Abstract
The aim of the study was to compare REM-dependent and REM-independent, obstructive sleep apnea syndrome (OSA) patients in relation to their daily sleepiness assessed by Epworth sleepiness scale (ESS). The study included 1863 consecutive patients, who were referred to a sleep centre with a presumed diagnosis of OSA. Following polysomnography, 292 patients fulfilled criteria for either REM-dependent OSA (REM-OSA, n = 102) or REM-independent OSA (nREM-OSA, n = 190). Both study groups were matched regarding sex and age. REM-OSA group had two times lower median apnoea-hypopnea index (AHI) compared to nREM-OSA (p < 0.001), yet day-time sleepiness measured by ESS was similar: median score 9.0 (6.0–11.0) and 8.0 (4.8–11.0), p = 0.109, respectively. Subsequent post-hoc ANCOVA analysis, with covariates (BMI, percent of total sleep time spent in REM stage, percent of total sleep time spent in the supine position), has shown statistically significant difference between study groups regarding AHI (p < 0.001) and no difference regarding ESS score (p = 0.063). Despite two times lower AHI, patients with REM-OSA present with similar day-time sleepiness as those with REM independent OSA. Daily sleepiness may be stronger associated with apneas/hypopneas occurring in REM than nREM sleep.
Similar content being viewed by others
Introduction
Obstructive sleep apnea syndrome (OSA) is characterized by recurrent episodes of apneas or hypopneas during sleep leading to intermittent hypoxemia and arousals. The severity of OSA is by convention assessed by apnea–hypopnea index (AHI)1. A few distinct phenotypes of OSA have been recognized with the main division lying between patients with sleep disordered breathing (SDB) independent of body position and sleep stage (usually obese with severe disease) and those with SDB dependent on supine position and/or REM sleep (usually non-obese, with mild to moderate disease)2,3.
Excessive daily sleepiness (EDS) is the one of leading complaints among patients with OSA and may be assessed by the Epworth sleepiness scale (ESS). It has been shown that EDS leads to impaired concentration, mood lability as well as other neurocognitive difficulties4. The relation between ESS score and the severity of OSA is still unclear as literature provides contradictory information with some researchers arguing in favour5,6,7 and some against8,9. It is conceivable that EDS may depend not solely on AHI index, but some more subtle disease indices. An ongoing and unresolved argument is pending, what are predictors of EDS, main culprits being sleep fragmentation due to arousals and intermittent hypoxemia, but also metabolic comorbidities, e.g. glucose intolerance10,11. Moreover, what is the contribution of EDS to prevalent cardiovascular or metabolic complications of OSA and why effects of CPAP treatment throughout the spectrum of the disease are so inconsistent12.
Based on clinical observation, we noted that some patients with substantial daily sleepiness presented with relatively low AHI calculated for total sleep, while SDB was confined to REM sleep (REM-dependent OSA, REM-OSA). Additionally, it has been shown that REM-dependent OSA is associated with cardiovascular and metabolic complications. Mokhlesi et al. reported that incidence of hypertension was strongly related to SDB in REM sleep13. Similarly, Grimaldi et al. found that worsening of glycemic control in type 2 diabetic patients was associated with SDB in REM sleep14. Thus, we hypothesise, that daily-sleepiness may be more related to SDB confined to REM sleep rather than occurring independently of sleep stage and the severity of the disorder defined by AHI alone. REM-OSA patients may have high AHI calculated exclusively for REM sleep, but due to usually fixed percentage of REM sleep in 20–25% range, their basal AHI calculated for total sleep time usually corresponds to mild or moderated disease. Therefore, to have a similar group for comparison in regard to AHI, but also other clinical metrics (e.g. age, sex, BMI) we decided to use mild and moderate (30 > AHI ≥ 5) sleep stage-independent OSA (nREM-OSA) as a control group. These patients are usually non-obese, presenting with positional disease, i.e. preponderance of SDB in the supine sleeping position.
Therefore, the aim of this study was to compare REM-OSA to nREM-OSA patients in respect to daily somnolence assessed by ESS15. We hypothesised, that SDB confined to REM results in greater EDS when compared to SDB not related to any sleep stage in patients with mild to moderate OSA severity.
