Abstract
Peanut is a popular food due to its high nutrient content. The effects of ZnCl2 on peanut seed germination, fatty acid and sugar contents, vitamin biosynthesis, antioxidant content, and Zn assimilation were evaluated in this study. Treatment with ZnCl2 significantly improved the germination rate, enhanced reactive oxygen species production and reduced the content of total fatty acids in peanut seed and sprout. However, ZnCl2 treatment did not reduce total sugar or total protein relative to the control. Germination promoted the biosynthesis of phenolics and resveratrol and increased the antioxidant capacity, as evaluated by Fe3+ reducing power and 2,2-diphenyl-1-picrylhydrazyl radical scavenging ability, especially under Zn stress conditions. The vitamin content decreased in the following order among treatments: germinated seeds with ZnCl2 treatment > germinated seeds without ZnCl2 treatment > dormant seeds. Interestingly, Zn content was approximately five times higher in the germinated ZnCl2-treated seeds compared to in the untreated germinated seeds and the dormant seeds. The results of this study provide a new method for producing healthy foods with enhanced vitamin content and antioxidant capacity.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Healthy and low-calorie diets are currently welcomed by consumers, which has led to an increase in the consumption of functional foods1,2,3,4. Foods with low levels of fatty acids (FAs) and sugar are expected to reduce energy production and benefit the health of consumers5,6.
Peanuts have a high content of nutrients, including plant protein, dietary fibre, unsaturated FAs, β-vitamins, vitamin E, Mg and numerous bioactive substances (e.g., flavonoids, resveratrol and plant sterols)7,8. Many overweight individuals avoid peanut consumption due to its high calorie content9. Peanut intake is thought to lead to weight gain and an increase in body mass index10,11.
A large amount of energy and resources are consumed during seed germination12, while a large amount of antioxidant substances are synthesized during this process13,14. Published data show that germination enhances the phenolic content and total antioxidant capacity (TAC) in many kinds of seeds13. In addition, abiotic stress treatment can enhance the content of antioxidant compounds in germinated seeds more than in dormant seeds15,16. For example, moderate concentrations of NaCl were found to enhance the biosynthesis of phenolics in radish sprouts15.
While heavy metals in food can have negative effects on human health17, certain heavy metals such as Zn, Fe and Cu are indispensable for human health at trace levels18,19,20. Among these beneficial elements, Zn is vital for human beings and particularly child development21,22. Although many efforts have been made to supplement foods with these beneficial elements, the results remain unsatisfactory19.
In this study, peanut seeds were soaked in ZnCl2 solution during the germination process. The contents of FAs, sugars, and vitamins along with the antioxidant capacities were compared between the dormant seeds and germinated seeds and sprouts under favourable and Zn stress conditions with the goal of answering two main questions: (1) Can Zn addition affect the nutrient content and antioxidant capacity of germinated peanut seeds and sprouts? (2) Can germination significantly improve Zn assimilation in germinated peanut seeds and sprouts? The results of this study provide a better understanding of how seed germination affects seed nutrient quality from the perspective of human health.
Results
Effects of Zn treatment on peanut seed germination, sprout growth, superoxide production and hydrogen peroxide accumulation
As shown in Fig. 1A, the germination ability of peanut seeds was significantly affected by ZnCl2. Compared to the control, treatment with a low concentration (20 mM) of ZnCl2 significantly increased the germination rate (GR) by 36%, 20% and 14% after imbibition for 24, 48 and 72 h, respectively (Fig. 1A; p < 0.05). However, high concentrations of ZnCl2 (100 and 200 mM) significantly decreased the GR by 12% and 44%, respectively, after imbibition for 72 h (Fig. 1A; p < 0.05). Thus, the Zn treatment concentration in subsequent experiments was 20 mM. Similarly, Zn treatment at 20 mM significantly enhanced sprout growth, particularly in the early stage (Fig. 1B). Compared to the water control, sprout length was increased by approximately 67%, 38%, 20%, 11% and 10% after 1, 2, 3, 4, and 5 d, respectively (Fig. 1B; p < 0.05).
Treatment with Zn also significantly enhanced superoxide (O2−) production and hydrogen peroxide (H2O2) accumulation in germinated seeds and sprouts compared to the water control. Zn increased O2− production by 81%, 51%, 35%, 31%, 18% and 7% after seed imbibition for 2, 8, 24, 48, 72 and 96 h, respectively (Fig. 1C; p < 0.05). Similar results were also observed for H2O2 accumulation in germinated seeds and sprouts after Zn treatment; for example, the H2O2 content was increased by approximately 68% and 41% compared to the control after Zn treatment for 2 and 8 h, respectively (Fig. 1D; p < 0.05).
