(N)] ([3H]-DPCPX). Membrane fractions (20 µg) prepared from human embryonic kidney (HEK) 293 cells expressing human adenosine A2B receptor (Perkin-Elmer) were incubated with 30.5 nM [3H]-DPCPX, test drugs, and assay buffer (50 mM HEPES pH 7.4, 5 mM MgCl2, 1 mM EDTA) in a 96-well microplate at room temperature for 1 h. A range of concentrations (3 nM to 100 µM) for each compound was tested in triplicate to generate competition curves. The samples were passed through a GF/C filter (Perkin-Elmer) pre-soaked for 1 h with 0.5% BSA and washed four times with wash buffer (5 mM HEPES pH 7.4, 5 mM MgCl2, 1 mM EDTA, 0.25% BSA). Filters were then dried for 30 min before attachment to a MeltiLex A (Perkin-Elmer). The radioactivity remaining on the filter was monitored with a MicroBeta Trilux microplate scintillation counter (Perkin-Elmer). Inhibitory binding constant (Ki) values were determined from IC50 and KD values according to the Cheng & Prusoff equation32. Ki and IC50 values were calculated by non-linear regression analysis using the equation for a sigmoid concentration-response curve (Graph-PAD Prism). The KD value for [3H]-DPCPX binding to A2BR was determined by saturation assay (KD = 10.9 nM). Non-specific binding was examined in the presence of PSB-1115 (10 µM). All experiments were performed in triplicate. Data were normalized to the results of each compound at 3 nM. The data represent the average of the values from at least three independent experiments.
Test drugs or control drugs (aminophylline, CGS21680, BAY60-6583, PSB-1115, NECA) (approximately 10 µM) were screened using 96-well microplates, each of which included a non-specific binding control (without [3H]-DPCPX), buffer control (without drug), and aminophylline and PSB-1115 as positive controls. Before drug screening, we confirmed that the Ki values of the positive control drugs were similar to those in previous reports and that a selective A2A agonist, CGS21680, had no affinity for the A2B receptor membrane. Each plate was normalized to the buffer control. From the screening, we selected drugs with higher affinity than aminophylline. Then, these candidates were tested at three different concentrations (0.1–10 µM) in triplicate. Finally, we identified 12 candidates that had equivalent binding affinity to aminophylline.
Calcium mobilization assay
Calcium mobilization was assayed using the FRIPR® calcium-6 assay kit (Molecular Devices) according to the manufacturer’s instructions. Chinese hamster ovary (CHO) cells stably expressing both human adenosine A2B receptor and Gα15 protein (GeneScript) were seeded in a 96-well microplate (poly-D-lysine-coated black plate) at a density of 3×104 cells/well with 100 µL Ham’s F12 containing heat-inactivated 10% FBS, 1% penicillin/streptomycin (Gibco), 400 µg/mL G418, and 100 µg/mL hygromycin. Cells were incubated for 24 h at 37 °C in 5% CO2. After that, 100 μL loading buffer containing 2.5 mM probenecid was added to each well, and incubation proceeded for another 2 h. A 20-μL test drug solution was added to the appropriate wells and the plates were kept at room temperature for 10 min. Cells were stimulated by the addition of 20 µL 1 µM adenosine solution or Hank’s balanced salt solution (HBSS) with an injector. Fluorescence intensity (F) was measured by a TECAN plate reader (excitation 485 nm; emission 525 nm; 80 cycles; single detection). Serial concentrations of test drugs dissolved in DMSO were prepared with HBSS (3 to 100 µM). Calcium mobilization (∆F/F) was calculated as follows: ∆F/F = [(maximum F value after adenosine treatment) – (maximum F value before adenosine treatment)] / (maximum F value before adenosine treatment). As these cells overexpress not only the A2B receptor but also the Gα15 protein, this cell line was suitable for the evaluation of A2B receptor binding activity.
cAMP accumulation assay
Colonic epithelial T84 cells (CCL-248; ATCC) were plated into a 24-well microplate at a density of 2×105 cells/well with 500 μL DMEM/Ham’s F12 (1:1 mixture) supplemented with 5% FBS and 1% penicillin/streptomycin. Cells were incubated for approximately 7 days at 37 °C in 5% CO2 until confluent. During preculture, the medium was changed twice a week. Confluent cells were washed with 300 μL HBSS containing 1 unit/mL adenosine deaminase (ADA) before further incubation for 2 h with HBSS without ADA. After that, cells were preincubated with 100 μL 50 µM rolipram for 15 min, and then with 100 μL adenosine receptor agonist and calcium blockers for another 15 min. During adenosine stimulation, ADA inhibitor EHNA (50 µM) was added with rolipram to avoid adenosine degradation. T84 cells were washed with HBSS and lysed with 250 μL 0.1 N HCl at room temperature for 20 min. Intracellular cAMP levels were determined by an enzymatic cAMP assay kit from Cayman Chemical Company (Ann Arbor, MI) according to the instruction manual.
