Abstract
In roots of Arabidopsis thaliana, Zn can be either loaded into the xylem for translocation to the shoot or stored in vacuoles. Vacuolar storage is achieved through the action of the Zn/Cd transporter HMA3 (Heavy Metal Atpase 3). The Col-0 accession has an HMA3 loss-of-function allele resulting in high shoot Cd, when compared to accession CSHL-5 which has a functional allele and low shoot Cd. Interestingly, both Col-0 and CSHL-5 have similar shoot Zn concentrations. We hypothesize that plants sense changes in cytosolic Zn that are due to variation in HMA3 function, and respond by altering expression of genes related to Zn uptake, transport and compartmentalisation, in order to maintain Zn homeostasis. The expression level of genes known to be involved in Zn homeostasis were quantified in both wild-type Col-0 and Col-0::HMA3CSHL-5 plants transformed with the functional CSHL-5 allele of HMA3. We observed significant positive correlations between expression of HMA3 and of genes known to be involved in Zn homeostasis, including ZIP3, ZIP4, MTP1, and bZIP19. The results support our hypothesis that alteration in the level of function of HMA3 is counterbalanced by the fine regulation of the Zn homeostasis gene network in roots of A. thaliana.
Similar content being viewed by others
Introduction
Zinc (Zn) is an essential element to plants. While Zn deficiency can cause stunted growth and decreased productivity, excessive Zn concentration in plant tissues can lead to toxicity. Therefore, plants evolved mechanisms to tightly control Zn uptake, tissue distribution and subcellular compartmentalization to maintain proper Zn homeostasis1,2. The main gene families implicated in Zn homeostasis are ZIP (Zinc-regulated/Iron-regulated Protein), which perform primary Zn uptake into cells3,4; Metal Tolerance Protein (MTP), involved in Zn detoxification into vacuoles5; ZIFL (Zinc-Induced Facilitator-Like), which are able to transport nicotianamine and phytosiderophores that bind Zn6; and HMA (Heavy Metal-Associated), with members that act as Zn efflux transporters from the cytoplasm into the vacuole or to the apoplast7,8,9. Yellow-Stripe Like proteins, which transport metal-phytosiderophore/nicotianamine complexes, are also thought to have a role in Zn homeostasis10. Moreover, the transcription factors bZIP19 and bZIP23 from A. thaliana were shown to be important in the Zn deficiency response11.
Plants may also accumulate elements with no known biological function, such as cadmium (Cd). High Cd concentration in leaves and seeds can make plants a source of Cd exposure for humans, and since Cd is a class I carcinogen, avoiding Cd accumulation in the edible tissue of plants is a key factor for food safety12,13. Thus, studies dissecting Cd transport in plants have described a number of key genes, especially in genetically tractable species such as A. thaliana and rice14,15. Importantly, it was shown that Cd transporters localized in root vacuolar membranes compartmentalize Cd in the vacuole, leading to lowered Cd availability in the root symplast, and lower xylem loading. Thus, increased accumulation of Cd into root vacuoles decreased the Cd concentration in leaves and seeds16,17. Examples of transporters capable of compartmentalising Cd into root vacuoles includes in A. thaliana AtABCC1 and AtABCC2, a duplicated gene pair that is able to transport Cd-phytochelatin complexes into root vacuoles18,19; the CAX-type proton antiporters, AtCAX2 and AtCAX4 from A. thaliana20,21; and AtHMA322.
The vacuolar transporter AtHMA3 and its orthologs in other species were shown to control Cd concentration in above-ground tissues. In A. thaliana, loss-of-function of AtHMA3 resulted in increased Cd sensitivity, whereas ectopic over-expression improved tolerance, establishing its role in Cd detoxification through Cd accumulation in root vacuoles22. AtHMA3 was shown to control natural variation in leaf Cd in A. thaliana with functional AtHMA3 alleles conferring low leaf Cd concentrations, whereas accessions with non-functional alleles showed increased leaf Cd concentrations7. Interestingly, Col-0, the most commonly used A. thaliana accession, has a non-functional truncated AtHMA3 sequence. Transformation of Col-0 with the genomic fragment containing a functional allele of AtHMA3 resulted in decreased leaf Cd concentration, demonstrating that root vacuole compartmentalization affects root-to-shoot translocation7. Similarly, in rice OsHMA3 natural variation was shown to control Cd accumulation in shoots and grains, with functional alleles restricting Cd root-to-shoot translocation by enhancing storage of Cd in root vacuoles, and with loss-of-function alleles showing the opposite phenotype9,23,24. Further, in Brassica rapa BjHMA3 has been established to control natural variation in leaf accumulation of Cd, similar to both A. thaliana and rice, with polymorphisms in the coding region of BjHMA3 driving variation in function25. Allelic variation of BrHMA3, shown to affect leaf Cd accumulation in B. rapa, was observed to have no effect on leaf Zn concentration. OsHMA3 ectopic over-expression resulted in increased Cd tolerance and lower Cd concentration in leaves and grains, but increased Cd concentration in roots23,26. Thus, HMA3 function is likely conserved in A. thaliana, B. rapa and rice, and is a major determinant controlling Cd movement from roots to above-ground tissues.
