Abstract
Small for gestational age (SGA) newborns are often born from hypertensive pregnancies. This study aimed to compare the systemic metabolism of cortisol (F) in pregnancies with SGA and appropriate for gestational age (AGA) infants, considering both the normotensive (NT) and hypertensive patients. We hypothesized that the disturbances in systemic metabolism of F in pre-eclampsia (PE) might be attributed not to hypertension only, but to SGA. The study included 117 pregnants in the third trimester, divided into groups: NT pregnancy and SGA neonate (SGA-NT); NT pregnancy and AGA neonate (AGA-NT; controls), and respective groups with PE: SGA-PE and AGA-PE. We assessed the glucocorticoid balance with the function of enzymes involved in systemic metabolism of F: 11β-hydroxysteroid dehydrogenase type 1 and 2 (11β-HSD1 and 11β-HSD2), 5α- and 5β-reductase. The enzymes’ functions were estimated with the levels of F, cortisone (E), and their metabolites in plasma or urine, which we measured with HPLC-FLD and HPLC-MS/MS. The plasma F/E and urinary free F/E (UFF/UFE) ratios correlated significantly only in patients with the normal function of 5α- and 5β-reductase. The increased function of 11β-HSD2 was noted in all pre-eclamptic pregnancies. Increased function of 5α- and 5β-reductase was specific only for SGA-PE pregnancies, and the function of 5α-reductase was dependent on fetal sex. The SGA-NT pregnancies with male fetuses trended towards the higher function of renal 11β-HSD2 and 5β-reductase; SGA-NT pregnancies with female fetuses lacked any systemic glucocorticoid imbalance. In conclusion, systemic metabolism of F is the most intensive in pre-eclamptic pregnancies complicated by SGA with female fetuses. Our study supports the hypothesis about the different origins of PE and idiopathic intrauterine growth restriction and suggests the sex-specific mechanisms responsible for fetal growth restriction.
Similar content being viewed by others
Introduction
Newborns born with the birth weight below the 10th percentile concerning their sex and gestational age (GA) at delivery are classified as small for gestational age (SGA). Low birth weight may result from the intrauterine growth restriction (IUGR) in which the fetus fails to achieve its full growth potential1. Both IUGR and SGA pregnancies correlate with the higher rates of neonatal morbidity and mortality, lower Apgar scores, placental abruption, pre-term deliveries, and stillbirth2,3. Moreover, lower birth weight increases the risk of cardiovascular, metabolic and mental disorders in adulthood1,4.
More than 60% of SGA pregnancies have an unexplained etiology and remain idiopathic2. Low birth weight may be a consequence of the constitutional factors such as lower maternal weight; SGA may also result from pathological conditions. Risk factors that promote lower birth weight could be of environmental, maternal, fetal, and placental origin1,5. Hypertensive pregnancy, especially pre-eclampsia (PE), is one of them. It attributes to 12–25% of all SGA/IUGR cases6.
Association between SGA/IUGR and PE and the disturbed activity and/or expression of placental 11β-hydroxysteroid dehydrogenase type 2 (11β-HSD2) has been established7,8,9,10,11,12. The enzyme protects the fetus from the high concentration of maternal cortisol (F) due to inactivation of F into cortisone (E)13. We have previously reported14,15 the increased apparent activity of renal 11β-HSD2 in women with gestational hypertension and PE – conditions which are often complicated by the SGA neonates16. Therefore, the question emerged whether the observed abnormalities occur only in the hypertensive pregnancy and if they also are present in IUGR.
A part of the answer came in 2018 with the study of Vasku et al.17. They analyzed serum F/E ratios in pregnant women and concluded about higher systemic 11β-HSD2 activity in PE without IUGR but not in IUGR without PE. However, the ratio of urinary free F (UFF) and free E (UFE) indicates the most reliably the 11β-HSD2 function18. Furthermore, the 11β-HSD2 is the only one of the four major enzymes involved in the metabolism of F; to evaluate the glucocorticoid balance properly, one should also consider the function of 11β-hydroxysteroid dehydrogenase type 1 (11β-HSD1), 5α- and 5β-reductase19,20,21,22.
