Abstract
We report the robust ferroelectric properties of (1 − x)(Bi1/2Na1/2)TiO3–x(Bi1/2K1/2)TiO3 (x = 33%) single crystals grown by a top-seeded solution growth process under a high oxygen-pressure (0.9 MPa) atmosphere. The sample exhibit a large remanent polarization of 48 μC/cm2 and a sizeable piezoelectric strain constant of 460 pm/V. Neutron powder diffraction structural analysis combined with first-principles calculations reveals that the large ferroelectric polarization comparable to PbTiO3 stems from the hybridization between Bi-6p and O-2p orbitals at a moderately negative chemical pressure.
Similar content being viewed by others
Introduction
A versatile structural framework of ABX3 perovskites delivers a wide variety of electronic properties1,2 such as superconductivity3,4,5, dielectric permitivity6,7,8,9, ionic conductivity10,11,12,13 and magneto-electric effects14,15. These functionalities arise from electron correlations and/or electron-lattice coupling, which can be designed by a diverse combination of the constituent elements2,16,17. For the A-site atoms, Bi is of considerable importance from fundamental and practical points of view, thanks to the strong interactions between Bi-6s(p) and X-p orbitals. For photovoltaic devices, Bi-based halides show a high efficiency derived from antibonding Bi-6s states near the valence band maximum18,19,20. Bismuth ferrite BiFeO3 is a rare multiferroic material at room temperature exhibiting a coupling between ferroelectric and antiferromagnetic orders, where the covalency arising from the Bi-6p and O-2p hybridization gives rise to a robust ferroelectric polarization21,22,23,24,25.
A combination of Bi with alkali metals on the A-site enables to accommodate higher-valent cations such as Ti4+ on the B site. Bismuth potassium titanate (Bi1/2K1/2)TiO3 [BKT] has ferroelectricity in a tetragonal structure (space group P4mm) at room temperature, which has attracted much attention as a lead-free piezoelectric material26. The solid solution with rhombohedral (Bi1/2Na1/2)TiO3 [BNT; space group R3c]26,27, i.e., the BKT–BNT system, displays a morphotropic phase boundary (MPB), where the ferroelectric structure changes dramatically and the piezoelectric activity is maximal28,29,30,31,32,33. There have been intense efforts on BKT-based ceramics to replace lead zirconate titanate (PZT) currently used for various applications34,35.
By contrast, few studies on BKT-based single crystals have been performed to date because of the difficulty in growing high-quality samples. At a temperature above 1300 K, BKT undergoes a thermal decomposition36, which makes the crystal growth difficult; to the best of our knowledge, the preparation of BKT crystals has never been achieved. Although some studies have reported the crystal growth of BKT-based solid solutions37,38,39, these crystals suffer from the problems arising from point defects. Owing to a high vapor pressure, Bi is apt to evaporate from the lattice leaving a vacancy of Bi (VBi”’), which is accompanied by the formation of oxygen vacancy (VO••)40. In addition, an oxidation treatment is required for as-grown samples but increases leakage currents to some extent because of p-type conduction, which prevents us from applying an electric field (E)41,42. Moreover, VO•• tends to accumulate at ferroelastic domain walls, which are strongly pinned and eventually clamped even under high fields43,44. For revealing the ferroelectric nature, it is desirable to develop a high-quality single crystal with a low concentration of VBi”’ where external fields can switch spontaneous polarization (Ps).
In this study, we report a growth of high-quality BKT-based single crystals exhibiting a complete switching of Ps, employing the top-seeded solution growth (TSSG) method under high-oxygen-pressure (high-Po2) atmosphere45,46. We chose a BKT-rich tetragonal phase in the BKT–BNT system. Our process enables us to obtain relatively large and high-performance single crystals with a large Ps of 48 μC/cm2 and a high piezoelectric strain constant (d33) of 460 pm/V. Structural analysis combined with first-principles calculations reveals that the robust ferroelectric polarization and resultant high d33 stem from the orbital interaction between Bi-6p and O-2p at a moderate chemical pressure.