Materials and Methods
Patients
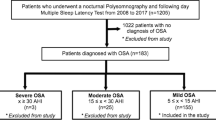
The study group consisted of 1863 consecutive patients, who were referred to Sleep and Respiratory Disorders Centre with presumptive diagnosis of OSA between January 2011 and December 2016.
All patients included in the study were assessed and investigated by authors and underwent diagnostic polysomnography (PSG); while scoring PSG studies, the authors were blinded for the clinical data. Overall, the prevalence of OSA in our cohort as defined by AHI ≥ 5/h was 76.6%, n = 1427. Furthermore, patients were excluded from the study if their total sleep time was shorter than three hours, if sleep time either in lateral or supine position was shorter than half an hour or if total REM sleep was shorter than half an hour. At the initial visit before PSG, patients underwent a standard examination and ESS was performed. Patients, who were not able to answer all 8 questions from ESS were excluded from study. Furthermore, as REM-OSA patients usually suffer from mild to moderate disease individuals with severe form of nREM-OSA (AHI ≥ 30, usually non-position dependent OSA) were also excluded.
Next, based on PSG results, patients were assigned into two subgroups if they presented with one of two distinct OSA phenotypes: REM-OSA (defined by AHIREM/AHInREM ≥ 2 and AHIREM ≥ 5/h) and nREM-OSA (defined by AHIREM/AHInREM < 1.5, and 30 > AHI ≥ 5/h) (Table 1). The latter group, usually, consisted of patients with positional disease, i.e. AHIback/AHIside ≥ 2 (Table 1). We used AHIREM/AHInREM < 1.5 as an inclusion criterion for nREM-OSA to better differentiate it from REM-OSA with AHIREM/AHInREM ≥ 2, as this ratio is a continuum in the cohort and we wanted to have a good discrimination between study groups in this respect. Patients, who did not fulfil these criteria were excluded from the study. Finally, based on the aforementioned inclusion and exclusion criteria we found 102 patients with REM-OSA and 190 patients with nREM-OSA eligible for the study. More information on the process of patients’ selection can be found in the Supplementary Information section.
Polysomnography
Patients were admitted to the sleep lab at 21:00 hours (±0.5 hour) and underwent physical examination (measurement of body mass, height, heart rate and blood pressure). A standard nocturnal polysomnography was performed by recording the following channels: electroencephalography (C4\A1, C3\A2), chin muscles and anterior tibialis electromyography, electrooculography, measurements of oro-nasal air flow (a thermistor gauge), snoring, body position, respiratory movements of chest and abdomen (piezoelectric gauges), unipolar electrocardiogram and haemoglobin oxygen saturation (SaO2) (Sleep Lab, Jaeger – Viasys, Hoechberg, Germany). Sleep stages were scored according to the criteria based on 30 s epoch standard10,11.
Apnea was attained with the reduction of air flow to less than 10% of the baseline for at least 10 s. Hypopnea was defined as at least 30% reduction of air flow for at least 10 s, accompanied by over 3% decrease in SaO2 or an arousal. EEG arousals were scored according to AASM guidelines16.
The study was conducted in accordance with the amended Declaration of Helsinki. All patients gave their informed consent to the sleep study. Ethics Committee of the Medical University of Lodz approved the study protocol (RNN/23/15/KE).
Statistical analysis
The data were analysed with Statistica 12 software. Data distribution was tested with the Shapiro-Wilk test. The Student’s T-test or Mann Whitney U were used to compare continuous variables in case of normal and non-normal distribution of data, respectively. The frequencies were compared with Chi2 test. Subsequent post-hoc ANCOVA analysis was performed including covariates, which were different between study groups. Results are reported as a mean ± standard deviation for normally distributed data and as a median with interquartile range (LQ – UQ) for non-normally distributed data. P < 0.05 was considered statistically significant.
Results
Out of 1863 patients, 292 were included in the final analysis of which 137 (46.9%) were men and the mean age of the group was 53.7 ± 10.6 years. Hundred and two individuals fulfilled criteria for REM-OSA and 190 for nREM-OSA.
There were no differences between the groups regarding sex, age, total sleep time (TST) or percentage of REM sleep. Groups differed regarding BMI and time spent in supine position during sleep. Characteristics of patients with REM-OSA and nREM-OSA is shown in Table 1.