Limited evidence suggests that AtHMA3 and OsHMA3 also have a role in Zn homeostasis. Although AtHMA3 does not complement a yeast mutant lacking the Zn vacuolar transporter zrc127, A. thaliana loss-of-function mutant athma3 shows slightly increased Zn sensitivity, whereas plants ectopically over-expressing AtHMA3 show increased Zn tolerance22. However, genetic transformation of A. thaliana Col-0, which contains a non-functional allele of AtHMA3, with a functional allele of AtHMA3 from the A. thaliana CSHL-5 genotype only changed Zn leaf concentration very slightly, suggesting that any functional impact of AtHMA3 was potentially buffered by other mechanisms to maintain shoot Zn concentration in the Col-0 accession7. Similarly, in rice ectopic over-expression of OsHMA3 did not significantly change shoots Zn concentrations. Maintenance of Zn homeostasis in shoots was attributed to increased expression of ZIP transporters in roots of the over-expressing line. Since Zn is an essential element, regulatory mechanisms may be activated to increase uptake in lines that have an enhanced function of HMA3 that is driving increased Zn compartmentalisation into root vacuoles26. However, these results in rice were observed in lines that were ectopically over-expressing OsHMA3 from the maize ubiquitin1 promoter, which also drives expression in shoots. This may not be a relevant physiological context, given that shoot expression of normally root expressed Zn transporters is known to produce unexpected results on Zn homeostasis28. In a recently published study, rice genotypes with functional OsHMA3 were compared with genotypes containing natural loss-of-function alleles, and the authors observed that plants with functional OsHMA3 were more tolerant to high Zn concentration and able to accumulate more Zn in roots, while maintaining similar Zn concentrations in shoots. This was due to up-regulation of Zn uptake genes in roots of genotypes with higher expression of HMA329. However, the data is based on four genotypes, and it is possible that other genetic factors are involved in the correlations observed. Thus, the question of whether HMA3 is a component of the Zn homeostasis network in plants, and further how this network is reorganized to accommodate varying levels of HMA3 function, remain to be answered.
In this work, we used previously reported transgenic Col-0 lines that have been transformed with a functional AtHMA3 allele, isolated from the CSHL-5 A. thaliana accession (collected from Cold Spring Harbor Lab, Long Island, NY), and expressed from its native promoter. We used these lines to address the question of how plants with an increased root vacuolar Zn sink, driven by enhanced activity of HMA3, regulate Zn homeostasis. We performed experiments using a hydroponic system to identify changes in expression of genes in roots known to be involved in Zn homeostasis. We found that variation in AtHMA3 expression in our lines is correlated with expression of key genes in the Zn regulatory gene network, providing a direct causal relationship between HMA3 activity and the status of the Zn transcriptional regulatory network. Our work highlights how A. thaliana plants can transcriptionally buffer Zn homeostasis when Zn compartmentalization in root vacuoles is altered through the modification of AtHMA3 activity.
Results
Zn leaf concentration is not associated with genetic variation in AtHMA3
Previously, we conducted genome wide association (GWA) studies using leaf Cd concentration and genotype data derived from the 256 K SNP-tilling array Atsnptile 1, containing 248,584 SNPs30, for 337 A. thaliana accessions7. We found a single peak in chromosome 4 with multiple SNPs associated with leaf Cd concentration (Fig. 1A). The causative gene was identified as AtHMA3, which was shown to be a major driver of variation in leaf Cd concentration in A. thaliana species-wide diversity7. Since evidence suggests that AtHMA3 transports Zn as well as Cd into vacuoles, we hypothesized that genetic variation in AtHMA3 could also be associated with leaf Zn concentration. We conducted GWA mapping using the genotypic and Zn leaf data from the same experiment as described in Chao et al.7. However, we found no SNPs significantly associated with variation in Zn in the region of AtHMA3 (Fig. 1B). Thus, functional/hypofunctional AtHMA3 alleles do not seem to control variation in leaf Zn concentration in the same way they control leaf Cd concentration.
Genome-wide association mapping of leaf cadmium (top) and zinc (bottom) of 206,088 SNPs across 340 A. thaliana accessions using a mixed model analysis. Horizontal blue line indicates suggestive significance (-log10(1e-5)), horizontal red line indicates genome-wide significance (−log10(1e-7.5)) (a). Chromosome 4 detail (b). Relative expression of AtHMA3 in roots of A. thaliana Col-0, CSHL-5 and Col-0::HMA3CSHL−5 lines. PEX4 and EF1-α4 were used as internal normalization standard across all samples and HMA3 expression levels were calculated by 2−ΔCT method52 (c). Concentration of cadmium (d) and zinc (e) in leaves. Bars correspond to the means of biological replicates and the error bars correspond to the standard error. Letters above bars indicate statistically significant differences using a one-way ANOVA with Tukey’s test using a 95% confidence interval.
One possible explanation for this intriguing finding is that plasticity in the Zn transcriptional network buffers the impact of AtHMA3 on Zn. In contrast, because plants do not require Cd, it is not sensed and therefore does not trigger transcriptional buffering. In order to test this hypothesis we used previously generated transgenic A. thaliana lines of the Col-0 accession, which has a naturally truncated, non-function allele of AtHMA3, and which we had transformed with the genomic fragment containing the functional AtHMA3 allele from the CSHL-5 A. thaliana accession (hereafter Col-0::HMA3CSHL-5), including its promoter, coding sequence and 3′ UTR7. We expect these lines to show AtHMA3 expression in tissues and at levels similar to the native AtHMA3 gene from CSHL-5. Variation in expression level should be derived from positional effects of transgene insertion and number of copies inserted.
We evaluated the expression of AtHMA3 in Col-0, CSHL-5 and the three transgenic homozygous lines of Col-0::HMA3CSHL-5 (lines #1, #2, and #3) (Fig. 1C). Across the transgenic lines, #3 showed the highest relative expression level: 2.5 and 4.2 times higher than #1 and #2 respectively. This range of variation in AtHMA3 expression in the Col-0::HMA3CSHL-5 transgenic lines allowed use to effectively test the impact of AtHMA3 function on the expression of genes involved in Zn homeostasis. Expression of the HMA3CSHL-5 allele in our three Col-0 transgenic lines was also found to be of a similar magnitude to its native expression level in CSHL-5 (Fig. 1C).