Results
Neutron diffraction data were collected47 for (1 − x)BNT–xBKT powders (x = 30–35%) prepared by solid-state reaction, and the powders were found to have a tetragonal P4mm structure. Figure 1(a) shows the fitting result of the Rietveld analysis for x = 30% measured at 295 K along with the calculated profile, the difference in intensity, and the peak positions. Our analysis leads to a profile reliability (Rp) factor of 5.8% and a weighted Rp (Rwp) factor of 9.9%. All reflections are indexed by a perovskite unit cell in tetragonal P4mm symmetry indicating a clear splitting of, e.g., 200 and 002 reflections, which is due to a tetragonal strain (c/a) of 1.016. The refined crystal structure with the atomic displacements is illustrated in Fig. 1(b) and the detailed structural parameters are summarized in Supplementary Information I.
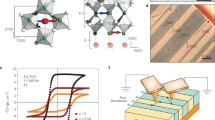
Refined crystal structure of tetragonal BNT-BKT. (a) Fitting results of the Rietveld refinement analysis of the NPD data for BNT–BKT (x = 30%). (b) Refined crystal structure for the tetragonal P4mm phase of BNT–BKT (x = 30%). (c) Polar displacements and (d) dipole moments of the constituent atoms along the c axis of the refined tetragonal P4mm phase of BNT-BKT (x = 30%).
Figure 1(c) shows the displacements of the constituent atoms along the c (polar [001]) axis. The off-center displacements are estimated from the hypothetic paraelectric structure, whose origin is set to the center of mass of the oxygen octahedron. The displacement of the A-site atoms (Bi0.50Na0.35K0.15) is as large as 0.034 nm, which is about twice that of the B-site atom (Ti). Figure 1(d) depicts the electric dipole moments of the constituent atoms estimated from the off-center displacements [Fig. 1(c)] and the averaged effective charges obtained by the DFT calculations. The cooperative displacements of the A- and B-site atoms lead to the parallel dipole moments of pA = 23.0 μC/cm2 for the A-site atoms and pB = 36.1 μC/cm2 for the B-site one. This dipole configuration provides a large Ps of 55.8 μC/cm2.
Our crystal-growth process provides a high-quality single crystal of x = 33% [Fig. 2(a,b)]. The crystal is transparent in yellow color with dimensions of 6 × 6 × 5 mm3. X-ray diffraction analysis shows that the crystallographic orientation coincides with that of the seed crystal, indicating an epitaxial growth from the seed. Figure 2(c) shows the leakage current density (J) at 298 K as a function of electric field (E) applied along [001], where the data of the crystal grown in the air (Po2 = 0.02 MPa) is also shown. The crystal grown at Po = 0.9 MPa exhibits a lower J by 1–2 orders of magnitude than that at Po2 = 0.02 MPa.
Grown crystal exhibiting a low leakage current. (a) Side and (b) perspective view of an as-grown single crystal of BNT-BKT (x = 33%) grown by the TSSG method at a PO2 of 0.9 MPa. (c) Leakage current densities along the [001] direction of BNT-BKT (x = 33%) single crystals grown at a PO2 of 0.02 and 0.9 MPa.
Figure 3 shows the polarization (P) and strain (S) properties at 298 K. The crystal (Po2 = 0.02 MPa) [Fig. 3(a)] exhibits a remanent polarization (Pr) of 32 μC/cm2 and a coercive field (Ec) of 23 kV/cm; because this crystal displays a high J of the order of ~10−6 A/cm2 at high fields, the leakage currents are comparable to the polarization-switching currents in the P-E measurements, leading to a blunted response. By contrast, the crystal (Po2 = 0.9 MPa) [Fig. 3(b)] features a well-saturated loop with a large Pr of 48 μC/cm2 and a low Ec of 18 kV/cm. The blue line in Fig. 3(b) indicates the P-E loop along [111] with a Pr of 30 μC/cm2. Provided that the application of E along [111] can lead to a complete switching of Ps (//[001]), the measurement along [111] yields Ps/√3 as a Pr (32.2 μC/cm2), which is close to the observed one (30 μC/cm2). Our measurements along [111] and [001] lead to a Ps of 50–52 μC/cm2, which is in good agreement with that (55.8 μC/cm2) determined by the NPD analysis with the DFT calculations.