AHI was 2 times higher for nREM-OSA than REM-OSA group (p < 0.001). To measure the impact of SDB occurring in REM sleep we calculated REM related AHI (AHIREM-related) dividing the number of REM apneas/hypopneas by TST and it was over 3 times higher in REM-OSA (p < 0.001). The polysomnography related variables and daytime sleepiness for REM- and nREM-OSA groups are presented in Table 1.
We subsequently divided the study groups into two subgroups: ESS < 10 and ESS ≥ 10 to find any factors that may be related to daily sleepiness. We observed no difference in the percentage of individuals scoring in the scale 10 or more between the groups. Moreover, metrics related to OSA, i.e. number of apneas/hypopneas, arousal index and desaturation parameters did not differ within subgroups (low vs high ESS) despite different median ESS scores. The differences in AHI and arousal index were similar between corresponding subgroups (low ESS REM-OSA vs nREM-OSA and high ESS REM-OSA vs nREM-OSA) to the differences noted between whole groups (Table 1). The results of this analysis are presented in Table 2.
Subsequent ANCOVA analysis (shown in Table 3) included variables that were different between groups: BMI, percent of TST spent in REM stage and percent of TST spend in supine position and were chosen as covariates for the purpose of the analysis. The analysis showed no difference between groups regarding EDS (p = 0.308), while severity of the disorder (AHI) remained statistically different (p < 0.001).
Discussion
We found that despite twice higher AHI in nREM- vs REM-OSA, both groups presented with similar daytime sleepiness based on ESS score, after adjusting for confounding variables. This result seems relevant as daily sleepiness represents an important aspect of OSA morbidity. This is not explicitly the confirmation of our hypothesis, because the study groups revealed similar daily sleepiness at different AHI levels, while we suspected the converse, i.e. the similar AHI levels but different ESS scores.
In previously published papers on relation of OSA severity and EDS, majority of them reported no correlation between the AHI index and ESS score9,17; similarly, we found no such correlation (data not shown). Therefore, other factors resulting in daytime sleepiness in OSA patients need to be addressed. As our study demonstrated, the ESS as a measure of day-time sleepiness was similar in study groups, despite lower AHI in REM-OSA group. Contrary to our findings, Punjabi et al.18 reported an increased risk of high EDS associated with SBD in non-REM sleep. However, EDS in the study was assessed by the Multiple sleep latency test, which even though considered to be more objective, focuses on different aspect of daily sleepiness than ESS. Furthermore, it has been suggested that EDS rather than being the effect of REM-dependent OSA, might be a result of lower O2 saturation during sleep among these patients19. However, we have not found any differences between our study groups regarding O2 saturation parameters, e.g. the mean SpO2 of desaturations and minimal SpO2, (Table 1, other saturation parameters not shown), which was also the case in other studies20. Similarly, when a subgroups with low and high EDS were compared, the difference in EDS could not be explained by saturation parameters or arousal index, as they did not differ (Table 2).
Thus, some other hypotheses are to be considered. It is plausible that non-interrupted REM is essential to the restorative properties of sleep; SDB in REM leads to arousals and as a consequence REM sleep fragmentation. The increased EDS in REM-OSA may be due to disruption of this phase continuity by SDB related arousals. Arousal index calculated for the total sleep time was lower in REM- vs nREM-OSA group, therefore, it could not be responsible for daily somnolence. Still, when we calculated AHI using only apneas/hypopneas that occurred in REM sleep, it was 3-fold higher in REM-OSA than in nREM-OSA, which could explain similar symptomatology despite lower total AHI. Hence, this indirectly supports the notion the integrity of REM sleep may be essential for refreshing properties of sleep and apneas/hypopneas occurring in this sleep stage may have greater impact on EDS.
The study groups were well matched regarding the clinical variables (Table 1) with the exception of BMI. REM-OSA presented with the mean BMI 2 kg/m2 higher than nREM-OSA group. It seems rather infeasible that this small difference might have accounted for the difference in daily-sleepiness, as ANCOVA analysis excluded this confounding effect.