The concentration of Cd in leaves of these transgenic lines was approx. 45% lower than in Col-0, with all three lines showing similar decreases in Cd concentration (Fig. 1D). Zn concentrations, on the other hand, were only slightly reduced in the Col-0::HMA3CSHL-5 genotype compared to Col-0, and only line #3 showing a statistically significant reduction (Fig. 1E). Interestingly, line #3 showed the highest AtHMA3 expression level among the transgenic lines (Fig. 1C). We found no change in concentrations of other elements in the Col-0::HMA3CSHL-5 genotype (Table S1). These observations establish the impact of AtHMA3 activity on both Cd and Zn concentration in leaves, reproducing in hydroponically cultivated plants that observed by Chao et al.7 in plants cultivated in soil.
Modulation of AtHMA3 activity leads to changes in expression of Zn homeostasis genes in roots
We next tested whether modulation of AtHMA3 function in the Col-0 accession induces transcriptional changes in known Zn-related genes in root tissues. We evaluated expression levels of AtZIP1, AtZIP2, AtZIP3, AtZIP4, AtZIP5, AtbZIP19, AtHMA4, AtNAS1, AtYSL2, AtPCR2, AtMTP1, AtMTP3 and AtZIF1 in all three Col-0::HMA3CSHL-5 lines. Regression analyses including only the complemented lines (i.e., we excluded non-transgenic Col-0 plants expression data) were used to identify any possible relationship between the expression of AtHMA3 and transcription of each selected gene.
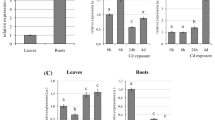
We found several genes involved in Zn homeostasis where the expression of the gene in roots was significantly correlated with the increasing expression of AtHMA3. These genes were the Zn plasma membrane transporters AtZIP3 and AtZIP4; the Zn effluxer AtPCR2; the vacuolar Zn transporter AtMTP1; and the putative Fe and Zn-phytosiderophore transporter AtYSL2 (Fig. 2A). Expression of the transcription factor AtbZIP19, which was shown to bind the AtZIP4 promoter, and is important for uptake under Zn deficiency, also showed a positive correlation with AtHMA3 expression (Fig. 2A). AtZIP1, AtZIP2, AtZIP5, AtHMA4, AtNAS1, AtMTP3 and AtZIF1 were also tested, but showed no significant correlation with AtHMA3 expression (Fig. 2B).
Correlation between root relative expression of AtHMA3 and Zn-related genes in transgenic lines. PEX4 and EF1-α4 were used as internal normalization standard across all samples and the expression levels were calculated by 2−ΔCT method52. The points in Fig. 2 refer to individual plants from the three complementation lines used in this work. Significant correlations (*P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.001) are shown as black diamonds (a) and non-significant correlations are shown as grey diamonds (b).
After identifying Zn homeostasis genes that show altered expression in response to AtHMA3 expression, we performed a Pearson’s correlation analysis to find other possible interactions across the genes studied. We found a significant strong correlation (r ≥ 0.7) between expression of AtZIP3 x AtMTP1, AtZIP4 x AtbZIP19, AtZIP4 x AtMTP1, AtZIP5 x AtZIP1, AtbZIP19 x AtYSL2, AtbZIP19 x AtMTP3, AtMTP1 x AtZIF1, AtMTP3 x AtNAS1, AtMTP3 x AtYSL2, AtNAS1 x AtYSL2, AtPCR2 x AtZIF1. This result and other significant correlations (0.56 ≤ r ≤ 0.69, classified here as moderate) are shown in Fig. 3.
To support the correlation analyses based on gene expression presented above, we also investigated the Protein-Protein Functional Associations (PPFA) network of AtHMA3. This network was generated using various public data sources from the STRING31 platform, relating to the 14 target genes we monitored in this study to see if the proteins we found to be correlated had any predicted functional associations with AtHMA3, either directly or indirectly. The PPFA network corroborated most of the results of the gene expression analyses: AtHMA3 is predicted to be functionally associated directly with AtMTP1, which in turn is associated with AtZIP3 and AtZIP4 through AtIAR1 (Fig. 4). AtZIP4, in turn is functionally associated with AtbZIP19. However, our experimental analysis differs from the PPFA network in that AtHMA3 expression is not correlated with AtZIP1/2/5, AtHMA4, AtNAS1, and AtMTP3 expression (Fig. 2b). Taken together, our correlation and PPFA network data support the conclusion that functional changes in AtHMA3 alter the expression of genes that are part of the transcriptionally co-regulated Zn homeostasis network.
Predicted Protein-Protein Association (PPFA) network performed using the STRING platform. The purple rectangles are proteins whose gene expression is significantly correlated with that of AtHMA3 and the yellow rectangles show proteins whose gene expression is not significantly correlated with that of AtHMA3, based on the real-time qPCR expression analysis performed in the present work. The white boxes represent proteins with predicted associations in STRING, but not assayed in this work.
Discussion
In A. thaliana, almost 2400 proteins have Zn-related functions32, including enzymes associated with antioxidant systems (Cu/Zn superoxide dismutase)33 and photosynthesis (carbonic anhydrase)34. Thus, cellular Zn concentration needs to be finely tuned. There is evidence that the vacuolar localised HMA3 transports Zn9,22,29, and that natural functional allelic variation exists for HMA3 in A. thaliana, rice and B. rapa7,24,25,35. Intriguingly, this variation in HMA3 functions appears to have very limited impact on Zn accumulation, and is primarily manifest as variation in Cd accumulation7,24,25,26,29,35. One plausible explanation for this is that changes in the transcriptional networks regulating Zn homeostasis buffer variation in HMA3 function. Here, we show that A. thaliana plants do transcriptionally buffer changes in Zn compartmentalization in root vacuoles as a direct consequence of modification of AtHMA3 function, in order to maintain a constant Zn concentration in leaves. Further, our data provides a clear answer to the previously posed question of ‘why plants expressing the functional form of the Cd/Zn transporter AtHMA3 (such as CSHL-5 genotype) show lower Cd concentration in the leaves compared to genotypes with non-functional AtHMA3 (such as Col-0), while Zn concentration is not significantly changed’7?