Polarization and strain properties. (a,b) Polarization properties of BNT-BKT (x = 33%) single crystals grown at (a) PO2 = 0.02 MPa and (b) PO2 = 0.9 MPa. The index beside each curve indicates the crystallographic direction of the polarization measurements. (c,d) Strain properties of BNT-BKT (x = 33%) single crystals grown at PO2 = 0.9 MPa under (c) bipolar and (d) unipolar electric fields along the [001] and [111] directions.
Figure 3(c) shows the S-E curves along [001] and [111]. The crystal (Po2 = 0.9 MPa) displays a clear butterfly loop in the bipolar measurement; a strain reaches up to ~0.3% along [001], which is larger than that along [111]. A piezoelectric strain constant (d*) estimated from the slope of the unipolar curve at E < 5 kV/cm is as high as 460 pm/V along [001], which is comparable to that34 for commercial PZT ceramics. The bipolar curve along [001] exhibits a large negative S of ~−0.8% at E = Ec (20 kV/cm), whereas that along [111] has a negative S that can be extrapolated from the slope. These results enable us to understand the mechanism of the polarization-switching dynamics under E. If the Ps vector is reversed by the 180° switching and a 90° domain structure does not participate in the process, the negative S is attributed solely to the converse piezoelectric effect. In this case, the slope of S with decreasing E remains constant until the 180°-Ps switching starts, and the negative S can be expected from d*. Provided that the polarization reversal proceeds through the 90°-Ps switching, i.e., the successive rotation of Ps by 90° mediated in a 90° domain state, this process is accompanied by a change in crystallographic configuration from E // c to E // a in each domain. Assuming that the state at E = Ec has a 90° domain structure composed of the domains in the E // c and E // a configurations, we estimate an averaged S of –(c − a)/2a to be −0.8%, which accords with the experiment (−0.8%).
Discussion
Here we discuss the origin of the structural difference between BKT with tetragonal P4mm and BNT with rhombohedral R3c. We investigate the external pressure (p) dependence of the free energy G for the BKT and BNT cells by density functional theory (DFT) calculations, the details of which are described in Calculation Method and Supplementary Information II. For both the cells, the R3c structure is stabilized at a higher p while the P4mm one is at a lower p, because the R3c structure with octahedral rotations of a−a−a− (Glazer notation) prefers a smaller cell volume (V) in the higher-p region. The equilibrium state (p = 0) was found in the R3c phase for the BNT cell with the R3c- P4mm boundary p (p1) at −2.21 GPa, while that appears in the P4mm phase with its p (p2) of 3.20 GPa. A partial substitution of K having a larger ionic radius (rion) for Na increases the average rion on the A site and V; a negative chemical pressure caused by the K substitution increases the phase-boundary p.
Although we did not find an anomaly in the bond valence sum (BVS) as a function of p (Supplementary Fig. SI), the cation-O bonds show prominent features; especially, the Bi-O bonds exhibit a reconstruction across the phase boundary. In the centrosymmetric structure, Bi is surrounded by twelve O atoms. In the R3c structure, Bi is displaced along [111], leading to four different lengths: the shortest Bi-O1 (×3), followed by Bi-O2 (×3), Bi-O2* (×3) and Bi-O1* (×3), where asterisk (*) denotes a longer bond. Essentially, Bi-O1 is independent of p at ~0.240 nm, whose length is in good agreement with the experiments48. By contrast, Bi-O2 is lengthened when p decreases, leading to a smaller BVS of Bi. In the P4mm structure, the displacement of Bi along [001] results in three different lengths: the shorter four, the intermediate four (Bi-O1) and the longer four. Moreover, the tetragonal P4mm accommodates the markedly short Bi-O2 of ~0.225 nm.