The scarce data concern the systemic function of these enzymes and the UFF/UFE in relation to SGA pregnancies. Disturbed expression or activity of 11β-HSD1 was observed in the placenta, fetal membranes, or decidua of PE/IUGR pregnancies23,24,25. We previously indicated the imbalance in the function of 5α/β-reductase in PE15; no published work discussed the issue in gestations complicated by SGA.
This study aimed to estimate and compare the apparent activities of 11β-HSD1, 11β-HSD2, 5α- and 5β-reductase in SGA and appropriate for gestational age (AGA) pregnancies. We hypothesized that the disturbances in systemic metabolism of F in PE might be attributed not to hypertension only, but to SGA, which often accompanies PE. Glucocorticoid balance was analyzed in both normotensives and pre-eclamptic women with an emphasis on any abnormalities specific to SGA condition. We assessed the function of the enzymes by the levels of plasma and urinary F, E, and their tetrahydro- and allo-tetrahydrometabolites (THF and allo-THF for F; THE and allo-THE for E) in urine.
Materials and Methods
Study population
The study was performed in accordance with the Declaration of Helsinki. The Ethical Committee at Poznan University of Medical Sciences approved the protocol (documents no. 954/11 and 1129/16). Each participant gave written informed consent before including in the project. We enrolled 117 pregnant women hospitalized in Gynecological and Obstetrics University Hospital of Poznan University of Medical Sciences between 2013 and 2016. All subjects were of Polish Caucasian origin and had a singleton pregnancy with GA ranging from 27 to 41.
The study population comprised women who gave birth to a child classified as SGA, based on the newborn’s birth weight (lower than the 10th percentile as defined by the American College of Obstetricians and Gynecologists26) according to the regional data27. The fact of SGA was suspected during pregnancy, considering the estimated fetal weight (pregnancies with IUGR26), but was finally confirmed after delivery. For comparative purposes, two types of patients were included: normotensive and pre-eclamptic ones. They constituted two different groups (SGA-NT and SGA-PE, respectively) and were analyzed separately.
The results of women from SGA groups were compared to AGA (newborn’s birth weight between 10th and 90th percentile for appropriate GA and sex)27. The AGA groups (AGA-NT and AGA-PE) were matched to proper SGA groups (SGA-NT and SGA-PE, respectively) regarding GA at sample collection, maternal pre-pregnancy BMI, maternal age, and parity. The exclusion criteria for all study groups comprised: stillbirth and congenital fetal defects, chromosomal anomalies, chronic infectious diseases, mental and chronic liver disorders, and metabolic and endocrine diseases (except for hypothyroidism and gestational diabetes). Patients who declared consuming a significant amount of licorice products or grapefruit juice, being active smokers, or with a history of alcohol or drug abuse were also not eligible. Thus, our SGA-NT group comprised women with idiopathic SGA (IUGR) babies while AGA-NT group consisted of healthy controls.
Despite the complexity of PE, we defined the condition as hypertension (blood pressure ≥ 140/90 mmHg from 2 measurements, at least 4 h apart) and new-onset of proteinuria ≥ 300 mg/24 h28. This approach is the most common in scientific research, and we maintained the criteria for better comparability with previously published results. BMI before pregnancy was calculated from self-reported weight and height, while prematurity was defined as giving birth before the 37th week of gestation. Women diagnosed with PE were treated with antihypertensive drugs, mostly with methyldopa (>94% of pre-eclamptic patients) - either as an independent therapy or in combination with other medications (nitrendipine, metoprolol, verapamil, magnesium sulfate) while those with hypothyroidism took levothyroxine.
Table 1 shows the general characteristics of patients. There were no significant differences between 4 study groups regarding maternal age, GA at sampling, nulliparity, infant’s sex, or frequency of suffering from gestational diabetes and hypothyroidism. Two patients from the AGA-PE group (10.5%) had asthma but did not take steroids during gestation. Six women from each of the pre-eclamptic groups (SGA-PE and AGA-PE) were previously diagnosed with chronic hypertension; the others suffered from pregnancy-induced hypertension. Prematurity was recognized in 73.7% of cases from SGA-PE group, 36.8% of AGA-PE, 28.6% of SGA-NT and only 6.4% of AGA-NT. Among PE patients there was a marked disparity in proteinuria of 2.11 (0.52–3.26) vs. 0.46 (0.35–1.05) g/24 h (medians (interquartile ranges)) for SGA-PE and AGA-PE group, respectively.