A recent study using rice natural accessions with functional and non-functional OsHMA3 alleles suggested that Zn homeostasis related genes have their steady-state level changed when OsHMA3 activity varies in order to maintain Zn concentrations in shoots29. Accessions with a functional OsHMA3 allele showed higher expression levels of OsZIP4, OsZIP5, OsZIP8, and OsZIP10 in roots compared to accessions with a non-functional OsHMA3 allele29. These results from Cai et al.29 agree with data from OsHMA3 over-expressing lines, which have decreased Cd concentration in shoots, but similar Zn concentrations compared to WT, which was attributed to up-regulation of OsZIP4, OsZIP5, OsZIP8, OsZIP9 and OsZIP1026. However, experiments with over-expressing lines can be misleading, since expression at high levels in all cells is not physiologically relevant. Experiments with natural accessions, although informative, should also be interpreted carefully, since natural accessions can vary at many genetic loci, making it difficult to draw conclusions about the contribution of any given locus from a small selection of accessions such as explored by Cai et al.29.
In our experiment with A. thaliana, we used the Col-0 accession which has a naturally occurring non-functional allele of AtHMA3, and transformed this accession with a functional allele from the CSHL-5 A. thaliana accession under control of its native promoter, to generate multiple independent Col-0::HMA3CSHL-5 lines, which were compared to wild type Col-0 plants as a control. In this experimental set up, the only genetic variation between the lines is the presence/absence of the functional AtHMA3 allele and its expression levels due to insertional effects and copy number variation. Using this variation in expression of the functional CSHL-5 AtHMA3 allele in the null hma3 Col-0 background, we were able to directly test the impact of AtHMA3 function on the Zn homeostasis transcriptional network.
We found that expression of functional AtHMA3 alleles in a background with no functional AtHMA3 promotes changes in expression of multiple genes involved in Zn homeostasis in roots. ZIP genes (AtZIP3 and AtZIP4; Fig. 2A) were upregulated. AtZIP3 is predominantly expressed in roots of Zn-deficient plants, whereas AtZIP4 is induced by Zn deficiency in both roots and shoots36. Induction of ZIP transporters was also observed in rice ectopically overexpressing a functional OsHMA3 allele, compared to genotypes with a loss-of-function allele26. Both can complement yeast mutants that are defective in Zn uptake3,11, indicating their role in maintaining Zn homeostasis, although their precise physiological role is unclear. Interestingly, two highly similar transcription factors essential for Zn deficiency responses in A. thaliana, AtbZIP19 and AtbZIP23, can bind to Zinc Deficiency Response Elements (ZDRE) in the promoter of both AtZIP4 and AtZIP3, and promote up regulation of expression11,37. Lilay et al.38 proposed that Zn modulated the activity of AtbZIP19 at the protein level, in the nucleus, where cellular Zn sufficiency represses its activity. Importantly, we found AtbZIP19 expression to be positively correlated with AtHMA3 expression (Fig. 2A), indicating that the higher the AtHMA3 function, the more the plants induce Zn deficiency-related genes, supporting our conclusion that increased compartmentalization of Zn into vacuoles induces expression of key Zn-related genes in order to maintain cytosolic Zn homeostasis.
The expression of functional AtHMA3 also showed a high correlation to AtMTP1 (Metal Tolerance Protein 1), related to Zn-excess. The AtMTP1 transporter is located at the vacuolar membrane, and is known to be involved in the detoxification of excess Zn through its compartmentalisation into vacuoles39,40. We also found correlation between AtHMA3 and AtPCR2 in our transgenic lines. AtPCR2 is a Zn exporter with a dual function, effluxing Zn into the rhizosphere and xylem under Zn excess41. Thus, increased expression of AtMTP1 and AtPCR2 upon higher AtHMA3 activity suggests that roots are sensing local Zn excess when HMA3 function is high. It also indicates that increased AtHMA3 function is not enough to sequester Zn in the vacuole when Zn uptake is higher due to increased expression of AtZIP3/AtZIP4.
We propose that functional copies of AtHMA3 result in increased Zn transport into root vacuoles. Cytosolic Zn availability decreases, leading to less Zn translocation from roots to shoots. Zn uptake transporters (AtZIP3 and AtZIP4) are up regulated, which could be a response to a shoot-derived signal28 or to lower local Zn status2. Increased Zn uptake leads to higher local Zn concentration in the root symplast, which causes increased expression of Zn excess responsive genes (Fig. 5). This transcriptional buffering of the Zn homeostasis network in response to variation in AtHMA3 function maintains Zn concentration in shoots. The expression simultaneously of both Zn deficiency and Zn excess genes in response to increasing vacuolar Zn compartmentalization (driven by AtHMA3) is reminiscing of the ‘zinc – shock’ hypothesis proposed in yeast where the vacuolar Zn transport gene ZRC1 is upregulated under Zn-limiting conditions42. Our model presented in Fig. 5 shows how A. thaliana plants would acclimate to increased Zn vacuolar sequestration upon higher HMA3 function. It is important to acknowledge that the model is based on the correlation analyses, and needs further functional validation.