To elucidate the origin of the phase stability, we investigate the electronic structures at p~13.4 GPa and −2.3 GPa; the high p stabilizes the R3c structure (Fig. 4a–c) while the low p does the P4mm phase (Fig. 4d–f). The conduction band is formed primality by Ti-3d, and the valence band has a dominant contribution of O-2p. We note that the marked density of states (DOS) of not only Ti-3d but also Bi-6p appears in the valence band; especially the hybridized states of Bi-6p and O-2p determine the bottom of the valence band. At p~13.4 GPa in the BNT cell, the R3c phase features a dominant contribution of the Bi-6p (px + py) derived states around the bottom with a minimum at −5.79 eV in the vicinity of the Γ point (the wavefunction is seen in Fig. 4c), which is lower by ~0.1 eV than the P4mm [Supplementary Fig. S2(a,b)]. We found that the stabilization of the R3c phase for BNT stems from the low-lying valence states arising from the Bi-6p and O-2p hybridization.
Density of states (DOS) calculations. DFT calculation results of (a–c) the R3c structure in the BNT cell at p = 13.4 GPa and (d–f) the P4mm structure in the BKT cell at p = −2.3 GPa; (a,d) partial DOS for Bi, O1 and O2 atom, (b,e) band structures, and (c,f) wave functions at the k points denoted by the dot marks in the band structures.
The similar feature is also seen in the BKT cell at p~−2.3 GPa; the mixed states of Bi-6p and O-2p dominate the bottom of the valence band. The maximal DOS of Bi-6p lies at ~−4.3 eV for the P4mm phase, which is higher than that (~−4.5 eV) for the R3c phase [Supplementary Fig. S2(c,d)]. However, the P4mm phase exhibits a markedly large DOS arising from the Bi-6p (px + py) and O-2p in-plane hybridization (see the wavefunction shown in Fig. 4f), which is due to a small band dispersion in the entire Brillouin zone. Indeed, the atomic partial charge of Bi-6p is 0.89 for the P4mm phase, which is larger than 0.85 for the R3c phase. We found that the P4mm phase of BKT is stabilized by a large DOS of the Bi-6p (px + py) states derived from the orbital interaction with O-2p.
In summary, we uncover the ferroelectric polarization and piezoelectric strain constant in the BKT-based single crystals grown by the high-PO2 TSSG process. These properties originate from a Ps of ~56 μC/cm2 with a c/a of ~1.6%; this Ps rivals that of PbTiO3 (70 μC/cm2). Our theoretical calculations show that the Bi-6p and O-2p hybridization at a moderately negative chemical pressure stabilizes the ferroelectric distortion in tetragonal symmetry. We could apply the high-PO2 process to other functional materials including Bi and/or K in bulk and film forms, here that we have developed high-quality BKT-based crystals by suppressing a defect formation reaction.
Methods
Experimental procedure
We prepared powders of (1 − x)BNT-xBKT (x = 30–35%) via solid-state reaction of the raw materials of Bi2O3 (99.99%), TiO2 (99.99%), Na2CO3 (99.99%), and K2CO3 (99.99%). These starting materials were mixed using ball milling with 100-μm beads and then calcined at 1,223 K for 4 h. The calcined powders were crushed by the ball milling and then calcined again at 1,423 K for 4 h to achieve a homogeneous solid solution.
For crystal structural anlyises, we performed time-of-flight (TOF) NPD measurements using a neutron powder diffractometer iMateria (BL20)47 at Japan Proton Accelerator Research Complex (J-PARC). NPD data in the d range of 0.05 < d/nm < 0.25 were collected with a high resolution Δd/d = 0.16%. The crystal structure was refined by the Rietveld method with a computer software Z-Rietveld49.
We adopted the high-oxygen-pressure top-seeded solution growth (high-Po2 TSSG) method to obtain high-quality BNT–BKT single crystals, the details of which are described in refs. 45,46,50. 70%BNT-30%BKT (x = 30%) powders were mixed with a Bi2O3-KF flux at a weight ratio of BNT-BKT: Bi2O3 (99.99%): KF (99%) = 10: 10: 2. The mixture was soaked at over 1,430 K for 4 h in a Pt crucible rotated at 10 rpm, and then slowly cooled to approximately 1,400 K to form the solution. We used a BNT seed crystal obtained by a conventional flux method, which was set with a {001} plane normal to the melt surface. The seed rotated at 20 rpm counter to the crucible rotation was dipped into the solution, held at the same height for 1–4 h, and then slowly pulled out at a rate of 0.1–0.2 mm h−1 for over 20 h during cooling (<0.5 °C h−1). Eventually, a grown bulk crystal was detached from the solution and then slowly cooled to room temperature. The chemical composition of the grown crystal was analysed by inductively coupled plasma-atomic emission- spectrometry, indicating that the chemical composition is BNT–33%BKT with a composition deviation less than 1%.