Effects of Zn treatment on the contents of total FA, total sugar and total protein
The effects of ZnCl2 treatment (20 mM) on the contents of nutrients (total FA, total sugar and total protein) were evaluated. As shown in Fig. 2, total FA, total sugar and total protein were respectively reduced by 75%, 83% and 65% in the germinated peanut seeds and sprouts compared to the dormant seeds (p < 0.05). Compared to the water control, ZnCl2 treatment decreased the content of total FA in germinated peanut seeds and sprout by 74% and increased the contents of total sugar and total protein by 20% and 9%, respectively (Fig. 2; p < 0.05).
Effects of Zn treatment on FA and sugar composition
Seven main FAs were detected in dormant and germinated peanut seeds and sprouts (Table 1), and their contents decreased in the following order: linolic acid > oleic acid > palmic acid > stearic acid > behenic acid > arachic acid > lignoceric acid. Compared to the dormant seeds, the contents of all these FAs decreased after germination. For example, the content of linolic acid was reduced by approximately 74% and 95% after germination in the water control and Zn-treated group, respectively (Table 1; p < 0.05).
Four main non-structural sugars were detected in dormant and germinated peanut seeds and sprouts (Table 2), and the contents decreased in the following order: starch > sucrose > glucose > fructose. Germination reduced the starch and sucrose contents but enhanced the glucose and fructose contents. For example, the sucrose and starch contents were respectively decreased by approximately 75% and 82% in ZnCl2-treated group compared to the dormant seeds (Table 2; p < 0.05).
Effects of Zn treatment on antioxidant capacity
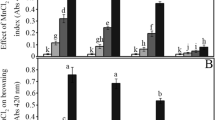
Compared to the dormant seeds, germination increased the antioxidant (e.g., total phenolics and resveratrol) content and antioxidant capacity, as evaluated by Fe3+ reducing power/TAC and 2,2-diphenyl-1-picrylhydrazyl (DPPH) radical scavenging capacity, and this effect was further enhanced by ZnCl2 treatment (Fig. 3). For example, total phenolic content was approximately 376% and 1145% higher in germinated peanut seeds and sprouts under favourable and Zn stress conditions, respectively, compared to in the dormant seeds (Fig. 3A; p < 0.05). Similarly, the resveratrol content was approximately eight and 25 times higher in the germinated seeds and sprouts in the water control and Zn-treated groups, respectively, compared to in the dormant seeds (Fig. 3B). The TAC content was approximately 9.6 times higher (water control) and 11.8 times higher (ZnCl2 treatment) in the germinated seeds and sprouts compared to in the dormant seeds (Fig. 3C; p < 0.05). ZnCl2 treatment enhanced the DPPH-radical scavenging capacity by 22% compared to the water control (Fig. 3D; p < 0.05).
Effects of ZnCl2 treatment on total phenolic content (A), resveratrol content (B), TAC (C) and DPPH-radical scavenging capacity (D) in peanut seeds and sprouts. Bars represent standard deviations of means (n = 3). Means followed by the same letter are not significantly different (p < 0.05) among treatments.
Effects of Zn treatment on vitamin and Zn contents
ZnCl2 treatment enhanced the biosynthesis of all studied vitamins with the exception of vitamin B1 (Table 3). The contents of vitamins A, B2, C, and E were increased by approximately 11.9, 7.5, 18, and 3.7 times in the germinated ZnCl2-treated seeds and sprouts compared to the dormant seeds (Table 3; p < 0.05). In contrast, the content of vitamin B1 decreased by approximately 45% and 36% after germination under favourable and Zn stress conditions, respectively, compared to the dormant seeds (Table 3; p < 0.05). Finally, the Zn content was approximately five times higher in the germinated ZnCl2-treated seeds compared to in the dormant seeds (Table 3; p < 0.05).
Discussion
Many studies have been carried out on the effects of germination on antioxidant contents and TAC in seeds14,15,23. Less data are available to show the correlations between nutrients (e.g., FAs and sugar) consumption and antioxidant biosynthesis during seed germination, especially under abiotic stress conditions.