Assessment of colonic fluid secretion and absorption
To determine the intestinal luminal fluid volume, a mouse intestinal loop model was used as described previously with some modifications2. Mice fasted for 18 h were anesthetized with 2% isoflurane. The intestinal tract was exposed using a 1-cm middle incision. The colon was ligated with silk approximately 1 cm distal to the caecal–colon junction. Then 100 μL drug solution or vehicle (saline containing 2.5% DMSO) was injected from the ligated point into the colonic lumen. Immediately, one loop of approximately 2 cm was prepared by ligating the colon around 3 cm distal to the caecal–colon junction. The tract was returned to the peritoneal cavity, and the abdominal incision was closed with sutures. Mice were allowed to recover from the anaesthesia. After 2 h, the colonic loop was removed, and its length and weight (including intraluminal contents) were measured. Then, the loop was opened and wiped to remove residual contents. The volume of residual intraluminal fluid was calculated as follows: Residual fluid volume (μL/cm) = [(isolated loop weight with intraluminal contents) – (isolated loop weight without intraluminal contents)] / loop length. Finally, data were normalized to 100% vehicle. Control mice were treated with saline containing 2.5% DMSO (vehicle) alone. For assessment of the inhibitory activity of nifedipine or PSB-1115 on adenosine-produced colonic water secretion, a 1 mM adenosine solution was used.
Homology modelling
As no experimental structures of A2B were available at the time, homology-based models were built for A2B. These were based on a crystal structure of the closely related A2A, which has 55% sequence identity to A2B in the transmembrane region. The crystal structure of A2A (PDB entry 5N2R33); complexed with an antagonist (PSB36) served as a structural template. The sequence of human A2B (UniProt entry P29275) was aligned to that of the A2A crystal structure using MATRAS34 Subsequently, the alignment in the second extracellular loop (ECL2) was manually edited (Supplementary Fig. 1). The C-terminus was excluded from the model because the coordinates were missing in the template. The cytochrome b562RIL fused between TM5 and TM6 was replaced with the corresponding wild-type amino acid sequence (QLRTELMD) of A2B. According to a mutagenesis study of the four cysteine residues in the ECL2, the C17145.50S mutant lost its capacity for high-affinity binding to the A2B antagonist, whereas cysteine-to-serine mutants in the other three positions behaved like the wild-type receptor35. Therefore, we considered only the C783.25–C17145.50 disulphide bond, which is highly conserved in class A G protein-coupled receptors. The antagonist PSB36 was incorporated and placed in the same position as in the A2A crystal structure. All modelling procedures were performed using Modeller v9.1336.
Docking simulation
The crystal structures of nifedipine found in the Crystallography Open Database37 were computationally docked into the A2B model using the sievgene/myPresto docking engine38 implemented in MolDesk ver. 1.139. The partial charge was derived by quantum chemical calculations using Gaussian0340 at the HF/6-31 G* level followed by RESP fitting41. The AMBER99 force field parameters42 were applied to A2B. All acidic residues (Asp and Glu) and all basic residues (Lys and Arg) were set to be negatively and positively charged, respectively. The His2807.43 residue was assumed to be positively charged whereas all other His residues were set to be neutrally charged and protonated at the δ-nitrogen atom. The binding pocket of A2B was specified as a collection of probe points, which were located as the heavy atoms of PSB36 modelled with A2B. Flexible docking was performed by twisting the rotatable bonds of nifedipine, while the coordinates of the A2B model remained fixed. The plausibility of docking was evaluated by the scores of the docking program. The best pose giving the highest score is shown in Fig. 6.
Statistical analysis
Data and statistical analysis were compiled using recommendations on experimental design and analysis in pharmacology43. Values obtained from animal experiments were derived from at least two independent experiments. All data are expressed as mean ± standard error of the mean (SEM). For the assessment of colonic fluid secretion, normalization was performed after collection of all data. Statistical analyses were performed using SPSS 24.0 software (IBM). One- or two-way ANOVA followed by Dunnett’s test or Tukey’s test were used to evaluate differences between more than two groups. Differences were considered significant at P < 0.05.
References
Bucheimer, R. E. & Linden, J. Purinergic regulation of epithelial transport. J. Physiol. 555, 311–321 (2004).
Weissmuller, T. et al. PMNs facilitate translocation of platelets across human and mouse epithelium and together alter fluid homeostasis via epithelial cell-expressed ecto-NTPDases. J. Clin. Invest. 118, 3682–3692 (2008).
Sohn, C. I., Park, H. J. & Gebhart, G. F. Adenosine receptor agonists modulate visceral hyperalgesia in the rat. Gut Liver 2, 39–46 (2008).