The single crystals obtained were annealed at 1173 K for 10 h in air to remove mechanical stress induced during the crystal growth. The annealed crystals were cut along the {001} and {111} plane into plates with a thickness of 0.2 mm, and then gold electrodes were sputtered onto the cut surfaces. We measured polarization and leakage current of the crystal at 298 K using a ferroelectric test system (Toyo Corporation; Model 6252 Rev. B), and strain properties using a laser Doppler displacement meter.
Calculation methods
DFT calculations were performed via the generalized gradient approximation51 with a plane wave basis set. The projector-augmented wave method52 was applied by the Vienna ab initio simulation package (VASP)53. We employed the gradient-corrected exchange-correlation functional of the Perdew-Burke-Ernzerhof revised for solids (PBEsol)54 and a plane-wave cut-off energy of 520 eV. The adopted mesh size of the k-point sampling grid was less than 5 nm‒1 for structural optimizations, 2.5 nm−1 for density-functional perturbation theory (DFPT) calculations. A rock-salt-like A-site ordering were adopted for constructing the Bi1/2Na1/2TiO3 and Bi1/2K1/2TiO3 cells55,56.
To obtain the Born effective charges, all the atomic positions were optimized in the Bi1/2Na1/2TiO3 and Bi1/2K1/2TiO3 cells under the constraints of the fixed lattice constants determined by the NPD analysis. Adopting a weighted average (mol %) of the Born effective charges (Zeff*) of the constituent atoms obtained in their respective Bi1/2Na1/2TiO3 and Bi1/2K1/2TiO3 cells, we estimated the averaged Zeff* of each atom in the BNT-BKT solid solutions. The calculations for the Bi1/2Na1/2TiO3 cell result in the following Zeff* values: 3.9 e for Bi, 1.1 e for Na, 6.1 e for Ti, −5.1 e for Oc, and −1.7 e for Oa, where e indicates the elementary charge of 1.602 × 10−19 C. We also found 4.0 e for Bi, 1.2 e for K, 6.0 e for Ti, −3.4 e for Oc, and −2.3 e for Oa for the Bi1/2K1/2TiO3 cell. Adopting a weighted averaging of the Born effective charges in the respective cells on the mol% base, we obtain the averaged effective Zeff* of each atom in BNT–30%BKT: 2.6 e for Bi0.50Na0.35K0.15 (A-site atom), 6.1 e for Ti, −3.6 e for Oc, and −2.3 e for Oa.
To evaluate phase stability, we calculated the total energy (U) per ABO3 unit cell as a function of the cell volume (V) for the Bi1/2Na1/2TiO3 and Bi1/2K1/2TiO3 cells in R3c and P4mm symmetries, and then analyzed by the Murnaghan equation of state57:
where U0, B0, B0′, and V0 are the total energy, the bulk modulus and its first derivative with respect to the hydrostatic pressure (p) and V at p = 0. Since the free energy (G) is expressed as G = U + pV, we can obtain the relation between G and p using the fitting parameters in Eq. 1. The arrangement of the A-site atoms in the cells lowers the symmetry, i.e., the space group of the rhombohedral changes from R3c to R3 and that of the tetragonal from P4mm to I4mm. For simplicity, the higher symmetry is used to denote the space group throughout this paper.
References
Bhalla, A. S., Guo, R. & Roy, R. The perovskite structure—a review of its role in ceramic science and technology. Mater. Res. Innov. 4, 3–26 (2000).
Saparov, B. & Mitzi, D. B. Organic–Inorganic Perovskites: Structural Versatility for Functional Materials Design. Chem. Rev. 116, 4558–4596 (2016).
Sleight, A. W., Gillson, J. L. & Bierstedt, P. E. High-temperature superconductivity in the BaPb1-xBixO3 systems. Solid State Commun 17, 27–28 (1975).
Murphy, D. W. et al. New superconducting cuprate perovskites. Phys. Rev. Lett. 58, 1888–1890 (1987).