In this study, the FA, sugar, vitamin, antioxidant, and Zn contents were compared between dormant and germinated peanut seeds and sprouts. As shown in Fig. 1, treatment with high concentrations of ZnCl2 (e.g., 100 and 200 mM) inhibited peanut seed germination compared to the water control. However, treatment with a low concentration of ZnCl2 (20 mM) significantly increased the seed GR compared to the water control (Fig. 1A). Thus, moderate Zn treatment can improve peanut seed germination. Interestingly, Zn treatment also improved seedling/sprout growth (Fig. 1B). We speculate that this phenomenon may be associated with Zn-mediated reactive oxygen species (ROS), which can accelerate seed germination and plant growth24,25,26. Thus, the O2− production and H2O2 content were investigated. Zn addition markedly increased O2− production and H2O2 content, particularly at the early stage of germination (Fig. 1C,D). Thus, the ability of Zn to promote seed germination and sprout growth is likely related to its effects on ROS production.
Peanut seeds can preserve a high percentage of energy compounds, including fat and sugar7,8. The effects germination with and without moderate Zn treatment on the total FA, total sugar and total protein contents were evaluated herein (Fig. 2). Germination significantly decreased the contents of total FA, total sugar and total protein compared to the dormant seeds, regardless of whether ZnCl2 was applied. The main types of FAs (e.g., oleic acid and linolic acid) and sugars (e.g., starch and sucrose) were further investigated (Tables 1 and 2). The main FAs were oleic acid, linolic acid, and palmic acid, especially in dormant peanut seed (Table 1). The contents of these FAs were decreased significantly by germination, particularly after ZnCl2 treatment (Table 1). The contents of non-structural carbohydrates (e.g., starch) were also reduced in the germinated seeds and sprouts, especially under Zn treatment conditions (Table 2). In contrast, the sucrose content was reduced more significantly in the germinated seeds without Zn treatment than in those with Zn treatment (Table 2). Furthermore, the contents of glucose and fructose increased after seed germination (Table 2). These results demonstrate that the changes in non-structural carbohydrates during germination depend on the type of sugar, which raises another interesting question: Why does Zn treatment further accelerate the degradation of FAs and starches during germination compared to the water control? One plausible explanation is that Zn-mediated ROS promoted seedling growth and metabolic activity, requiring more energy (e.g., adenosine triphosphate) to be produced via FA and starch degradation.
High contents of antioxidants are indispensable for deterring seed ageing27,28. Thus, oxidative stress is required for seed dormancy release26,29. It seems that a large amount of antioxidants may be consumed after seed germination. To confirm this speculation, the antioxidant contents (e.g., total phenolics and resveratrol) and antioxidant capacity were compared between the dormant and germinated seeds and sprouts (Fig. 3). Germination was found to significantly enhance antioxidant content and capacity (Fig. 3), in accordance with a previous report30 showing that seed germination can significantly enhance the accumulation of total phenolics and resveratrol in sprouts. Interestingly, treatment with 20 mM ZnCl2 further enhanced the antioxidant content and antioxidant capacity (Fig. 3). Compared to the water control, ZnCl2 treatment increased the total phenolic and resveratrol contents in germinated peanut seeds and sprouts by approximately 2.6 and 3 times, respectively (Fig. 3A,B). Similarly, a past study found that abiotic stress (ultraviolet radiation and H2O2) significantly increased resveratrol biosynthesis in peanut plant31. Accordingly, greater TAC and higher DPPH radical scavenging were observed in the ZnCl2-treated seeds compared to in the water control group (Fig. 3C,D). This effect might be explained by Zn-mediated ROS, which can promote antioxidant biosynthesis in plants32.
Seed germination was found to increase the contents of vitamins A, B1, B2, C and E compared to the dormant seeds, especially under Zn treatment (Table 3). Interestingly, certain vitamins (e.g., vitamins C and E) are known antioxidants32. This suggests that high quantities of antioxidant and metabolite molecules are required for peanut seed germination, especially after Zn treatment. The findings show that ZnCl2 treatment not only reduced FA accumulation, but also enhanced the antioxidant, vitamin and Zn contents in germinated peanut seeds and sprouts. Zn supplementation can be extended to other seed germination-derived foods. Thus, Zn treatment can be used to produce healthy foods in the future.
