Abstract
Scarcity of fresh water in arid and semi-arid regions means that we must use more saline waters for irrigation and develop tools to improve crop salt tolerance. The objectives of our study were to (1) Evaluate fruit production, salt tolerance and ion composition of eggplant cv Angela, both nongrafted and when grafted on tomato cv Maxifort rootstock and (2) Evaluate eggplant specific toxicity effect of Cl− and Na+ ions under saline conditions. We salinized the irrigation water with either a Na+-Ca2+- Cl− composition typical of coastal Mediterranean ground waters as well as a mixed Na+-Ca2+-SO42− Cl− type water, a composition more typical of interior continental basin ground. For each water type we evaluated 5 different salinity (osmotic) levels of –0.003 (control), –0.15, –0.30, –0.45 and –0.60 MPa. There were no statistically significant differences in the fruit yield relative to the water type, indicating that Cl− ion toxicity is not a major factor in eggplant yield associated with salinity. This conclusion was confirmed by the determination that leaf Cl content was not correlated with relative yield. The electrical conductivity of the saturation extract (ECe) at which yield is predicted to be reduced by 50% was 4.6 dS m−1 for the grafted plants vs. 1.33 dS m−1 for the nongrafted plants. The relative yield was very well correlated to leaf Na concentrations regardless of grafting status, indicating that Na is the toxic ion responsible for eggplant yield loss under saline conditions. The increased salt tolerance of cv Angela eggplant when grafted onto tomato Maxifort rootstock is attributed to a reduced Na uptake and increased Ca and K uptake with Maxifort rootstock.
Similar content being viewed by others
Introduction
Semiarid regions of the world have a scarcity of good-quality water. Competitive demand for fresh water among urban, industrial, and agricultural sectors has led researchers to focus on using marginal waters for agricultural production1. There have been many research efforts to improve the salt tolerance of crops by traditional breeding programs. However, commercial success has been very limited as salt tolerance is complex genetically and physiologically2,3. Factors limiting plant growth under saline conditions may vary among crops, requiring crop specific information before traditional breeding or marker assisted breeding can be successful. Furthermore, lack of general public acceptance of genetic engineering for crop improvement means that other approaches to improving yield under saline conditions need to be considered3.
Increased tolerance to salinity was related to reduced Na+ in shoot tissues with no change in Cl− 4 reduced concentrations of Na+ and Cl− 3, and increased K+ with lower Na+ and Cl− 5. Surprisingly a large percentage of salinity studies on horticultural or agronomic crops use NaCl as the sole salinizing agent. The use of these single salt salinizing compositions may limit the extent to which the results can transferred to field conditions, especially if the salinity damage is caused by specific toxic ions rather than osmotic effects. The same argument can be made for the anion as well as cation composition6. Munns and Testor7 considered that several processes were involved with salt tolerance, primarily the effects of osmotic stress and secondarily tissue ion tolerance.
Most published salinity and grafting experiments were conducted solely with NaCl as the salinizing salt3,8,9. Penella et al.10 conducted a field experiment with grafted and nongrafted pepper using a mostly NaCl irrigation water at EC = 7.5 dS m−1. The salt tolerance of eggplant has been investigated earlier, primarily with NaCl as the salinizing solution. Assaha et al.11 measured a 49% decrease in vegetative growth when eggplant was irrigated for 14 d with 50 mM NaCl, Heuer et al.12 in a field experiment determined that fruit yield decreased above ECe = 1.1 dS m−1. Based on his salinity response equation we calculate that he had a 50% yield loss at ECe = 8.3 dS m−1. Hannachi et al.13 measured differences in salt tolerance among four eggplant cultivars. Akinci et al.14 determined that NaCl salinity decreased germination and seedling dry weight of three eggplant cultivars while leaf Na increased (leaf Cl not determined). We found no studies that investigated specific anion effects in relation to salinity tolerance of grafted eggplant in terms of yield. Earlier studies on tomato have found no significant effects comparing self-grafted and nongrafted rootstock of tomato indicating that grafting per se had no impact on fruit yield9,15, or qualitative fruit parameters and measured fruit ion composition15. These findings are consistent with the results of Fernandez-Garcia et al.16 that xylem and phloem vessels are formed through the graft union 8 d after grafting. Under saline conditions there were no differences in tomato fruit yield between nongrafted and self grafted plants3,9, indicating that salt tolerance is also unaffected by grafting per se.