Working model of how AtHMA3 function changes the Zn homeostasis transcriptional network. (A) Zn homeostasis in roots of plants with hypo-functional/non-functional AtHMA3. Zn in the symplast is maintained low, and Zn is also stored in the root vacuoles. (B) Zn homeostasis changes in roots of plants with higher AtHMA3 function. Higher AtHMA3 function results in (1) higher vacuolar Zn storage, and a consequent lowering of Zn symplastic concentration, which result in (2) decreased Zn root-to-shoot translocation and decreased cytosolic Zn concentration. (C) As a result of decreased Zn root-to-shoot translocation and presumable lower Zn concentration in shoots and/or roots, (3) Zn uptake transporters are up regulated, which (4) re-establish Zn symplastic concentration, Zn xylem and therefore maintain Zn shoot concentration. (D) As a result of sustained up regulation of Zn uptake transporters, roots might undergo local, transient Zn excess in the cytoplasm, which explain (5) up regulation of Zn vacuolar detoxification transporters. The order of event may be different than presented. Changes in ‘Zn’ font size represent higher/lower Zn concentrations. Changes in the transparency of the text represent variation in the expression of transporters (less transparent more expression).
The model proposed in Fig. 5 is supported by the STRING-based PPFA Network that allowed us the identification of functional associations between AtHMA3 and all the genes we show experimentally to be co-regulated with AtHMA3, either through direct or indirect associations. This network analysis also shows that the experimentally determined AtHMA3 driven co-regulation network (that includes bZIP19, ZIP4, ZIP3, MTP1 and YSL2) is a subnetwork of a more complete Zn homeostasis network that also links into iron (Fe) and manganese (Mn) homeostasis, through such genes as IRT1 (high affinity iron uptake transporter43), and MTP8 (AT3G58060; vacuolar Mn transporter44,45).
Zn homeostasis is finely controlled through complex transcriptional buffering regulated by Zn sensing, and this buffering is able to compensate for alterations in the strength of the vascular Zn sink in roots. However, this is not the case for Cd, where leaf Cd concentration is strongly affected by alteration in the strength of the root vascular Cd sink. We conclude that this difference between Zn and Cd is due to the fact that plants are not able to sense Cd in the same way as Zn. They are therefore unable to modulate transcriptional networks to buffer leaf Cd in the same way they can for Zn. This leads to the intriguing idea that Cd can act as a ‘tracer’ to reveal hidden genetic variation in the Zn homeostatic network. A clear example of this is the use of Cd accumulation to identify functional variation in the vacuolar Zn transporter HMA3 in A. thaliana, B. rapa, and rice7,9,23,24,25.
Methods
Plant materials and growth conditions
Seeds from A. thaliana Col-0 and three lines expressing the functional allele of HMA3 from CSHL-5 (collected from Cold Spring Harbor Lab, Long Island, NY) in the Col-0 background (Col-0::HMA3CSHL-5) were cultivated for 4 weeks in nutrient solution containing 10% Hoagland solution in which Fe was replaced by Fe-HBED [N,N9-di(2-hydroxybenzyl)ethylenediamine-N,N9-diacetic acid monohydrochloride hydrate; Strem Chemicals, Inc.]. We also added a non-toxic Cd concentration of 0.05 μM [as Cd(NO3)]. The plants were kept in a climate-controlled room for growth with a photoperiod of 10 h light (100 mmol m−2 s−1)/14 h dark, humidity of 60% and temperature ranging from 19 to 22 °C. After 4-weeks growth, roots were harvested and used for RNA extraction and shoots were harvested for elemental analysis, performed as described by Chao et al.7. We performed two independent experiments using a randomized block design, and similar results were obtained. Here we show the results of one experiment, which had five biological replicates for Col-0 and transgenic lines Col-0::HMA3(1) and Col-0::HMA3 (2), and four biological replicates for line Col-0::HMA3 (3).
Genome-wide association mapping
GWA mapping was performed using the GWA-Portal46. Elemental concentrations of Cd and Zn were determined by ICP-MS as described here and in Chao et al.7, for 340 accessions from the core set in that work. The 340 accessions were genotyped using the custom-designed SNP-tilling array Atsnptile 130, resulting in 206,088 SNPs between both phenotype and genotyped accession sets. The GWA analysis was done using an accelerated mixed model implemented in the program AMM, described previously47.
Selection of target genes
The following genes which expression has been previously shown to be regulated by Zn deficiency in A. thaliana were chosen for this study: bZIP1911, ZIF16,40, ZIP13,11,48,49, ZIP311,36, ZIP411,36,49, and ZIP511. We have also evaluated the expression of ZIP236, HMA48, MTP128,39,50, MTP351, NAS16, PCR241, and YSL249 genes, due to their known functions in Zn homeostasis.
Quantitative real-time PCR
Total RNA was extracted from roots using TRIzol Plus RNA Purification kit (Invitrogen Life Technologies). After DNA removal with DNAse I (Invitrogen), two micrograms of total RNA was used to synthesize first strand cDNA with Transcriptor First Strand cDNA Synthesis Kit (Roche). Quantitative real-time PCR was performed using SensiFASTTM SYBR® Hi-ROX Kit (Bioline), using the first strand cDNA as a template, on a Real-Time PCR System (ABI StepOnePlus, Applied Biosystems, USA). Primers for qRT-PCR were designed to cover an exon-exon junction using the Primer-BLAST platform (NCBI). Primer sequences are shown in Table S2. For the analysis, PEX4 (AT5G25760) and EF1-α4 (AT5G60390) were both used as internal normalization standards across all samples. Expression levels were calculated using the 2−ΔCT method52. For all samples analysed primer efficiencies were corrected using the Miner algorithm53.