In conclusion, seed germination reduced the total FA, total protein and total sugar contents while enhancing antioxidant and vitamin contents in peanut seeds compared to the dormant seeds. Treatment with a low concentration of ZnCl2 improved seed germination, further reduced the contents of energy-storing compounds, further enhanced antioxidant content, and led to Zn assimilation. Thus, treatment with ZnCl2 offers a new way to increase the antioxidant capacity and Zn content during seed germination.
Materials and Methods
Seed treatment
Peanut seeds (kainong 70; Arachis hypogaea Linn.) were obtained from a seed distributor in Zhengzhou, China. The seeds were sown in plastic boxes and placed in a seed germinator at room temperature. Germination trials were conducted in plastic boxes equipped with 4-cm-deep sand with a particle diameter of approximately 0.5 mm. The sand was moistened with distilled water or water containing different concentrations of ZnCl2. Before seed germination, half of the control seeds were moistened with distilled water for 30 min. After treatment, the seeds were transferred to plastic boxes.
This experiment was divided into two treatment groups. In group 1, four concentrations of ZnCl2 (0, 20, 100 and 200 mM) were applied to the water-moistened seeds. In group 2, the Zn-treated peanut seeds were placed in ZnCl2-soaked sand for germination. All assays were replicated at least three times, and each replicate was carried out on 50 seeds. When the radicle emerging from the peanut seed reached a length of 1 cm, the seeds and sprouts were collected for subsequently assay.
GR and sprout length assay
GRs were calculated as the percentage of germinated peanut seeds after sowing for different time periods33,34. Sprout length was measured using Vernier callipers.
O2 − and H2O2 content assays
O2− production was determined by monitoring nitrate formation from hydroxylamine in the presence of O2− generators, as described by Elstner and Heupel35. Seed and sprout tissues were homogenized with liquid nitrogen in a chilled pestle and mortar with 1 mL of 65 mM Ki-phosphate buffer (pH 7.8). The homogenate was centrifuged at 5,000 × g for 10 min at 4 °C. Ki-phosphate buffer (0.45 mL; pH 7.8) and 10 mM hydroxylamine hydrochloride (50 μL) were added to the supernatant. After developing this mixture at room temperature for 20 min, 0.5 mL of the mixture was added to a solution (0.5 mL) containing 17 mM anaphthaleneamine at room temperature for 20 min. The mixture was vortexed and centrifuged at 1,500 × g for 5 min. The absorbance of the pink aqueous phase was then recorded at 530 nm. A standard curve of NO2− was used to calculate the production rate of O2− from the chemical reaction of hydroxylamine and O2−.
The H2O2 contents in treated peanut seed were determined using a previously reported method36. H2O2 production in peanut seeds and sprouts was determined using the oxidation xylenol orange assay, which is based on the oxidation of Fe2+ ions by peroxide followed by colorimetric detection of the reaction of Fe3+ with the sodium salt of xylenol orange. One millilitre of assay reagent (25 mM FeSO4 and 25 mM (NH4)2SO4 dissolved in 2.5 M H2SO4) was added to 100 mL of 125 μM xylenol orange and 100 mM sorbitol. The collected tissue was ground and centrifuged at 8000 × g for 5 min. The supernatant (100 μL) was added to 1 mL of xylenol orange reagent. After 30 min of incubation, the absorbance of the Fe3+–xylenol orange complex was recorded at 560 nm using a spectrophotometer.
Assay for total phenolics content
The peanut seeds and sprouts were collected for the measurement of total phenolics. The content of total phenolics was determined using Folin–Ciocalteu reagent37. Two grams of sample was extracted for 2 h with 20 mL of 80% methanol containing 1% hydrochloric acid at 25 °C on an orbital shaker at 200 rpm. The mixture was centrifuged at 1000 × g for 20 min, and the supernatant was transferred to 100-mL vials. The supernatants were combined and used for the total phenolics assay. The extract (1 mL) was mixed with 7.5 mL of Folin–Ciocalteu reagent and allowed to stand at room temperature for 5 min. Next, 7.5 mL of 60 g·L−1 sodium bicarbonate solution was added to the mixture. After allowing to stand for 90 min at room temperature, the absorbance was recorded at 725 nm. The results are expressed as ferulic acid equivalents.
Resveratrol extraction and assay
Resveratrol was determined using the method of Fettig and Hess with modification38. Peanut seeds and sprouts were protected from light during analysis. The free form of resveratrol was extracted by agitating the frozen sample powder in methanol for 16 h at 25 °C. Resveratrol was separated using a μBondapak C18 column and assayed with a fluorescence detector (excitation wavelength of 330 nm and emission wavelength of 374 nm). Samples from three replications were analysed.