The objective of our study was to (1) Evaluate the yield and salt tolerance of eggplant, cv Angela, under both nongrafted conditions and when grafted on tomato cv Maxifort rootstock and (2) Evaluate eggplant specific toxicity effect of Cl− and Na+ ions and K/Na relatons under saline conditions. The effects of salt ion composition on plant salt tolerance are important for improved prediction of crop response to salinity under field conditions and for identifying specific ion toxicity for future salt tolerance breeding.
Material and Methods
The study was carried out in a greenhouse within 30 sand tanks (1.2 length × 0.6 width × 0.5 m deep) in USDA Salinity Laboratory., Riverside, California. Two grafted and two nongrafted seedlings were sown in each sand tank. The sand tanks were flood irrigated with the same amount of water (approximately 130 L) once a day. The daily flushing of the sand means that the rootzone salinity was maintained essentially equal to the irrigation water (reservoir) salinity. Drainage water returned to the same reservoir (1,500 L) after each irrigation. Irrigation water quality checks performed daily (EC and pH) and weekly sampled and analyzed to monitor ion content. Addition of deionized water was generally sufficient to maintain ion composition (with minor nitric acid and KCl addition to maintain pH, nitrate and K concentrations. Micronutrients were also reapplied approximately midway through the experiment. Salinity treatments were initiated two weeks after planting. Grafted and nongrafted eggplant seedling were purchased from Bevo Farms (Milner, BC, Canada). Eggplant seedlings consisted of nongrafted cv Angela and grafted cv Angela scions onto cv Maxifort tomato rootstock. Salinity treatments were undertaken at osmotic potential (OP) levels of –0.003 (control), –0.15, –0.30, –0.45 and –0.60 MPa.
In order to examine the effects of different source of salts at the same OP level, we prepared SO42−Cl− and Cl− irrigation waters to represent two water types. The salt treatments were prepared for the two water types, either equal (in mmolc L−1) concentrations of Ca2+ and Na+ with Cl− as the anion, designated as a Cl− water, or a mixed salt solution better representing arid zone of the world, designated as a SO42−Cl− water. The SO42−Cl− water had a relatively high SO42− concentration, Na+ > Ca2+ at high salinity, and increasing Mg2+ with increasing salinity17. The calculations to obtain equal osmotic values for the two water types were made using the Extract Chem model18. The composition of irrigation waters is shown in Table 1. Modified half Hoagland’s solution (plant nutrient solution) included 0.17 mmol L−1 KH2PO4, 0.75 mmol L−1 MgSO4∙7H2O, 2.0 mmol L−1 KNO3, and 0.25 mmol L−1 CaSO4. 2H2O with micronutrients, also expressed in mmol L−1, of 0.34 KH2PO4, 0.050 Fe (as sodium ferric diethylenetriamine pentaacetate), 0.023 H3BO3, 0.005 MnSO4, 0.0004 ZnSO4, 0.0002 CuSO4, and 0.0001 H2MoO4.
Irrigation water composition including Na, K, Ca, Mg, S, Fe, Mn, Cu, and Zn determined using PerkinElmer Optima 3300DV ICP OES (inductively coupled plasma optical emission spectroscopy) (PerkinElmer Corp, Waltham, MA, USA), and Cl by amperometric titration using a Labconco chloridometer (Labconco, Kansas City MO, USA) and NO3− by spectrophotometrically using a Hitachi model 100-20 (Hitachi Corp, Japan) at a 210 nm wavelength. The plant and fruit samples were washed in deionized water, dried in a forced-air oven at 70 °C for 72 h, and ground in a Wiley mill to pass a 60 mesh screen. Total S, total P, Ca, Mg, Na, and K of the leaf and fruit tissue were determined from nitric–perchloric acid digests of the tissues by ICP OES. The fruit and leaf Cl− was determined on nitric–acetic acid extracts by amperometric titration. Statistical analyses of all data were performed using SPSS 16.0 (SPSS Inc., Chicago, IL, USA). The salt tolerance models suggested by Maas and Hoffman19 and van Genuchten and Hoffman20 were used to evaluate the salinity tolerance for grafted and nongrafted plant respectively.
Results
Yield
The yield data, presented in Table 2 show higher fruit yields for Maxifort grafted plants relative to nongrafted plants at all osmotic pressures. The statistical results on the yield data indicate that there were no significant differences in yield based on water type (p < 0.05) thus subsequent analysis considered the entire data set.