Protein-protein functional association network
The PPFA for the 14 A. thaliana genes (AtHMA3, AtZIP1, AtZIP2, AtZIP3, AtZIP4, AtZIP5, AtbZIP19, AtHMA4, AtNAS1, AtYSL2, AtPCR2, AtMTP1, AtMTP3 and AtZIF1) were obtained from STRING v11.031. The network is built on the association score calculated by STRING, which are calculated based on the following sources of evidence: text-mining, neighbourhood, experiments, gene fusion, databases, co-occurrence and co-expression. We highlight that associations do not necessarily mean that proteins are physically binding each other, but jointly contributing to a shared function. We generated our network using a minimum association score of 0.7 (high confidence) and a maximum number of 10 associations for the first shell, and export to cytoscape v3.7. AtPCR2 was excluded from the final analysis because it had no associations in our network.
Statistical analysis
The experiment was conducted using a randomized block design, with five Col-0 and 14 transgenic Col-0::HMA3CSHL-5 plants (biological replicates) derived from 3 independent Col-0::HMA3CSHL-5 lines. We used five replicate plants for lines Col-0::HMA3(1) and Col-0::HMA3(2), and four plants for line Col-0::HMA3(3). The investigation of differences in AtHMA3 expression and leaf Zn and Cd between the genotypes was carried out using the ANOVA F-test (p ≤ 0.05) test. The correlation between expression of AtHMA3 and the target genes was evaluated using linear regression. We performed normality tests for all variables and residues of regression, and the p-value was corrected using the Benjamini-Hochberg test (P ≤ 0.023). Pearson’s correlation coefficient was used to estimate correlations across all the target genes tested in this study.
References
Ricachenevsky, F. K., Menguer, P. K., Sperotto, R. A. & Fett, J. P. Got to hide your Zn away: Molecular control of Zn accumulation and biotechnological applications. Plant science: an international journal of experimental plant biology 236, 1–17, https://doi.org/10.1016/j.plantsci.2015.03.009 (2015).
Sinclair, S. A. et al. Systemic Upregulation of MTP2- and HMA2-Mediated Zn Partitioning to the Shoot Supplements Local Zn Deficiency Responses. The Plant cell 30, 2463–2479, https://doi.org/10.1105/tpc.18.00207 (2018).
Milner, M. J., Seamon, J., Craft, E. & Kochian, L. V. Transport properties of members of the ZIP family in plants and their role in Zn and Mn homeostasis. Journal of experimental botany 64, 369–381, https://doi.org/10.1093/jxb/ers315 (2013).
Ricachenevsky, F. K. et al. Elemental Profiling of Rice FOX Lines Leads to Characterization of a New Zn Plasma Membrane Transporter, OsZIP7. Frontiers in plant science 9, 865, https://doi.org/10.3389/fpls.2018.00865 (2018).
Ricachenevsky, F. K., Menguer, P. K., Sperotto, R. A., Williams, L. E. & Fett, J. P. Roles of plant metal tolerance proteins (MTP) in metal storage and potential use in biofortification strategies. Frontiers in plant science 4, 144, https://doi.org/10.3389/fpls.2013.00144 (2013).
Haydon, M. J. et al. Vacuolar nicotianamine has critical and distinct roles under iron deficiency and for zinc sequestration in Arabidopsis. The Plant cell 24, 724–737, https://doi.org/10.1105/tpc.111.095042 (2012).
Chao, D. Y. et al. Genome-wide association studies identify heavy metal ATPase3 as the primary determinant of natural variation in leaf cadmium in Arabidopsis thaliana. PLoS genetics 8, e1002923, https://doi.org/10.1371/journal.pgen.1002923 (2012).
Hussain, D. et al. P-type ATPase heavy metal transporters with roles in essential zinc homeostasis in Arabidopsis. The Plant cell 16, 1327–1339, https://doi.org/10.1105/tpc.020487 (2004).
Miyadate, H. et al. OsHMA3, a P1B-type of ATPase affects root-to-shoot cadmium translocation in rice by mediating efflux into vacuoles. The New phytologist 189, 190–199, https://doi.org/10.1111/j.1469-8137.2010.03459.x (2011).
Schaaf, G. et al. A putative function for the arabidopsis Fe-Phytosiderophore transporter homolog AtYSL2 in Fe and Zn homeostasis. Plant & cell physiology 46, 762–774, https://doi.org/10.1093/pcp/pci081 (2005).
Assuncao, A. G. et al. Arabidopsis thaliana transcription factors bZIP19 and bZIP23 regulate the adaptation to zinc deficiency. Proceedings of the National Academy of Sciences of the United States of America 107, 10296–10301, https://doi.org/10.1073/pnas.1004788107 (2010).
Nawrot, T. et al. Environmental exposure to cadmium and risk of cancer: a prospective population-based study. The Lancet. Oncology 7, 119–126, https://doi.org/10.1016/S1470-2045(06)70545-9 (2006).
Nordberg, G. F. Historical perspectives on cadmium toxicology. Toxicology and applied pharmacology 238, 192–200, https://doi.org/10.1016/j.taap.2009.03.015 (2009).
Clemens, S., Aarts, M. G., Thomine, S. & Verbruggen, N. Plant science: the key to preventing slow cadmium poisoning. Trends in plant science 18, 92–99, https://doi.org/10.1016/j.tplants.2012.08.003 (2013).
Huang, X. Y. & Salt, D. E. Plant Ionomics: From Elemental Profiling to Environmental Adaptation. Molecular plant 9, 787–797, https://doi.org/10.1016/j.molp.2016.05.003 (2016).
Peng, J. S. & Gong, J. M. Vacuolar sequestration capacity and long-distance metal transport in plants. Frontiers in plant science 5, 19, https://doi.org/10.3389/fpls.2014.00019 (2014).