He, T. et al. Superconductivity in the non-oxide perovskite MgCNi3. Nature 411, 54–56 (2001).
Scott, J. F. High-dielectric constant thin films for dynamic random access memories (DRAM). Annu. Rev. Mater. Sci. 28, 79–100 (1998).
van Dover, R. B., Schneemeyer, L. F. & Fleming, R. M. Discovery of a useful thin-film dielectric using a composition-spread approach. Nature 392, 162–164 (1998).
Kishi, H., Mizuno, Y. & Chazono, H. Base-Metal Electrode-Multilayer Ceramic Capacitors: Past, Present and Future Perspectives. Jpn. J. Appl. Phys. 42, 1–15 (2003).
Cowley, R. A., Gvasaliya, S. N., Lushnikov, S. G., Roessli, B. & Rotaru, G. M. Relaxing with relaxors: a review of relaxor ferroelectrics. Adv. Phys 60, 229–327 (2011).
Medvedev, D. A., Lyagaeva, J. G., Gorbova, E. V., Demin, A. K. & Tsiakaras, P. Advanced materials for SOFC application: Strategies for the development of highly conductive and stable solid oxide proton electrolytes. Prog. Mater. Sci. 75, 38–79 (2016).
Zhang, K. et al. Nanostructured Mn-based oxides for electrochemical energy storage and conversion. Chem. Soc. Rev. 44, 699–728 (2015).
Teraoka, Y., Zhang, H. M., Okamoto, K. & Yamazoe, N. Mixed ionic-electronic conductivity of La1−xSrxCo1−yFeyO3−δ perovskite-type oxides. Mater. Res. Bull. 23, 51–58 (1988).
Mizusaki, J., Arai, K. & Fueki, K. Ionic conduction of the perovskite-type halides. Solid State Ionics 11, 203–211 (1983).
Srinivasan, G., Rasmussen, E. T., Levin, B. J. & Hayes, R. Magnetoelectric effects in bilayers and multilayers of magnetostrictive and piezoelectric perovskite oxides. Phys. Rev. B 65, 134402 (2002).
Van Aken, B. B., Palstra, T. T. M., Filippetti, A. & Spaldin, N. A. The origin of ferroelectricity in magnetoelectric YMnO3. Nat. Mater. 3, 164–170 (2004).
Li, W. et al. Chemically diverse and multifunctional hybrid organic–inorganic perovskites. Nat. Rev. Mater. 2, 16099 (2017).
Filip, M. R. & Giustino, F. The geometric blueprint of perovskites. Proc. Natl. Acad. Sci. 115, 5397–5402 (2018).
Singh, T., Kulkarni, A., Ikegami, M. & Miyasaka, T. Effect of Electron Transporting Layer on Bismuth-Based Lead-Free Perovskite (CH3NH3)3Bi2I9 for Photovoltaic Applications. ACS Appl. Mater. Interfaces 8, 14542–14547 (2016).
Miller, N. C. & Bernechea, M. Research Update: Bismuth based materials for photovoltaics. APL Mater. 6, 84503 (2018).
Park, B.-W. et al. Bismuth Based Hybrid Perovskites A3Bi2I9 (A: Methylammonium or Cesium) for Solar Cell Application. Adv. Mater. 27, 6806–6813 (2015).
Ravindran, P., Vidya, R., Kjekshus, A., Fjellvåg, H. & Eriksson, O. Theoretical investigation of magnetoelectric behavior in BiFeO3. Phys. Rev. B 74, 224412 (2006).
Ramesh, R. & Spaldin, N. A. Multiferroics: progress and prospects in thin films. Nat. Mater. 6, 21–29 (2007).
Zeches, R. J. et al. A Strain-Driven Morphotropic Phase Boundary in BiFeO3. Science 326, 977–980 (2009).
Picozzi, S. & Ederer, C. First principles studies of multiferroic materials. J. Phys. Condens. Matter 21, 303201 (2009).
Yoneda, Y., Kitanaka, Y., Noguchi, Y. & Miyayama, M. Electronic and local structures of Mn-doped BiFeO3 crystals. Phys. Rev. B 86, 184112 (2012).