Grafting, salinity and interactions of grafting x salinity had significant effects on yield at p < 0.05 level (Table 2). The highest yield was obtained from the grafted control treatment with 11.16 kg plant−1. In our experiment, Maxifort grafted plants had yield decreases of 6.8, 53, 66 and 80%, relative to the control, for salinity levels of −0.15, −0.30, −0.45 and −0.60 MPa, respectively. The significant interaction of grafting x salinity is evidenced by the data that grafted plants had slightly higher yields under control and much higher yields under OP levels of −0.15 to −0.45 MPa (corresponding to EC values of 4 to 12 dS m−1). For the control treatment in the absence of salt stress, the yield difference was 22.8% greater for the Maxifort grafted plants as compared to nongrafted plants. The yield differences between Maxifort grafted and nongrafted plants were much more prominent under moderate and high salinity, with the yield differences at 3.98 and 8.26 dSm−1 being 80.5 and 68.1%, respectively. Thus, in our experiment, the positive effect induced by grafting tomato (cv Maxifort) rootstock on eggplant fruit salt tolerance increased with the level of salt stress.
Ion uptake in leaves and fruits
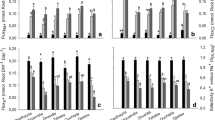
Statistical examination revealed that there was a significant (p < 0.001) difference in Na+ shoot content between Maxifort grafted and nongrafted plants, with grafted plants having lower Na+ uptake (Table 3). There were no statistical differences in Na+ content in leaves related to water type (data not shown) so the data were combined. This is not unexpected as the two water compositions have similar Na+ ion compositions (Table 1). In the control treatment sodium uptake for grafted and nongrafted plants was not statistically different (Table 3). Increasing irrigation water salinity led to an increase in Na+ uptake by both grafted and nongrafted plants, and at any salinity level Na+ uptake in leaves was significantly and much lower for grafted plants (Table 3). Similarly, fruit Na+ contents were not significantly different for water type but were significantly greater in nongrafted as compared to grafted plants (Table 4). Except for the control treatment, Cl− content in leaves were higher for treatments irrigated with Cl− as compared to SO42—Cl− waters, again consistent with the water compositions. Calcium accumulation in leaves was affected by both water type and grafting (Table 3). The Cl− salt treatment had slightly (12.4%) but significantly greater Ca2+ on average as compared to the SO42+ – Cl− treatment (Table 3), consistent with the greater Ca2+ in the Cl− salt irrigation waters. The average leaf Ca2+ content of grafted eggplants was much greater (40%) and highly significantly different than nongrafted plants, p < 0.001 (Table 3). Statistical analyses revealed that the mean Ca2+ content of the fruit was greater for grafted as compared to nongrafted plants (Table 4). The fruit Ca2+ content decreased with salinity (Table 4).
Leaf Mg2+ contents were affected by salinity, water type and grafting. There were grafting x salinity and salt x salinity interactions (Table 3). The Mg2+ content for grafting was greater than for nongrafted plants (Table 3). Consistent with leaf Mg accumulation, average fruit Mg2+ concentration was also higher in the SO42−Cl− salt treatments (Table 4). Leaf and fruit S accumulation were also affected by water type, as SO42−Cl− water treatments had higher S in leaves and fruits as compared to the Cl− irrigation water treatments (Tables 3 and 4).
Leaf K+ concentrations were significantly and much higher in the grafted plants as compared to the nongrafted plants (Table 3) for the controls as well as for all salinity treatments, however K+ was in all instances above deficiency levels, (<20–50 mg kg−1 vegetative dry weight, Marschner21) and there were no visual symptoms of K+ deficiency.
The P content in leaves was in general not significantly greater in nongrafted Angela plants as compared to Maxifort grafted plants across all salinity concentrations (Table 3) nor was there any indication of P deficiency. Fruit P concentrations were not significantly different as related to salinity or grafting, but were slightly greater on average in Cl as compared to SO4 type irrigation waters (Table 4).
Leaf Cl contents were affected by salinity, grafting and water type. Consistent with increased Cl content in the Cl type waters, these treatments had increased leaf Cl. Nongrafted plants had significantly greater leaf Cl concentrations and Cl content increased with salinity (Table 3). In contrast Cl content in fruit from nongrafted plants was significantly lower than Cl content in Maxifort grafted plants (Table 4).
Discussion
The lack of a significant difference in fruit yield between the Cl− and the SO42−Cl− irrigation water types means that Cl− as a specific toxic ion was not an important factor affecting eggplant yield under saline conditions. The much greater Cl− concentration in the Na+-Ca2+-Cl− irrigation water did not cause a significant decrease in yield.