Ricachenevsky, F. K., de Araujo Junior, A. T., Fett, J. P. & Sperotto, R. A. You Shall Not Pass: Root Vacuoles as a Symplastic Checkpoint for Metal Translocation to Shoots and Possible Application to Grain Nutritional Quality. Frontiers in plant science 9, 412, https://doi.org/10.3389/fpls.2018.00412 (2018).
Mendoza-Cozatl, D. G. et al. Tonoplast-localized Abc2 transporter mediates phytochelatin accumulation in vacuoles and confers cadmium tolerance. The Journal of biological chemistry 285, 40416–40426, https://doi.org/10.1074/jbc.M110.155408 (2010).
Park, J. et al. The phytochelatin transporters AtABCC1 and AtABCC2 mediate tolerance to cadmium and mercury. The Plant journal: for cell and molecular biology 69, 278–288, https://doi.org/10.1111/j.1365-313X.2011.04789.x (2012).
Koren’kov, V. et al. Enhanced Cd2+ -selective root-tonoplast-transport in tobaccos expressing Arabidopsis cation exchangers. Planta 225, 403–411, https://doi.org/10.1007/s00425-006-0352-7 (2007).
Korenkov, V., King, B., Hirschi, K. & Wagner, G. J. Root-selective expression of AtCAX4 and AtCAX2 results in reduced lamina cadmium in field-grown Nicotiana tabacum L. Plant biotechnology journal 7, 219–226, https://doi.org/10.1111/j.1467-7652.2008.00390.x (2009).
Morel, M. et al. AtHMA3, a P1B-ATPase allowing Cd/Zn/Co/Pb vacuolar storage in Arabidopsis. Plant physiology 149, 894–904, https://doi.org/10.1104/pp.108.130294 (2009).
Ueno, D. et al. Gene limiting cadmium accumulation in rice. Proceedings of the National Academy of Sciences of the United States of America 107, 16500–16505, https://doi.org/10.1073/pnas.1005396107 (2010).
Yan, J. et al. A loss-of-function allele of OsHMA3 associated with high cadmium accumulation in shoots and grain of Japonica rice cultivars. Plant, cell & environment 39, 1941–1954, https://doi.org/10.1111/pce.12747 (2016).
Zhang, L. et al. F-Y Variation in the coding region of the heavy metal ATPase BrHMA3 controls natural variation in cadmium accumulation in widely eaten Brassica rapa vegetables. Journal Experimental Botany In Press (2019).
Sasaki, A., Yamaji, N. & Ma, J. F. Overexpression of OsHMA3 enhances Cd tolerance and expression of Zn transporter genes in rice. Journal of experimental botany 65, 6013–6021, https://doi.org/10.1093/jxb/eru340 (2014).
Gravot, A. et al. AtHMA3, a plant P1B-ATPase, functions as a Cd/Pb transporter in yeast. FEBS letters 561, 22–28, https://doi.org/10.1016/S0014-5793(04)00072-9 (2004).
Gustin, J. L. et al. MTP1-dependent Zn sequestration into shoot vacuoles suggests dual roles in Zn tolerance and accumulation in Zn-hyperaccumulating plants. The Plant journal: for cell and molecular biology 57, 1116–1127, https://doi.org/10.1111/j.1365-313X.2008.03754.x (2009).
Cai, H., Huang, S., Che, J., Yamaji, N. & Ma, J. F. A tonoplast-localized OsHMA3 plays an important role in maintaining Zn homeostasis in rice. Journal of experimental botany, https://doi.org/10.1093/jxb/erz091 (2019).
Baxter, I. et al. A coastal cline in sodium accumulation in Arabidopsis thaliana is driven by natural variation of the sodium transporter AtHKT1;1. PLoS genetics 6, e1001193, https://doi.org/10.1371/journal.pgen.1001193 (2010).
Szklarczyk, D. et al. STRING v11: protein-protein association networks with increased coverage, supporting functional discovery in genome-wide experimental datasets. Nucleic acids research 47, D607–D613, https://doi.org/10.1093/nar/gky1131 (2019).
Broadley, M. R., White, P. J., Hammond, J. P., Zelko, I. & Lux, A. Zinc in plants. The New phytologist 173, 677–702, https://doi.org/10.1111/j.1469-8137.2007.01996.x (2007).
Kliebenstein, D. J., Monde, R. A. & Last, R. L. Superoxide dismutase in Arabidopsis: an eclectic enzyme family with disparate regulation and protein localization. Plant physiology 118, 637–650, https://doi.org/10.1104/pp.118.2.637 (1998).
Fabre, N., Reiter, I. M., Becuwe-Linka, N., Genty, B. & Rumeau, D. Characterization and expression analysis of genes encoding alpha and beta carbonic anhydrases in Arabidopsis. Plant, cell & environment 30, 617–629, https://doi.org/10.1111/j.1365-3040.2007.01651.x (2007).
Liu, C. L. et al. Natural variation in the promoter of OsHMA3 contributes to differential grain cadmium accumulation between Indica and Japonica rice. J Integr Plant Biol, https://doi.org/10.1111/jipb.12794 (2019).
Grotz, N. et al. Identification of a family of zinc transporter genes from Arabidopsis that respond to zinc deficiency. Proceedings of the National Academy of Sciences of the United States of America 95, 7220–7224 (1998).
Inaba, S. et al. Identification of putative target genes of bZIP19, a transcription factor essential for Arabidopsis adaptation to Zn deficiency in roots. The Plant journal: for cell and molecular biology 84, 323–334, https://doi.org/10.1111/tpj.12996 (2015).
Lilay, G. H., Castro, P. H., Campilho, A. & Assuncao, A. G. L. The Arabidopsis bZIP19 and bZIP23 Activity Requires Zinc Deficiency - Insight on Regulation From Complementation Lines. Frontiers in plant science 9, 1955, https://doi.org/10.3389/fpls.2018.01955 (2018).