Samples and methods
Each patient provided one fasting blood sample and a 24-hour urine collection. The blood was collected in the morning (at 7.00–8.00 a.m.) on the day when the urine collection was completed. The blood sample was centrifuged at 1740 × g for 10 min, and plasma was collected. Both plasma and urine were stored at −25 °C until analyzed. The HPLC-FLD method was used to determine the total concentrations of F and E in plasma, as described in detail elsewhere14. Urinary UFF and UFE, as well as total amounts of F, E, THF, allo-THF, THE, and allo-THE, were measured with HPLC-MS/MS method29. For total steroids, assays were preceded by enzymatic hydrolysis (37 °C, 20 h) with β-glucuronidase from Helix pomatia. When the determined steroids were below the lower limit of quantitation or the limit of detection, we applied the previously described procedure15. Based on the available literature18,20,21,22,30,31, the apparent activity of enzymes involved in the metabolism of F was assessed by: UFF/UFE for 11β-HSD2 function; (THF + allo-THF)/(THE + allo-THE) noted as THFs/THEs for global 11β-HSD activity; allo-THF/F for 5α-reductase; and THF/F for 5β-reductase. In calculations regarding 5α- and 5β-reductase function, we used only the total glucocorticoids. This decision was made because of our previous observations15 concerning the disturbed conjugation degree of certain steroids with glucuronic and sulphuric acid in pre-eclamptic pregnancies.
Additionally, the F metabolic clearance was calculated by (THFs + THEs)/UFF ratio18; the net glucocorticoid balance by plasma F/E ratio32; the daily secretion of F by the sum of THFs, THEs, total F and total E in urine31.
Statistics
The statistical analysis was carried out using Statistica 13 software (Statsoft Inc., Tulsa, OK, USA). The continuous variables were expressed, depending on the results of the Shapiro-Wilk test, as a mean ± SD or a median score (interquartile range), while the categorical data as a number of subjects (%). The differences between groups were checked with ANOVA, Kruskal-Wallis/Mann-Whitney U test, or χ2 test for normally distributed, non-parametric, and categorical data, respectively. The simple correlations were assessed with the Spearman test. In each analysis, a P-value < 0.05 was considered significant.
The use of the Kruskal-Wallis test enabled to identify both the influence of PE/SGA on F metabolism as well as the interaction between PE and SGA. However, patients with PE presented higher pre-pregnancy BMI compared with normotensive individuals. Therefore, the forward multiple regression analyses were performed (selection F = 1, elimination F = 0) to particularize the steroid results from the Kruskal-Wallis test.
Results
Glucocorticoid balance in pre-eclamptic pregnancies
The analysis of glucocorticoid balance concerning fetal sex did not show significant differences in the three groups (AGA-NT, AGA-PE, and SGA-PE; supplementary data - Figs. S1–S6). Therefore, for pre-eclamptic pregnancies, we made all further comparisons for combined groups (comprising both female and male fetuses). Figure 1 presents the calculated parameters reflecting the function of 11β-HSD2, global 11β-HSD, 5α- and 5β-reductase, as well as the overall glucocorticoid balance in the body (AGA-NT vs. AGA-PE vs. SGA-PE). The detailed results (levels of plasma and urinary steroids and calculated ratios) are shown in supplementary Table S1.
Glucocorticoid balance in pregnant women: normotensive with appropriate for gestational age newborn (AGA-NT), as well as pre-eclamptic with appropriate or small for gestational age newborns (AGA-PE and SGA-PE, respectively). The function of renal 11β-HSD2 (reflected by the ratio of urinary UFF/UFE – A), systemic 11β-HSD (THFs/THEs – B), hepatic 5α- and 5β-reductase (allo-THF/F - C; THF/F - D, respectively), overall balance between F and E (plasma F/E - E) as well as the F clearance [(THFs + THEs)/UFF - F]. Boxplots present: median (middle point), interquartile range (box), and range. Outliers were excluded according to Tuckey’s method. The detailed P-values for between-group differences are presented in the figure.