Feoktistov, I. & Biaggioni, I. Adenosine A2B receptors. Pharmacol. Rev. 49, 381–402 (1997).
Chandrasekharan, B. P. et al. Adenosine 2B receptors (A(2B)AR) on enteric neurons regulate murine distal colonic motility. FASEB J. 23, 2727–2734 (2009).
Antonioli, L. et al. Role of the A(2B) receptor-adenosine deaminase complex in colonic dysmotility associated with bowel inflammation in rats. Br. J. Pharmacol. 171, 1314–1329 (2014).
Asano, T. & Takenaga, M. Adenosine A2B Receptors: An Optional Target for the Management of Irritable Bowel Syndrome with Diarrhea? J. Clin. Med. 6, (2017).
Asano, T. et al. Aminophylline suppresses stress-induced visceral hypersensitivity and defecation in irritable bowel syndrome. Sci. Rep. 7, 40214 (2017).
Warren, C. A. et al. Contribution of adenosine A(2B) receptors in Clostridium difficile intoxication and infection. Infect. Immun. 80, 4463–4473 (2012).
Camilleri, M. Intestinal secretory mechanisms in irritable bowel syndrome-diarrhea. Clin Gastroenterol Hepatol 13, 1051–1057; quiz e1061–1052, (2015).
Thiagarajah, J. R., Donowitz, M. & Verkman, A. S. Secretory diarrhoea: mechanisms and emerging therapies. Nat. Rev. Gastroenterol. Hepatol. 12, 446–457 (2015).
Watson, M. J. et al. The Cystic Fibrosis Transmembrane Conductance Regulator (CFTR) Uses its C-Terminus to Regulate the A2B Adenosine Receptor. Sci. Rep. 6, 27390 (2016).
Strohmeier, G. R., Reppert, S. M., Lencer, W. I. & Madara, J. L. The A2b adenosine receptor mediates cAMP responses to adenosine receptor agonists in human intestinal epithelia. J. Biol. Chem. 270, 2387–2394 (1995).
Barrett, K. E., Huott, P. A., Shah, S. S., Dharmsathaphorn, K. & Wasserman, S. I. Differing effects of apical and basolateral adenosine on colonic epithelial cell line T84. Am. J. Physiol. 256, C197–203 (1989).
Kolachala, V. L., Obertone, T. S., Wang, L., Merlin, D. & Sitaraman, S. V. Adenosine 2b receptor (A2bR) signals through adenylate cyclase (AC) 6 isoform in the intestinal epithelial cells. Biochim. Biophys. Acta 1760, 1102–1108 (2006).
Mizushima, T. Identification of a molecular mechanism for actions of existing medicines and its application for drug development]. Yakugaku Zasshi 132, 713–720 (2012).
Nichols, J. M., Maiellaro, I., Abi-Jaoude, J., Curci, S. & Hofer, A. M. “Store-operated” cAMP signaling contributes to Ca2+-activated Cl−secretion in T84 colonic cells. Am. J. Physiol. Gastrointest. Liver Physiol 309, G670–G679 (2015).
Christofi, F. L. et al. Differential gene expression of adenosine A1, A2a, A2b, and A3 receptors in the human entericnervous system. J. Comp. Neurol. 439, 46–64 (2001).
Müller, C. E. & Jacobson, K. A. Recent developments in adenosine receptor ligands and their potential as novel drugs. Biochim. Biophys. Acta 1808, 1290–1308 (2011).
Thimm, D. et al. Ligand-specific binding and activation of the human adenosine A(2B) receptor. Biochem. 52, 726–740 (2013).
Peeters, M. C. et al. Domains for activation and inactivation in G protein-coupled receptors–a mutational analysis of constitutive activity of the adenosine A2B receptor. Biochem. Pharmacol. 92, 348–357 (2014).
Morel, N. et al. The action of calcium channel blockers on recombinant L-type calcium channel alpha1-subunits. Br. J. Pharmacol. 125, 1005–1012 (1998).
Bharucha, A. E., Pemberton, J. H. & Locke, G. R. 3rd American Gastroenterological Association Technical Review on Constipation. Gastroenterology 144, 218–238 (2013).
Chiarioni, G., Scattolini, C., Bonfante, F., Brentegani, M. T. & Vantini, I. Effect of nifedipine on mouth-to-cecum transit of liquid meal in normal subjects. Dig. Dis. Sci. 38, 1022–1025 (1993).
Zar, M. & Gooptu, D. Effect of nifedipine on the contractile responses of human colonic muscle. Br. J. Clin. Pharmacol. 16, 339–340 (1983).
Eckle, T. et al. Cardioprotection by ecto-5′-nucleotidase (CD73) and A2B adenosine receptors. Circulation 115, 1581–1590 (2007).