Antioxidant capacity assay
The peanut seeds and sprouts collected for antioxidant capacity assay were weighed and immediately frozen in liquid N2. Dry samples (1 g) were ground into a powder in liquid N2 using a mortar and pestle, transferred to 100 mL of 90% (w/v) methanol/water solution, and incubated at room temperature for 24 h in the dark. The extracts were filtered, the filtrates from each replicate were pooled, and the solvent was evaporated under vacuum at 45 °C using a rotary evaporator. The resulting crude extracts were stored in a desiccator at 4 °C until being analysed for TAC and ROS scavenging capacity.
TAC was evaluated by ferric reducing ability of plasma assay39. The DPPH radical scavenging method was used to evaluate the ROS scavenging capacity40,41. DPPH solution in methanol (50 μM) was freshly prepared, and 2.9 mL of this solution was mixed with 100 μL of extract. The samples were incubated for 30 min at 37 °C in a water bath, and the decrease in absorbance at 515 nm was measured (AE). The DPPH solution was used as a blank sample, and its absorbance was also measured (AB). The DPPH radical scavenging capacity was calculated as follows: percentage of inhibition = [(AB−AE)/AB] × 100%.
Total FA and composition assay
Peanut seeds and sprouts were collected for total FA assay. The total FA content and composition were determined using the method of Andersen et al.42.
Total sugar and non-structural carbohydrate assay
Peanut seeds and sprouts were collected for total sugar and non-structural carbohydrate assay. The levels of glucose, sucrose, fructose, starch (amyloglucosidase was used to hydrolyse starch) and total sugar were determined by high-performance liquid chromatography (HPLC)43. Peanut seed and sprout tissues (2 g) were fixed in 96% ethanol, and carbohydrates were extracted with 80% ethanol. The extracts were purified by a solid-phase extraction technique. Soluble carbohydrates (glucose, sucrose and fructose) were analysed using a Diasorb-130-Amin column (250 × 4 mm) packed with 6-µm-diameter particles. A refractometer was used as a detector, and an acetonitrile:water mixture (v:v = 70:30) was used as the eluent at a flow rate of 0.6 mL·min−1. The D-forms of fructose, glucose and sucrose were used as standards. The amount of soluble carbohydrates was calculated using the absolute calibration method.
Vitamin and Zn assays
Peanut seeds and sprouts were collected for vitamin A, B1, B2, C and E determination using HPLC according to the method of Feliciano et al.44. The Zn content was assayed using the method of Deng et al.41 Zn-treated peanut seeds and sprouts were collected, washed, air-dried and ground into powder using a mortar and pestle. For Zn extraction, digestion tubes were acid-washed and dried. Each dried powdered sprout sample (1 g each) was added to a digestion tube followed by the addition of 10 mL of a mixture of HNO3, HClO4 and H2SO4 (volume ratio = 5:1:1) to each tube. After 12 h, the tubes were placed in a digestion block at 80 °C for approximately 1 h. The temperature was then increased slowly to 120 °C–130 °C. When digestion was complete, the solutions were cooled, filtered and diluted to 100 mL with doubly deionized water. The filtrates were assayed for Zn using atomic absorption spectrometry (Analyst 700, Perkin Elmer, USA).
Data analysis
The experiments were conducted in a completely randomized design. Three replicates were analysed for each treatment. All data were analysed by Duncan’s multiple range test (p < 0.05) using SPSS 13.0 software.
References
Siro, I., Kápolna, E., Kápolna, B. & Lugasi, A. Functional food. Product development, marketing and consumer acceptance—A review. Appetite 51, 456–467 (2008).
Bigliardi, B. & Galati, F. Innovation trends in the food industry: the case of functional foods. Trends Food Sci. Technol. 31, 118–129 (2013).
Vidal-Valverde, C. et al. New functional legume foods by germination: effect on the nutritive value of beans, lentils and peas. Eur. Food Res. Technol. 215, 472–477 (2002).
El-Adawy, T., Rahma, E., El-Bedawey, A. & El-Beltagy, A. Nutritional potential and functional properties of germinated mung bean, pea and lentil seeds. Plant Foods Human Nutr. 58, 1–13 (2003).