Our study showed roughly 5.5% of patients suffering from OSA presented a pure REM-dependent phenotype, while, some studies suggested that the prevalence of this phenotype might be higher, reaching up to 10%21. This lower prevalence of REM-OSA phenotype might be related to strict inclusion criteria for this group that we used. There is evidence to support OSA patients with higher EDS respond better to CPAP treatment, reducing other comorbidities (e.g. high blood pressure)22 and improving quality of life21,23. Therefore, due to a higher morbidity in REM dependent OSA it may be justified to consider CPAP treatment at lower AHI levels.
Limitations of the study
In order to compare REM-OSA and nREM-OSA phenotypes we enrolled patients with SDB confined to REM sleep (AHIREM/AHInREM ≥ 2) and a control group with SDB not dependent on sleep stage (AHIREM/AHInREM < 1.5) within similar, mild to moderate, AHI range. Nevertheless, it should be underlined that the group selection was not perfect due to intrinsic properties of nREM-OSA phenotype. While, pure REM-OSA does exist, with patients presenting with SDB exclusively in REM sleep, in nREM-OSA SDB occurs in REM and nREM sleep to the similar extent, thus there is no a pure nREM-OSA phenotype to use for comparison. Therefore, we compared REM-OSA phenotype with a mixed one, i.e. no sleep stage dependent one. However, the simulated AHI calculated exclusively from all apneas/hypopneas that occurred only in REM sleep was 3-times higher in REM-OSA than in nREM-OSA which defends our group selection criteria and study design.
Matching the groups with respect to AHI and other clinical variables was another problem affecting the study design and analysis. REM-OSA patients usually have similar night to night AHI which depends mainly on rather constant REM sleep percentage and is in the mild-moderate range. In mild to moderate nREM-OSA group it can be highly variable and depends on percentage of sleep spent in a supine position, as majority of these patients have positional OSA with the ratio AHIback/AHIside ≥ 2. This implicates that their disease is better described by AHI range (from low AHIside to high AHIback) than a single value and night to night AHI in this group is variable and it cannot be predicted from a single PSG night. Therefore, our groups differed in respect to AHI but had similar daily sleepiness per ESS, which was converse to the study hypothesis.
In conclusion, this study contributes to the pending discussion on predictors of OSA morbidity. Apart from implicated quantitative indices of disease severity, e.g. AHI, arousals or desaturations, we posit that qualitative differences in OSA phenotypes related to SDB occurring in different sleep stages may independently explain the severity of daily sleepiness.
References
Gabryelska, A., Łukasik, Z. M., Makowska, J. S. & Białasiewicz, P. Obstructive Sleep Apnea: From Intermittent Hypoxia to Cardiovascular Complications via Blood Platelets. Front Neurol 9, 635 (2018).
Zinchuk, A. V., Gentry, M. J., Concato, J. & Yaggi, H. K. Phenotypes in obstructive sleep apnea: A definition, examples and evolution of approaches. Sleep Med Rev 35, 113–123 (2017).
Mokros, Ł. et al. High Negative Predictive Value of Normal Body Mass Index for Obstructive Sleep Apnea in the Lateral Sleeping Position. J Clin Sleep Med 14, 985–990 (2018).
Vaessen, T. J. A., Overeem, S. & Sitskoorn, M. M. Cognitive complaints in obstructive sleep apnea. Sleep Med Rev 19, 51–58 (2015).
Lee, S. J., Kang, H. W. & Lee, L. H. The relationship between the Epworth Sleepiness Scale and polysomnographic parameters in obstructive sleep apnea patients. Eur Arch Otorhinolaryngol 269, 1143–1147 (2012).
Gottlieb, D. J. et al. Relation of sleepiness to respiratory disturbance index: the Sleep Heart Health Study. Am. J. Respir. Crit. Care Med. 159, 502–507 (1999).
Sun, Y. et al. Polysomnographic characteristics of daytime sleepiness in obstructive sleep apnea syndrome. Sleep Breath 16, 375–381 (2012).
Sauter, C. et al. Excessive daytime sleepiness in patients suffering from different levels of obstructive sleep apnoea syndrome. J Sleep Res 9, 293–301 (2000).
Haba-Rubio, J., Janssens, J.-P., Rochat, T. & Sforza, E. Rapid eye movement-related disordered breathing: clinical and polysomnographic features. Chest 128, 3350–3357 (2005).