Earlier, Savvas and Lenz22 found no difference in eggplant yield between plants salinized with NaCl and those salinized with nutrient solution. They concluded that eggplant response to salinity was related to osmotic rather than toxic ion effects. However they examined only a control and one salinity level (EC = 4.7 dS m−1) and under the saline treatments (with or without added nutrients) they found only a 19% total fruit yield loss relative to the control. Thus the yield loss under salt stress in their experiment may not have been sufficiently large to detect differences in yield related to ion composition. In our experiment the yield loss of grafted plants at EC = 4.0 dS m−1 was also low, only 7% yield loss relative to control, and also not significantly different from the control (Table 2).
In our experiment, eggplant grafted to cv. Maxifort tomato rootstock had increased yield under control conditions relative to nongrafted eggplant, but the relative differences were much greater under saline conditions. The increased differences in eggplant yield that we observed with increasing stress, has been observed earlier in tomato when one genotype was grafted onto another more tolerant rootstock3,9,23. Compared with the control, the yield decreases for Maxifort grafted plants were 6.8, 53, 66.5, 80% while they were 76.5, 80, 82 and 90% for nongrafted plants, respectively (Table 2).
Salinity tolerance
Salt tolerance can often be adequately described on the basis of two parameters: threshold, and slope20. Typically these data are expressed as ECe, the EC of a saturation extract as shown in Eq. 1
Where Y/Yo is relative yield, a is the threshold EC salinity level at which yield starts to decline, b is the slope of the response curve expressed as % yield loss per dS m−1 and ECe is the EC of the saturation extract. In our experimental sand tank system the relation between ECiw and ECe (saturated paste) was calculated as ECe = 0.472 ECiw24. As seen in Fig. 1a, the Maas-Hoffman model well represented the salinity response for grafted plants (R2 = 0.96). The model provides a salinity threshold value of 0.42 dS m−1 with a 12.2% slope. The predicted ECe for 50% yield is 4.6 dS m−1. The Maas-Hoffman model was not satisfactory for representing the salinity response of nongrafted plants, primarily because the yield loss was very extensive between the control and the first salinity treatment and the data were highly non-linear. In this instance we analyzed the response using the van Genuchten and Hoffman20 model, shown below
where αs is the dimensionless stress response function (relative yield), h is the osmotic stress and h50 is the stress value at which there is a 50% yield loss, and b is an empirical fitting parameter. Using this model, expressed in terms of ECe rather than OP, we optimized the EC50 to the observed biomass data at the different salinities using TableCurve 2D version 5.025. The 50% yield loss corresponded to ECe = 1.33 dSm−1 (Fig. 1b) and the model fit resulted in a good prediction (R2 = 0.90). The yield data clearly showed that the Maxifort grafted plants had significantly greater yield than nongrafted plants (Table 2) and the salt tolerance analysis showed that salt tolerance was also greater for grafted plants (Fig. 1). The increased salt tolerance in Maxifort grafted plants is evidenced by the calculated 50% yield loss at 4.6 dS m−1 as compared to a 50% yield loss at 1.33 dS m−1 in the nongrafted Angela eggplant.
In a short term experiment Assaha et al.11 measured a 49% decrease in leaf dry weight for eggplant irrigated with 50 mM NaCl solutions relative to the non-saline control. This value corresponds to an approximate ECe of 2.5 dS m−1, but direct comparison with our data was not possible as they measured vegetative growth not fruit yield. In a field experiment, the relative yield of eggplant cv Black Oval, was reported as 72% of control at an ECe = 4.7 dS m−1 12 thus somewhat more salt tolerant than even our grafted plants. These comparisons suggest that either varietal differences in eggplant salt tolerance are quite large, and/or that under greenhouse conditions in our study, there was less stress from other abiotic factors thus salt stress was more evident than under field conditions. Since earlier research established that grafting per se does not affect yield and water and ion uptake, we attribute the effect of grafting to increased salt tolerance of Maxifort tomato as compared to Angela eggplant. Tomato appears to be more salt tolerant than eggplant. For example under experimental conditions similar to this study, Big Dena tomato had a 50% fruit yield loss at ECe = 6.6 dS m−1 17 as compared to ECe = 1.33 for Angela eggplant (Fig. 2). Grafting Maxifort rootstock onto Big Dena tomato resulted in 50% tomato yield loss at ECe 5.7 dS m−1 17 a value close to that found for eggplant grafted to Maxifort rootstock in this study (ECe = 4.6 dS m−1). Thus the salt tolerance response of Maxifort grated eggplant is similar to the response of Maxifort grafted on another tomato variety.