Drewnowski, A. & Specter, S. Poverty and obesity: the role of energy density and energy costs. Amer. J. Clin. Nutr. 79, 6–16 (2004).
Drewnowski, A., Darmon, N. & Briend, A. Replacing fats and sweets with vegetables and fruits—a question of cost. Amer. J. Public Health 94, 1555–1559 (2004).
Rusydi, M. & Azrina, A. Effect of germination on total phenolic, tannin and phytic acid contents in soy bean and peanut. Int. Food Res. J. 19, 673–677 (2012).
Arya, S., Salve, A. & Chauhan, S. Peanuts as functional food: a review. J. Food Sci. Technol. 53, 31–41 (2016).
Andersen, P. & Gorbet, D. Influence of year and planting date on fatty acid chemistry of high oleic acid and normal peanut genotypes. J. Agric. Food Chem. 50, 1298–1305 (2002).
Drewnowski, A. Energy density, palatability, and satiety: implications for weight control. Nutr. Rev. 56, 347–353 (1998).
Rolls, B., Drewnowski, A. & Ledikwe, J. Changing the energy density of the diet as a strategy for weight management. J. Amer. Diet. Assoc. 105, 98–103 (2005).
Raymond, P., Al-Ani, A. & Pradet, A. ATP production by respiration and fermentation, and energy charge during aerobiosis and anaerobiosis in twelve fatty and starchy germinating seeds. Plant Physiol. 79, 879–884 (1985).
Cevallos-Casals, B. & Cisneros-Zevallos, L. Impact of germination on phenolic content and antioxidant activity of 13 edible seed species. Food Chem. 119, 1485–1490 (2010).
Gan, R. et al. Bioactive compounds and bioactivities of germinated edible seeds and sprouts: An updated review. Trends Food Sci. Technol. 59, 1–14 (2017).
Yuan, G., Wang, X., Guo, R. & Wang, Q. Effect of salt stress on phenolic compounds, glucosinolates, myrosinase and antioxidant activity in radish sprouts. Food Chem. 121, 1014–1019 (2010).
Lim, J., Park, K., Kim, B., Jeong, J. & Kim, H. Effect of salinity stress on phenolic compounds and carotenoids in buckwheat (Fagopyrum esculentum M.) sprout. Food Chem. 135, 1065–1070 (2012).
Notten, M., Oosthoek, A., Rozema, J. & Aerts, R. Heavy metal concentrations in a soil–plant–snail food chain along a terrestrial soil pollution gradient. Environ. Pollut. 138, 178–190 (2005).
Wasantwisut, E. et al. Iron and zinc supplementation improved iron and zinc status, but not physical growth, of apparently healthy, breast-fed infants in rural communities of northeast Thailand. J. Nutr. 136, 2405–2411 (2006).
Underwood, B. Overcoming micronutrient deficiencies in developing countries: is there a role for agriculture? Food Nutr. Bull. 21, 356–360 (2000).
Vuori, E., Mäkinen, S., Kara, R. & Kuitunen, P. The effects of the dietary intakes of copper, iron, manganese, and zinc on the trace element content of human milk. Amer. J. Clin. Nutr. 33, 227–231 (1980).
Bhatnagar, S. & Taneja, S. Zinc and cognitive development. Brit. J. Nutr. 85, S139–S145 (2001).
Black, M. Zinc deficiency and child development. Amer. J. Clin. Nutr. 68, 464S-469S (1998).
Deng, B., Zhang, Y., Yang, K. & Li, Z. Changes in non-enzymatic antioxidant capacity and lipid peroxidation during germination of white, yellow and purple maize seeds. Pak. J. Bot. 48, 607–612 (2016).
Rodrıguez, A., Grunberg, K. & Taleisnik, E. Reactive oxygen species in the elongation zone of maize leaves are necessary for leaf extension. Plant Physiol. 129, 1627–1632 (2002).
Liszkay, A., van der Zalm, E. & Schopfer, P. Production of reactive oxygen intermediates (O2 −, H2O2, and ˙OH) by maize roots and their role in wall loosening and elongation growth. Plant Physiol. 136, 3114–3123 (2004).
Bailly, C., El-Maarouf-Bouteau, H. & Corbineau, F. From intracellular signaling networks to cell death: the dual role of reactive oxygen species in seed physiology. Comptes Rendus Biol. 331, 806–814 (2008).