Eckle, T., Grenz, A., Laucher, S. & Eltzschig, H. K. A2B adenosine receptor signaling attenuates acute lung injury by enhancing alveolar fluid clearance in mice. J. Clin. Invest. 118, 3301–3315 (2008).
Yang, D. et al. The A2B adenosine receptor protects against inflammation and excessive vascular adhesion. J. Clin. Invest. 116, 1913–1923 (2006).
Grenz, A. et al. The Reno-Vascular A2B Adenosine Receptor Protects the Kidney from Ischemia. PLoS Med. 5, e137 (2008).
Kilkenny, C. et al. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br. J. Pharmacol. 160, 1577–1579 (2010).
McGrath, J. C. & Lilley, E. Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br. J. Pharmacol. 172, 3189–3193 (2015).
Cheng, Y. & Prusoff, W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem. Pharmacol. 22, 3099–3108 (1973).
Cheng, R. K. Y. et al. Structures of Human A1 and A2A Adenosine Receptors with Xanthines Reveal Determinants of Selectivity. Structure 25, 1275–1285 e1274 (2017).
Kawabata, T. MATRAS: A program for protein 3D structure comparison. Nucleic Acids Res. 31, 3367–3369 (2003).
Schiedel, A. C. et al. The four cysteine residues in the second extracellular loop of the human adenosine A2B receptor: role in ligand binding and receptor function. Biochem. Pharmacol. 82, 389–399 (2011).
Sali, A. & Blundell, T. L. Comparative protein modelling by satisfaction of spatial restraints. J. Mol. Biol. 234, 779–815 (1993).
Merkys, A. et al. COD::CIF::Parser: an error-correcting CIF parser for the Perl language. J. Appl. Crystallogr. 49, 292–301 (2016).
Fukunishi, Y., Mikami, Y. & Nakamura, H. Similarities among receptor pockets and among compounds: analysis and application to in silico ligand screening. J. Mol. Graph. Model. 24, 34–45 (2005).
MolDesk ver.1.1, IMSBIO Co., Ltd., Tokyo, https://www.moldesk.com/ (2018).
Gaussian 03, Revision E.01. Frisch, M. J. et al. Gaussian, Inc., Wallingford CT, http://gaussian.com/ (2004).
Bayly, C. I., Cieplak, P., Cornell, W. & Kollman, P. A. A well-behaved electrostatic potential based method using charge restraints for deriving atomic charges: the RESP model. J. Phys. Chem. 97, 10269–10280 (1993).
Wang, J., Cieplak, P. & Kollman, P. A. How well does a restrained electrostatic potential (RESP) model perform in calculating conformational energies of organic and biological molecules? J. Comput. Chem. 21 1049–1074. 10.1002/1096-987X(200009)21:12%3C1049::AID-JCC3%3E3.0.CO;2-F (2000).
Curtis, M. J. et al. Experimental design and analysis and their reporting: new guidance for publication in BJP. Br. J. Pharmacol. 172, 3461–3471 (2015).
van der Hoeven, D. et al. A role for the low-affinity A2B adenosine receptor in regulating superoxide generation by murine neutrophils. J. Pharmacol. Exp. Ther. 338, 1004–1012 (2011).
Acknowledgements
The authors acknowledge Ayumi Kanada for technical assistance with the in vitro experiments. We also thank the Isotope Research Institute at St. Marianna University for help with the receptor binding assay. This work was supported by Grants-in-Aid for Young Scientists from the Japan Society for the Promotion of Science (JSPS KAKENHI grant number JP18K15033). Finally, we thank Edanz Group for editing a draft of this manuscript.
Author information
Authors and Affiliations
Contributions
Conception and design: T.A., K.T., T. Mizushima, and M.T. Conducted experiments: T.A., M.W., T. Mashimo, and Y.N. Analysis and interpretation: T.A., K.T., N.Y., Y.F., T. Mizushima, and M.T. Drafting and critically revising the manuscript: Y.F., T. Mizushima, M.T., and T.A.
Corresponding authors
Ethics declarations
Competing interests
T.M. and Y.N. are employees of LTT Bio-Pharma Co., Ltd., and T.M. is the chairman and director of the company. T.A. and M.T. are affiliated to an endowed research division of LTT Bio-Pharma Co., Ltd. All other authors have no conflict of interest to declare.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Asano, T., Noda, Y., Tanaka, KI. et al. A2B adenosine receptor inhibition by the dihydropyridine calcium channel blocker nifedipine involves colonic fluid secretion. Sci Rep 10, 3555 (2020). https://doi.org/10.1038/s41598-020-60147-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-60147-7
- Springer Nature Limited
This article is cited by
-
Pharmacological characterization of DPTN and other selective A3 adenosine receptor antagonists
Purinergic Signalling (2021)