Smolenskii, G. A., Isupov, V. A., Agranovskaya, A. I. & Krainik, N. N. New ferroelectrics of complex composition. IV. Sov. Phys. Solid State 2, 2651–2654 (1961).
Jones, G. O. & Thomas, P. A. Investigation of the structure and phase transitions in the novel A-site substituted distorted perovskite compound Na0.5Bi0.5TiO3. Acta Crystallogr., Sect. B 58, 168–178 (2002).
Elkechai, O., Manier, M. & Mercurio, J. P. Na0.5Bi0.5TiO3–K0.5Bi0.5TiO3 (NBT-KBT) system: A structural and electrical study. Phys. Status Solidi A 157, 499–506 (1996).
Sasaki, A., Chiba, T., Mamiya, Y. & Otsuki, E. Dielectric and Piezoelectric Properties of (Bi0.5Na0.5)TiO3–(Bi0.5K0.5)TiO3 Systems. Jpn. J. Appl. Phys. 38, 5564–5567 (1999).
Nagata, H., Yoshida, M., Makiuchi, Y. & Takenaka, T. Large Piezoelectric Constant and High Curie Temperature of Lead-Free Piezoelectric Ceramic Ternary System Based on Bismuth Sodium Titanate-Bismuth Potassium Titanate-Barium Titanate near the Morphotropic Phase Boundary. Jpn. J. Appl. Phys. 42, 7401–7403 (2003).
Izumi, M., Yamamoto, K., Suzuki, M., Noguchi, Y. & Miyayama, M. Large electric-field-induced strain in Bi0.5Na0.5TiO3–Bi0.5K0.5TiO3 solid solution single crystals. Appl. Phys. Lett. 93, 242903 (2008).
Morishita, A., Kitanaka, Y., Izumi, M., Noguchi, Y. & Miyayama, M. Enhanced piezoelectric properties in (Bi0.5K0.5)TiO3–(Bi0.5Na0.5)TiO3 ferroelectric single crystals. J. Adv. Dielectr. 1, 63–69 (2011).
Zhao, S., Li, G., Ding, A., Wang, T. & Yin, Q. Ferroelectric and piezoelectric properties of (Na,K)0.5Bi0.5TiO3 lead free ceramics. J. Phys. D. Appl. Phys. 39, 2277–2281 (2006).
Shrout, T. R. & Zhang, S. J. Lead-free piezoelectric ceramics: Alternatives for PZT? J. Electroceramics 19, 113–126 (2007).
Rödel, J. et al. Perspective on the Development of Lead-free Piezoceramics. J. Am. Ceram. Soc. 92, 1153–1177 (2009).
König, J. et al. The thermal decomposition of K0.5Bi0.5TiO3 ceramics. J. Eur. Ceram. Soc. 29, 1695–1701 (2009).
Yi, X. et al. Flux growth and characterization of lead-free piezoelectric single crystal [Bi0.5(Na1−xKx)0.5]TiO3. J. Cryst. Growth 281, 364–369 (2005).
Zhao, M. L. et al. Piezoelectric properties and structural phase transitions of naturally polarized (Na0.75K0.25Bi)0.5TiO3 crystal. Appl. Phys. Lett. 92, 142902 (2008).
Teranishi, S. et al. Giant strain in lead-free (Bi0.5Na0.5)TiO3-based single crystals. Appl. Phys. Lett. 92, 182905 (2008).
Nagata, H. Electrical properties and tracer diffusion of oxygen in some Bi-based lead-free piezoelectric ceramics. J. Ceram. Soc. Japan 116, 271–277 (2008).
Babu, J. B., Madeswaran, G., Chen, X. L. & Dhanasekaran, R. Effect of oxygen vacancies on ferroelectric behavior of Na1/2Bi1/2TiO3–BaTiO3 single crystals. Mater. Sci. Eng. B 156, 36–41 (2009).
Noguchi, Y., Teranishi, S., Suzuki, M. & Miyayama, M. Electric-field-induced giant strain in Bi0.5Na0.5TiO3-based single crystals: Influence of high-oxygen-pressure annealing. J. Ceram. Soc. Jpn. 117, 32–36 (2009).