Deng, B., Zhang, Y., Yang, K. & Li, Z. The differential antioxidant capacity of watermelon flesh at different maturity stages and its inhibitory effects on seed aging may explain the significance of fruit flesh colors. Acta Physiol. Plant. 39, 139–145 (2017).
Pinzino, C. et al. Aging, free radicals, and antioxidants in wheat seeds. J. Agric. Food Chem. 47, 1333–1339 (1999).
Ogawa, K. & Iwabuchi, M. A mechanism for promoting the germination of Zinnia elegans seeds by hydrogen peroxide. Plant Cell Physiol. 42, 286–291 (2001).
Limmongkon, A. et al. Antioxidant activity, total phenolic, and resveratrol content in five cultivars of peanut sprouts. Asian Pac. J. Trop. Biomed. 7, 332–338 (2017).
Chung, I., Park, M., Chun, J. & Yun, S. Resveratrol accumulation and resveratrol synthase gene expression in response to abiotic stresses and hormones in peanut plants. Plant Sci. 164, 103–109 (2003).
Gill, S. & Tuteja, N. Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol. Biochem. 48, 909–930 (2010).
Zhang, Y., Shi, H. & Deng, B. Mutagen-induced phytotoxicity in maize seed germination is dependent on ROS scavenging capacity. Sci. Rep., https://doi.org/10.1038/s41598-018-32271-y (2018)
Yang, K., Zhang, Y., Zhu, L., Li, Z. & Deng, B. Omethoate treatment mitigates high salt stress inhibited maize seed germination. Pestic. Biochem. Physiol. 144, 79–82 (2018).
Elstner, E. & Heupel, A. Inhibition of nitrite formation from hydroxylammonium-chloride: simple assay for superoxide dismutase. Anal. Biochem. 70, 616–620 (1976).
Gay, C., Collins, J. & Gebicki, J. Hydroperoxide assay with the ferric-xylenol orange complex. Anal. Biochem. 273, 149–155 (1999).
Singleton, V. & Rossi, J. Colorimetry of total phenolics with phosphomolybdic- phosphotungstic acid reagents. Amer. J. Enol. Vitic. 16, 144–158 (1965).
Fettig, S. & Hess, D. Expression of a chimeric stilbene synthase gene in transgenic wheat lines. Transg. Res. 8, 179–189 (1999).
Benzie, I. & Strain, J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: the FRAP assay. Anal. Biochem. 239, 70–76 (1996).
Deng, B., Guo, M., Liu, H., Tian, S. & Zhao, X. Inhibition of autophagy by hydroxychloroquine enhances antioxidant nutrients and delays postharvest fruit senescence of Ziziphus jujuba. Food Chem. 296, 56–62 (2019).
Deng, B., Yang, K., Zhang, Y. & Li, Z. Can heavy metal pollution defend seed germination against heat stress? Effect of heavy metals (Cu2+, Cd2+ and Hg2+) on maize seed germination under high temperature. Environ. Pollut. 216, 46–52 (2016).
Andersen, P., Hill, K., Gorbet, D. & Brodbeck, B. Fatty acid and amino acid profiles of selected peanut cultivars and breeding lines. J. Food Compos. Anal. 11, 100–111 (1998).
Glyad, V. Determination of monosaccharides, disaccharides, and oligosaccharides in the same plant sample by High-Performance Liquid Chromatography. Russ. J. Plant Physiol. 49, 277–282 (2002).
Feliciano, R. et al. Characterization of traditional and exotic apple varieties from Portugal. Part 1–Nutritional, phytochemical and sensory evaluation. J. Funct. Foods 2, 35–45 (2010).
Acknowledgements
This work was financially supported by grants from the National Natural Science Foundation of China (Grant no. 31471525) and Key program of NSFC-Henan United Fund (No. U1704232). We would like to thank the native English speaking scientists of Elixigen Company (Huntington Beach, California) for editing our manuscript.
Author information
Authors and Affiliations
Contributions
K.Z., C.Z. and M.Y. performed all experiments involving peanuts; K.Z. and D.Y. performed data acquisition and analysis; and D.Y. conceived and designed the study, wrote and revised the manuscript, and approved the final version for submission.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Zhao, K., Zhao, C., Yang, M. et al. ZnCl2 treatment improves nutrient quality and Zn accumulation in peanut seeds and sprouts. Sci Rep 10, 2364 (2020). https://doi.org/10.1038/s41598-020-59434-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-020-59434-0
- Springer Nature Limited