Ion uptake in leaves and fruits
As there is little data on the relation of tissue ion composition and salt tolerance for eggplant we primarily must relate our data to findings reported for other solanaceae species (tomato and pepper). Low accumulation of Na+ and/or Cl− in the shoot is frequently a characteristic of salt tolerant grafted tomato plants3,4,17,26. However Al-Harbi et al.27 found no decrease in Na+ or Cl− contents on grafted tomato and Penella et al.10 found that grafting a salt tolerant pepper rootstock on a commercial pepper variety increased yield and photosynthesis parameters but did not decrease Na+ and Cl- leaf ion content as compared to nongrafted pepper. Penella et al.28 determined increased salt tolerance of grafted pepper as compared to nongrafted pepper and significantly greater Cl− but not Na+ in the leaves of nongrafted plants. Although these experiments did not evaluate different irrigation water compositions, it seems most likely that the specific response to grafting pepper and tomato to ion uptake depends on the specific characteristics of the rootstock relative to the scion and might not be generalized, as commented earlier by others29.
The shoot ion content results from our experiment, where nongrafted plants had significantly higher Na+ concentrations are consistent with Na+ ion toxicity as yield and salt tolerance differences were greater for the grafted plants. Trends in fruit Na+ content in our experiment were also consistent with the leaf Na+ content. Grafting with Maxifort rootstock restricted Na+ accumulation in eggplant fruits. The differences in Na uptake between grafted and nongrafted plants may thus be an explanation of the increased salt tolerance of Maxifort grafted eggplants. This result is also consistent with the finding that cv Big Dena tomato scion grafted onto cv Maxifort tomato rootstock tends to exclude Na+ ions relative to cv Big Dena tomato15. Also, Bai et al.30 reported that eggplant grafted onto S. torvum had lower leaf Na+ and Cl− contents than did self-rooted plants under NaCl stress. These results are in contrast to Almeida et al.31 who in a three week experiment did not find a correlation between Na+ concentrations in the leaves and vegetative growth of 23 tomato accessions. They concluded that the relationship between Na+ concentration in the cells and tissue tolerance to salinity may vary among accessions. Our results are also in contrast to those of Huertas et al.32 who determined that in a five day experiment transgenic tomato with overexpression of SISOS2 gene was associated with higher Na+ content in leaves and higher growth relative to control. However Rivero et al.33 determined that tomato plants maintained higher K+/Na+ ratios, and reduced Na+ as well as better physiological response in combined heat-salinity stress treatments as compared to salinity only treatments. Their four day experiment on 30 d old plants could not evaluate the long term effects on biomass and yield. However, they did measure increased CO2 assimilation and increased transpiration in the combined stress treatments, consistent with greater K+/Na+ ratios less adverse salinity effects. Assaha et al.11 compared vegetative growth and ion uptake of eggplant and huckleberry under two levels of NaCl. The smaller decrease in relative growth of huckleberry as compared to eggplant was attributed to the lower leaf Na+ concentration in huckleberry however they did not evaluate leaf Cl− concentrations.
Leaf Cl− content in our experiment increased with increasing salinity but Maxifort grafted plants had lower leaf Cl− as compared to nongrafted plants at all salinity levels. As found for Na+, the Cl− levels were approximately double in the nongrafted as compared to grafted plants. On this basis alone we cannot distinguish Na+ from Cl− ion toxicity. These results are thus similar to those found by Estan et al.3 for grafted tomato and Bai et al.30 for eggplant. Penella et al.28 observed that grafted plants (irrigated with 40 mM NaCl) that were more salt tolerant than other grafted combinations of pepper, had lower Na and Cl content in the scion. They attributed the improved salt tolerance on ability to restrict toxic ions Na+ and Cl− ions from either entering the roots or being transported to the leaves rather than through synthesis of osmotically active metabolites28. Other researchers have also determined that more salt tolerant cultivars of rootstocks of grafted plants had reduced leaf Na and Cl content for pepper13, for tomato9,34 reduced Na for tomato (Cl not analyzed)35, reduced Na but not reduced Cl for tomato17. With the exception of Semiz and Suarez17, earlier studies on tomato, eggplant and pepper used a single salt as the salinizing solution (almost always NaCl), thus they could not evaluate relative toxicity of Na+ and Cl− ions.