The total secretion of glucocorticoids [(THFs + THEs + F + E)/UCr ratio] was similar in all groups (Table S1). Both pre-eclamptic groups (AGA-PE and SGA-PE) presented higher apparent activity of 11β-HSD2 (lower UFF/UFE; Fig. 1A), higher metabolic clearance of F [higher (THFs + THEs)/UFF; Fig. 1F] and lower plasma F/E (Fig. 1E) compared with healthy controls (AGA-NT group). Pre-eclamptic pregnancies complicated by the SGA baby (SGA-PE group) differed significantly from those with the AGA neonates (AGA-PE) and showed: the increased function of 5α-reductase (higher allo-THF/F; Fig. 1C), 5β-reductase (higher THF/F; Fig. 1D) and changed global 11β-HSD (higher THFs/THEs; Fig. 1B).
Glucocorticoid balance in SGA pregnancies
The analysis of glucocorticoid balance concerning fetal sex showed significant differences only in the SGA-NT group (supplementary data – Figs. S1–S6). Therefore, we made all further comparisons separately for pregnancies with female or male fetuses. Results of Kruskal-Wallis analyses (AGA-NT vs. SGA-NT vs. SGA-PE) concerning fetal sex are presented in Fig. 2. Detailed results are attached in supplementary materials (Tables S2 and S3).
Glucocorticoid balance in pregnant women: normotensive with appropriate or small for gestational age newborn (AGA-NT and SGA-NT, respectively), as well as pre-eclamptic with small for gestational age newborns (SGA-PE). The results are presented separately for pregnancies with female or male fetuses. The function of renal 11β-HSD2 (UFF/UFE - A), systemic 11β-HSD (THFs/THEs - B), hepatic 5α- and 5β-reductase (allo-THF/F - C; THF/F - D, respectively), overall balance between F and E (plasma F/E - E) as well as the F clearance [(THFs + THEs)/UFF - F]. Boxplots present: median (middle point), interquartile range (box), and range. Outliers were excluded according to Tuckey’s method. The detailed P-values for between-group differences are presented in the figure.
The total secretion of glucocorticoids was similar in all three groups (Tables S2 and S3). The trend towards the higher function of 11β-HSD2, which is characteristic for pre-eclamptic pregnancies, can also be observed in SGA-NT, but only with male fetuses (P = 0.052; Fig. 2A). A similar situation can be observed with plasma F/E (P = 0.102; Fig. 2E). The female SGA-NT group presents values of urinary UFF/UFE (Fig. 2A) and plasma F/E (Fig. 2E) comparable to AGA-NT and significantly higher than female SGA-PE. Metabolic clearance of F is significantly higher only in pre-eclamptic pregnancies (SGA-PE), SGA-NT pregnancies are comparable to AGA-NT (both with female and male fetuses; Fig. 2F). Increased functions of 5α- and 5β-reductases, observed in SGA-PE pregnancies, are determined by fetal sex. Increased function of 5α-reductase (Fig. 2C) is specific only for pre-eclamptic pregnancies (SGA-PE) with female fetuses. On the contrary, the function of 5β-reductase (Fig. 2D) is increased in both female and male SGA-PE pregnancies. SGA-NT group with only male fetuses showed increased function of 5β-reductase (P = 0.052). Tables S4–S6 show the results from multiple regression analyses, which confirm and complete the Kruskal-Wallis test.
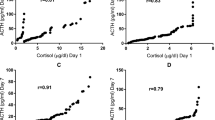
Figure 3 presents the results of the Spearman test; plasma F/E correlates with urinary UFF/UFE ratio in the AGA-NT group (R = 0.479), AGA-PE group (R = 0.651) and SGA-NT subgroup with female babies (R = 0.593), but neither in SGA-PE group nor in SGA-NT subgroup with male babies.
The correlations between plasma F/E ratio and urinary UFF/UFE ratio in AGA-NT (asterisks; A), AGA-PE (empty circles; B), SGA-PE (full circles; C), SGA-NT group with FEMALE fetuses (empty triangles; D), and SGA-NT group with MALE fetuses (full triangles; E). The Spearman test showed significant correlations between calculated parameters in AGA-NT (R = 0.479; P = 0.001), AGA-PE (R = 0.651; P = 0.002), and in SGA-NT with FEMALE fetuses (R = 0.593; P = 0.020). No correlation was observed either in SGA-PE group or in SGA-NT with MALE fetuses.