Bialasiewicz, P., Pawlowski, M., Nowak, D., Loba, J. & Czupryniak, L. Decreasing concentration of interstitial glucose in REM sleep in subjects with normal glucose tolerance. Diabet. Med. 26, 339–344 (2009).
Bialasiewicz, P., Czupryniak, L., Pawlowski, M. & Nowak, D. Sleep disordered breathing in REM sleep reverses the downward trend in glucose concentration. Sleep Med. 12, 76–82 (2011).
Garbarino, S. et al. Obstructive Sleep Apnea With or Without Excessive Daytime Sleepiness: Clinical and Experimental Data-Driven Phenotyping. Front Neurol 9, 505 (2018).
Mokhlesi, B. et al. Obstructive sleep apnea during REM sleep and hypertension. results of the Wisconsin Sleep Cohort. Am. J. Respir. Crit. Care Med. 190, 1158–1167 (2014).
Grimaldi, D., Beccuti, G., Touma, C., Van Cauter, E. & Mokhlesi, B. Association of obstructive sleep apnea in rapid eye movement sleep with reduced glycemic control in type 2 diabetes: therapeutic implications. Diabetes Care 37, 355–363 (2014).
Johns, M. W. A new method for measuring daytime sleepiness: the Epworth sleepiness scale. Sleep 14, 540–545 (1991).
Kapur, V. K. et al. Clinical Practice Guideline for Diagnostic Testing for Adult Obstructive Sleep Apnea: An American Academy of Sleep Medicine Clinical Practice Guideline. JCSM 13, 479–504 (2017).
Oksenberg, A., Arons, E., Nasser, K., Vander, T. & Radwan, H. REM-related obstructive sleep apnea: the effect of body position. JCSM 6, 343–348 (2010).
Punjabi, N. M. et al. The association between daytime sleepiness and sleep-disordered breathing in NREM and REM sleep. Sleep 25, 307–314 (2002).
Mediano, O. et al. Daytime sleepiness and polysomnographic variables in sleep apnoea patients. Eur. Respir. J. 30, 110–113 (2007).
Sunnetcioglu, A., Sertogullarından, B., Ozbay, B., Gunbatar, H. & Ekin, S. Obstructive sleep apnea related to rapid-eye-movement or non-rapid-eye-movement sleep: comparison of demographic, anthropometric, and polysomnographic features. J Bras Pneumol 42, 48–54 (2016).
Gillman, A., Roebuck, T., Ho, S., van Braak, E. & Naughton, M. T. Comparison of supine-only and REM-only obstructive sleep apnoea. Sleep Med. 13, 875–878 (2012).
Fava, C. et al. Effect of CPAP on blood pressure in patients with OSA/hypopnea a systematic review and meta-analysis. Chest 145, 762–771 (2014).
Weaver, T. E. et al. Continuous positive airway pressure treatment of sleepy patients with milder obstructive sleep apnea: results of the CPAP Apnea Trial North American Program (CATNAP) randomized clinical trial. Am. J. Respir. Crit. Care Med. 186, 677–683 (2012).
Acknowledgements
Supported by Medical University of Lodz grant no. 503/1-079-06/503-11-001-18.
Author information
Authors and Affiliations
Contributions
A.G. and P.B. created the concept of the study and performed statistical analysis. A.G. and P.B. collected the data, created the data base and wrote the manuscript. P.B. revised the paper.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Gabryelska, A., Białasiewicz, P. Association between excessive daytime sleepiness, REM phenotype and severity of obstructive sleep apnea. Sci Rep 10, 34 (2020). https://doi.org/10.1038/s41598-019-56478-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-56478-9
- Springer Nature Limited
This article is cited by
-
The relationship between sleep quality, daytime sleepiness, and rapid eye movement obstructive sleep apnea (REM-OSA)
Sleep and Breathing (2023)
-
Neurocognitive, mood changes, and sleepiness in patients with REM-predominant obstructive sleep apnea
Sleep and Breathing (2023)
-
Sleep-physiological correlates of brachycephaly in dogs
Brain Structure and Function (2023)
-
Characteristics of rapid eye movement-related obstructive sleep apnea in Thai patients
Scientific Reports (2022)
-
Comorbidities in Clinical and Polysomnographic Features of Obstructive Sleep Apnea: A Single Tertiary Care Center Experience
Journal of Epidemiology and Global Health (2022)