Robels, U. & Arlt, G. Domain wall clamping in ferroelectrics by orientation of defects. J. Appl. Phys. 73, 3454–3460 (1993).
Kitanaka, Y., Noguchi, Y. & Miyayama, M. Oxygen-vacancy-induced 90°-domain clamping in ferroelectric Bi4Ti3O12 single crystals. Phys. Rev. B 81, 94114 (2010).
Kitanaka, Y., Noguchi, Y. & Miyayama, M. High-Performance Ferroelectric Bi4Ti3O12 Single Crystals Grown by Top-Seeded Solution Growth Method under High-Pressure Oxygen Atmosphere. Jpn. J. Appl. Phys. 49, 09MC06 (2010).
Kitanaka, Y., Onozuka, H., Noguchi, Y. & Miyayama, M. High-Performance Ferroelectric Bi0.5Na0.5TiO3 Single Crystals Grown by Top-Seeded Solution Growth Method under High-Pressure Oxygen Atmosphere. Ferroelectrics 414, 24–29 (2011).
Ishigaki, T. et al. IBARAKI materials design diffractometer (iMATERIA)—Versatile neutron diffractometer at J-PARC. Nucl. Instrum. Methods Phys. Res., Sect. A 600, 189–191 (2009).
Aksel, E. et al. Local atomic structure deviation from average structure of Na0.5Bi0.5TiO3: Combined x-ray and neutron total scattering study. Phys. Rev. B 87, 104113 (2013).
Oishi, R. et al. Rietveld analysis software for J-PARC. Nucl. Instrum. Methods Phys. Res. Sect. A 600, 94–96 (2009).
Onozuka, H., Kitanaka, Y., Noguchi, Y. & Miyayama, M. Crystal Growth and Characterization of (Bi0.5Na0.5)TiO3–BaTiO3 Single Crystals Obtained by a Top-Seeded Solution Growth Method under High-Pressure Oxygen Atmosphere. Jpn. J. Appl. Phys. 50, 09NE07 (2011).
Langreth, D. C. & Perdew, J. P. Theory of nonuniform electronic systems. I. Analysis of the gradient approximation and a generalization that works. Phys. Rev. B 21, 5469–5493 (1980).
Blöchl, P. E. Projector augmented-wave method. Phys. Rev. B 50, 17953–17979 (1994).
Kresse, G. & Hafner, J. Ab initio molecular-dynamics simulation of the liquid-metal–amorphous-semiconductor transition in germanium. Phys. Rev. B 49, 14251–14269 (1994).
Perdew, J. P. et al. Restoring the Density-Gradient Expansion for Exchange in Solids and Surfaces. Phys. Rev. Lett. 100, 136406 (2008).
Kitanaka, Y. et al. Polarization twist in perovskite ferrielectrics. Sci. Rep. 6, 32216 (2016).
Groszewicz, P. B. et al. Reconciling Local Structure Disorder and the Relaxor State in (Bi1/2Na1/2)TiO3-BaTiO3. Sci. Rep. 6, 31739 (2016).
Murnaghan, F. D. Finite Deformation of an Elastic Solid. (Wiley, 1951).
Acknowledgements
We acknowledge Prof. T. Ishigaki and Prof. A. Hoshikawa for technical assistance for neutron powder diffraction measurements at the Japan Proton Accelerator Research Complex (2014A0282, 2014B0068). This research is partly supported by Japan Society for the Promotion of Science (Grant Number 17H06239, 18K14303). We thank Mr. A. Morishita for sample preparations and electric measurements.
Author information
Authors and Affiliations
Contributions
Y.N. conceived and initiated the project. M.M. directed the research. Y.N. and Y.K. carried out the theoretical study. Y.K. carried out the experiments and the structural analysis. Y.N. and Y.K. wrote the manuscript. All authors participated in the discussion of the obtained results.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kitanaka, Y., Noguchi, Y. & Miyayama, M. Uncovering ferroelectric polarization in tetragonal (Bi1/2K1/2)TiO3–(Bi1/2Na1/2)TiO3 single crystals. Sci Rep 9, 19275 (2019). https://doi.org/10.1038/s41598-019-55576-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-55576-y
- Springer Nature Limited