In our experiment we can evaluate the relative importance of Na+ vs Cl− ions by examination of the results of irrigation with two water types. The SO42−Cl− water type had yields that were not statistically different across salinity levels from the Cl− type waters. Thus the Maxifort grafted plants had greater salt tolerance than nongrafted Angela eggplant under both water types, providing strong evidence that Na is the growth limiting toxic ion under these salinity levels. Furthermore, analysis of the relationship between leaf Na+ and relative yield and leaf Cl− and relative yield, evaluating different salinities, grafting and water types, shows that leaf Cl− was not a predictor of eggplant yield (Fig. 2a) This statistical analysis is consistent with the determination that the Cl− salt treatment waters did not have significantly different yield than the SO42−Cl− salt treatment waters. We conclude that Cl− ion toxicity is not the major source of yield loss in eggplant.
In contrast to the lack of a relationship between leaf Cl− and relative yield, there was a good relationship between leaf Na+ and relative yield, representing all four treatments. The yields, and salt tolerance of these treatments were very different yet as shown in Fig. 2b (R2 = 0.83), relative yield was well predicted by leaf Na+ concentration (across water types, salinity and grafting). These data strongly indicate that Na+ ion is the yield limiting toxic ion in eggplant and the Maxifort grafted plants increased salt tolerance relative to nongrafted Angela can be attributed to improved Na+ exclusion of the Maxifort rootstock.
Many studies have indicated that maintenance of a high K/Na ratio is critical to salt tolerance, uptake9,13,35,36. Li et al.37 examined a salt tolerant and salt sensitive eggplant cultivar under 200 mM NaCl. They observed that the salt tolerant cultivar had a higher K/Na ratio in the leaves as well as upregulation of seven genes related to K+ transport. They concluded that K uptake is more important than Na exclusion because only one Na transporter was regulated in a similar fashion. However, rather than counting number of genes upregulated, if we examine the ion content in there study we find approximately equal changes in Na and K. We calculate that at the end of their experiment (23 d of exposure to salinity), the salt tolerant cultivar had 50% of the Na content and 180% of the K content of the salt sensitive cultivar. In our study grafted plants had 47% and 40% of the Na content of the nongrafted plants at the two highest salinity levels, respectively and 152 and 130% of the K leaf content of nongrafted plants respectively. Thus greater K content as well as lower Na content in eggplant is associated with increased salt tolerance. The relationship between K/Na vs. fruit yield is shown in Fig. 3 for all treatments. There is a relationship of decreasing yield below K/Na ratios of 22 but the relationship is not as good as that between Na and yield (Fig. 2b). The increase in leaf K was much greater than the decrease in leaf Na at each salinity level (Table 2) so it is not simply a case of improved K/Na selectivity. Our results are consistent with the findings of others that salt tolerant varieties or salt tolerant grafted plants had increased K/Na selectivity. The grafting of Maxifort rootstock on Angela thus results in substitution of K+ for Na+ ions, which is beneficial to adaption to saline conditions, minimizing Na+ toxicity while maintaining osmotic adjustment.
The increased Ca2+ uptake in grafted plants was associated with a corresponding decrease in Na+ uptake, suggesting that the mechanism reducing Na+ uptake in grafted plants was also related to Ca2+/Na+ selectivity. The greater uptake of Mg2+ by grafted plants is also explained by uptake mechanisms that exclude monovalent (Na+) ions relative to divalent (Ca2+ and Mg2+) ions. The increased Mg2+ uptake observed in the SO42−Cl− water treatments is explained by the greater Mg2+ content in those irrigation waters relative to the Cl− waters. Although high concentrations of K+, considered luxuriant uptake, can lead to Ca2+ and Mg2+ deficiency31, in our experiment high K+ in the Maxifort grafted plants was also associated with increased uptake of Ca2+ and Mg2+ and decreased uptake of Na+. Our leaf K values for nongrafted cv Angela plants was in agreement with results observed by Unlukara, et al.38 for nongrafted eggplant cv Kemer.
The increased salt tolerance of eggplant grafted to tomato cv. Maxifort is attributed primarily to decreased Na+ leaf ion concentration and increased K+ and Ca2+ leaf concentrations. The lack of a difference in yield between plants irrigated with Cl− as opposed to SO42−Cl− waters and the lack of a correlation between leaf Cl− and fruit yield strongly indicates that the improved salt tolerance of eggplant grafted plants is not related to Cl− ion toxicity. Future improvements in eggplant salt tolerance can be focused on improved Na+ ion exclusion mechanism or genes related to that process.