Desbrosses-Fonrouge, A. G. et al. Arabidopsis thaliana MTP1 is a Zn transporter in the vacuolar membrane which mediates Zn detoxification and drives leaf Zn accumulation. FEBS letters 579, 4165–4174, https://doi.org/10.1016/j.febslet.2005.06.046 (2005).
Haydon, M. J. & Cobbett, C. S. A novel major facilitator superfamily protein at the tonoplast influences zinc tolerance and accumulation in Arabidopsis. Plant physiology 143, 1705–1719, https://doi.org/10.1104/pp.106.092015 (2007).
Song, W. Y. et al. Arabidopsis PCR2 is a zinc exporter involved in both zinc extrusion and long-distance zinc transport. The Plant cell 22, 2237–2252, https://doi.org/10.1105/tpc.109.070185 (2010).
MacDiarmid, C. W., Milanick, M. A. & Eide, D. J. Induction of the ZRC1 metal tolerance gene in zinc-limited yeast confers resistance to zinc shock. The Journal of biological chemistry 278, 15065–15072, https://doi.org/10.1074/jbc.M300568200 (2003).
Barberon, M. et al. Monoubiquitin-dependent endocytosis of the iron-regulated transporter 1 (IRT1) transporter controls iron uptake in plants. Proceedings of the National Academy of Sciences of the United States of America 108, E450–458, https://doi.org/10.1073/pnas.1100659108 (2011).
Chu, H. H. et al. The Arabidopsis MTP8 transporter determines the localization of manganese and iron in seeds. Scientific reports 7, 11024, https://doi.org/10.1038/s41598-017-11250-9 (2017).
Eroglu, S., Meier, B., von Wiren, N. & Peiter, E. The Vacuolar Manganese Transporter MTP8 Determines Tolerance to Iron Deficiency-Induced Chlorosis in Arabidopsis. Plant physiology 170, 1030–1045, https://doi.org/10.1104/pp.15.01194 (2016).
Seren, U. GWA-Portal: Genome-Wide Association Studies Made Easy. Methods in molecular biology 1761, 303–319, https://doi.org/10.1007/978-1-4939-7747-5_22 (2018).
Seren, U. et al. GWAPP: a web application for genome-wide association mapping in Arabidopsis. The Plant cell 24, 4793–4805, https://doi.org/10.1105/tpc.112.108068 (2012).
Becher, M., Talke, I. N., Krall, L. & Kramer, U. Cross-species microarray transcript profiling reveals high constitutive expression of metal homeostasis genes in shoots of the zinc hyperaccumulator Arabidopsis halleri. The Plant journal: for cell and molecular biology 37, 251–268 (2004).
van de Mortel, J. E. et al. Large expression differences in genes for iron and zinc homeostasis, stress response, and lignin biosynthesis distinguish roots of Arabidopsis thaliana and the related metal hyperaccumulator Thlaspi caerulescens. Plant physiology 142, 1127–1147, https://doi.org/10.1104/pp.106.082073 (2006).
Kobae, Y. et al. Zinc transporter of Arabidopsis thaliana AtMTP1 is localized to vacuolar membranes and implicated in zinc homeostasis. Plant & cell physiology 45, 1749–1758, https://doi.org/10.1093/pcp/pci015 (2004).
Arrivault, S., Senger, T. & Kramer, U. The Arabidopsis metal tolerance protein AtMTP3 maintains metal homeostasis by mediating Zn exclusion from the shoot under Fe deficiency and Zn oversupply. The Plant journal: for cell and molecular biology 46, 861–879, https://doi.org/10.1111/j.1365-313X.2006.02746.x (2006).
Livak, K. J. & Schmittgen, T. D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 25, 402–408, https://doi.org/10.1006/meth.2001.1262 (2001).
Zhao, S. & Fernald, R. D. Comprehensive algorithm for quantitative real-time polymerase chain reaction. Journal of computational biology: a journal of computational molecular cell biology 12, 1047–1064, https://doi.org/10.1089/cmb.2005.12.1047 (2005).
Acknowledgements
We would like to thank Paulina Flis and John Danku for ICP-MS analysis. This work was supported by the grant BBSRC (BB/L000113/1) to DES and by the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001 to APB.
Author information
Authors and Affiliations
Contributions
Conceived and designed the experiments: A.P.B. and D.E.S. Performed the experiments: A.P.B. Analysed the data: A.P.B., F.K.R., M.W., D.E.S. and T.D. Contributed reagents/materials/analysis tools: D.E.S. Wrote the paper: A.P.B., D.E.S. and F.K.R. All authors have read and approved the final version of this manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Pita-Barbosa, A., Ricachenevsky, F.K., Wilson, M. et al. Transcriptional plasticity buffers genetic variation in zinc homeostasis. Sci Rep 9, 19482 (2019). https://doi.org/10.1038/s41598-019-55736-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-55736-0
- Springer Nature Limited
This article is cited by
-
The stoichiometry of soil macro and microelements plays a critical role in regulating Camellia oleifera nutrient accumulation and production
Journal of Soils and Sediments (2024)
-
Constitutive expression of bZIP19 with the Zn sensor motif deleted in Arabidopsis leads to Zn-specific accumulation and no visible developmental penalty
Plant and Soil (2024)
-
Heavy metal ATPase 3 expression controls zinc homeostasis in natural accessions of Arabidopsis thaliana
Theoretical and Experimental Plant Physiology (2022)
-
Genomic characterization of ZIP genes in pigeonpea (CcZIP) and their expression analysis among the genotypes with contrasting host response to pod borer
Physiology and Molecular Biology of Plants (2021)