References
Semiz, G. D., Suarez, D. L., Ünlükara, A. & Yurtseven, E. Interactive effects of salinity and N on pepper (Capsıcum Annuum L.) yield, water use efficiency and root zone and drainage salinity. J Plant Nutr 37, 595–610 (2014).
Flowers, T. J. Improving crop salt tolerance. J Exp Bot 55, 307–319 (2004).
Estan, M. T., Martinez-Rodriguez, M. M., Perez-Alfocea, F., Flowers, T. J. & Bolarin, M. C. Grafting raises the salt tolerance of tomato through limiting the transport of sodium and chloride to the shoot. J Exp Bot 56, 703–712 (2005).
Goreta, S., Bucevic-Popovic, V., Selak, G. V., Pavel-Vrancıc, M. & Perica, S. Vegetative growth superoxide dismutase activity and ion concentration of salt-stressed watermelon as influenced by rootstock. J Agri Sci 146, 695–704 (2008).
Huang, Y., Tang, R., Cao, Q. & Bie, Z. Improving the fruit yield and quality of cucumber by grafting onto the salt tolerant rootstock under NaCl stress. Sci Hortic 122, 26–31 (2009).
Grattan, S. R. & Grieve, C. M. Salinity - Mineral nutrient relations in horticultural crops. Sci Hortic 78, 127–157 (1999).
Munns, R. & Testor, M. Mechanisms of salinity tolerance. Plant Cell Environ 59, 651–681 (2008).
Santa-Cruz, A., Martínez-Rodríguez, M. M., Charter, J. & Bolarin, M. C. Response of plant yield and ion contents to salinity in grafted tomato plants. Acta Horti 559, 413–417 (2001).
Martinez-Rodriguez, M. M. et al. The effectiveness of grafting to improve salt tolerance in tomato when an ‘excluder’ genotype is used as scion. Environ Exp Bot 63, 392–401 (2008).
Penella, C. et al. Salt tolerant rootstock increases yield of pepper under salinity through maintenance of photosynthetic performance and sinks strength. J Plant Physiol 193, 1–11 (2016).
Assaha, D., Ueda, A. & Saneoka, H. Comparison of growth and mineral accumulation of two solanaceous species, Solanuum scabrum Mill.(huckleberry) and S. melongena L. (eggplant) under salinity stress. Soil Sci Plant Nutr 59, 912–920 (2013).
Heuer, B., Meiri, A. & Shalhevet, J. Salt tolerance of eggplant. Plant Soil 95, 9–13 (1986).
Hannachi, S. & Van Labeke, M. Salt stress affects germination, seedling growth and physiological responses differentially in eggplant cultivars (Solanum melongia L.). Sci. Hortic. 228, 56–65 (2018).
Akinci, I., Akinci, S., Yilmaz, K. & Dikici, H. Response of eggplant varieties (Solanum melongnea) to salinity in germination and seedling stages. New Zeal J Crop Hort. 32, 193–200 (2004).
Khah, E. M., Kakava, E., Mavromatis, A., Chachalla, D. & Goulas, C. Effect of grafting on growth and yield of tomato (Lycopersicon esculentul Mill.) in greenhouse and open-field. J. Applied Horticulture 8, 3–7 (2006).
Fernandez-Garcia, N., Carvajal, M. & Olmos, E. Graft union in tomato plants: Peroxidase and Catalase involvement. Annals Bot. 93, 53–60 (2004).
Semiz, G. D. & Suarez, D. L. Tomato salt tolerance: Impact of grafting and water composition on yield and ion relations. Turk J Agric For 39, 876–886 (2015).
Suarez, D. L. & Taber, P. Extractchem. Available at https://www.ars.usda.gov/research/software/?modeCode=20-36-05-00 (2012).
Maas, E. V. & Hoffman, G. J. Crop salt tolerance: Current assessment. J Irrig Drain Div ASCE 103, 115–134 (1977).
van Genuchten, M. T. & Hoffman, G. J. Analysis of crop salt tolerance data, in Soil salinity under irrigation: Processes and management: Ecology studies 51, Shainberg, I, & Shalhevet, J, eds., Springer-Verlag, New York, 258–271(1984).
Marschner, A. S. Mineral Nutrition of Higher Plants (Academic Press. San Diego 889 pp, 1995).
Savvas, D. & Lenz, F. Effects of NaCl or nutrient-induced salinity on growth, yield, and composition of eggplants grown in rockwool. Sci Hort 84, 37–44 (2000).
Colla, G., Rouphael, Y., Leonardi, C. & Bie, Z. Role of grafting in vegetable crops grown under saline conditions. Scientia Hort 127, 147–155 (2010).
Cornacchione, M. V. & Suarez, D. L. Emergence, forage production, and ion relations of alfalfa in response to saline waters. Crop Sci 55, 444–457 (2015).
Grieve, C. M., Grattan, S. R. & Maas, E. V. Plant salt tolerance. In: Wallender WW, Tanji KK (eds) ASCE Manual and Reports on Engineering Practice No. 71 Agricultural Salinity Assessment and Management (2nd edition) ASCE, Reston VA. Chapter 13 pp 405–459. (2012).
Santa-Cruz, A., Martínez-Rodríguez, M. M., Perez-Alfocea, F., Romero-Aranda, R. & Bolarin, M. C. The rootstock effect on the tomato salinity response depends on the shoot genotype. Plant Sci 162, 825–831 (2002).
Al-Harbi, A., Hejazi, A. & Al-Omran, A. Responses of grafted tomato (Solanum lycopersiocon L) to abiotic stress in Saudi Arabia. Saudi J Biol Sci. 24, 1274–1280 (2017).
Penella, C. et al. Grafting pepper onto tolerant rootstocks: An environmental-friendly technique overcome water and salt stress. Sci Hortic 226, 33–41 (2017).
Martinez-Ballesta, M., Alcaraz-Lopez, C., Muries, B., Mota-Cadenas, C. & Carvajal, M. Physiological aspects of rootstock-scion interactions. Sci. Hortic 127, 112–118 (2010).
Bai, L. P., Zhou, B. L., Li, N., Huo, S. F. & Fu, Y. W. The ion absorption and transportation of grafted eggplants (Solanum melongena L.) under NaCl stress. Plant Physiol Commun 41, 767–769 (2005).
Almeida, P., Feron, R., de Boer, G. & de Boer, A. Role of Na+, K+, Cl−, proline and sucrose concentrations in determining salinity tolerance and their correlation with expression of multiple genes in tomato. AoB Plants 6, plu039, https://doi.org/10.1093/aobpla/plu039. (2014).
Huertas, R. et al. Overexpression of SISOS2 (SICIPK24) confers salt tolerance to transgenic tomato. Plant Cell Environ 36, 1467–1482 (2012).
Rivero, R. et al. The combined effect of salinity and heat reveals a specific physiological, biochemical and molecular response in tomato plants. Plant Cell Environ 37, 1059–1073 (2014).
Fernandez-Garcia, N., Martinez, V. & Carvajal, M. Effect of salinity on growth, mineral composition, and wáter relations of grafted tomato plants. J. Plant Nutr. Soil Sci 167, 616–622 (2004).
Di Gioia, F., Signore, A., Serio, F. & Santamaria, P. Grafting improves tomato salinity tolerance through sodium partitioning within the shoot. HortSci 48, 855–862 (2013).
Penella, C. et al. Some rootstocks improve pepper tolerance to mild salinity through ionic regulation. Plant Sci. 230, 12–24 (2015).
Li, J. et al. Comparative transcriptome analysis reveals K+ transporter gene contributing to salt tolerance in eggplant. BMC Plant Biology 19, 67, https://doi.org/10.1186/s12870-019-1663-8 (2019).
Unlukara, A., Kurunç, A., Kesmez, G. D., Yurtseven, E. & Suarez, D. L. Effects of Salinity on Eggplant (Solanum Melongena L.) Growth and Evapotranspiration. Irrig. and Drain 59, 203–214 (2010).
Author information
Authors and Affiliations
Contributions
Gülüzar Duygu Semiz and Donald L. Suarez contributed equally to the conception and design of the experiment. Gülüzar Duygu Semiz and Donald L. Suarez contributed equally to the data collection, interpretation, and writing of the manuscript. Gülüzar Duygu Semiz prepared artwork.
Corresponding author
Ethics declarations
Competing interests
The authors have no competing interests as defined by Nature Research, or other interests that might be perceived to influence the results and/or discussion reported in this paper.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Semiz, G.D., Suarez, D.L. Impact of Grafting, Salinity and Irrigation Water Composition on Eggplant Fruit Yield and Ion Relations. Sci Rep 9, 19373 (2019). https://doi.org/10.1038/s41598-019-55841-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-55841-0
- Springer Nature Limited