Discussion
Our previous studies revealed the increased function of systemic 11β-HSD214,15, 5α-reductase, and 5β-reductase15 in PE compared with a healthy pregnancy. Here, we present novel findings on the functions of 5α- and 5β-reductase. The increase in functions of 5α- and 5β-reductase are characteristic for pre-eclamptic gestations only with SGA baby and additionally are associated with the fetal sex. The increase in 5α-reductase function relates to SGA-PE pregnancies only with female fetuses, while the function of 5β-reductase increases in all SGA-PE pregnancies. The apparent activity of 11β-HSD2 increases in all pregnancies with PE, irrespectively either of the growth of the fetus (both SGA-PE and AGA-PE) or the fetal sex.
The function of 11β-HSD2 in PE increases regardless of the SGA condition: the UFF/UFE ratio was significantly lower in both pre-eclamptic groups (SGA-PE and AGA-PE) in comparison with healthy controls (Fig. 1A). Our results are consistent with those presented by Vasku et al.17 and Jayasuriya et al.33, and detail the glucocorticoids’ profile in pre-eclamptic pregnancy. The advantage of our study is that we included the two cohorts of pre-eclamptic women: those who gave birth to SGA (SGA-PE), and AGA neonates (AGA-PE).
The urinary THFs/THEs ratio was higher in the SGA-PE group than in controls and AGA-PE (Fig. 1B). Provided the normal activities of 11β-HSD2 and 5α/β-reductase, the F and E metabolites ratio usually predicts the function of 11β-HSD120,22,31. In our study, the conclusions about 11β-HSD1 cannot be derived from the THFs/THEs - the THFs/THEs value in SGA-PE is impacted by the higher systemic activities of 11β-HSD2 (Fig. 1A), 5α-reductase (Fig. 1C) and 5β-reductase (Fig. 1D). Finken et al. reported similar situation when comparing the F metabolism in women and men22. The authors attributed the higher THFs/THE ratio rather to the different activity of 5β-reductase than to the abnormal activity of 11β-HSD1.
In the non-pregnant population, approx. 50% of F, secreted by the adrenal cortex, appear in the urine as A-ring metabolites19. A sum of THFs, THEs and the total amount of F and E in urine emerged as a reliable parameter to assess the daily production of F31. This index was similar in all study groups (Tables S1–S3). It suggests that despite the increased systemic metabolism of F in pre-eclamptic gestation (reflected in the higher clearance of F; Fig. 1F), the overall secretion of F remains unchanged.
The SGA-PE group, but not AGA-PE, manifested lower plasma F concentration (Table S1). Such results may indicate the blunted HPA axis in SGA-PE. The limitation of this assumption is a single measurement of total plasma F (morning sample); therefore, it needs verification in the future. Some authors noted the decreased plasma F in PE33,34,35, but one has never assessed this phenomenon in relation to the coexisting PE and SGA. Vasku et al. demonstrated similar levels of serum F in PE without IUGR and healthy controls17. This finding is in agreement with our observations for the AGA-PE group.
Scarce literature data concern the systemic F metabolism in women with idiopathic IUGR. Idiopathic IUGR was characterized by normal values of serum F/E ratio17,33, plasma E/F ratio10, or serum levels of A-ring metabolites of F and E17. It complies with our findings from plasma and urine for the SGA-NT group with female fetuses. None of the cited authors10,17,33 analyzed glucocorticoid balance in idiopathic IUGR pregnancy concerning fetal sex. Our results indicate that it may be fetal-sex-specific: we revealed the trend towards increased function of renal 11β-HSD2 (Fig. 2A) and 5β-reductase (Fig. 2D) in SGA-NT subgroup with males (Tables S2–S3). No such trend occurred in SGA-NT subgroup with female fetuses. Differences in maternal glucocorticoid metabolism, which are fetal-sex-dependent, add to the topic on sex-specific differences in early-life programming of HPA axis36.
IUGR and PE might have similar etiology as they both result from placental insufficiency. On the other hand, PE and idiopathic IUGR have different risk factors37, and associate with various abnormalities38,39,40. Our study supports the hypothesis on the different origins of IUGR and PE and suggests the various molecular mechanisms responsible for growth restriction depending on the fetal sex. The data on sex-specific differences in function or expression of 11β-HSD1 and 11β-HSD2 are inconclusive. Some authors reported a lack of sex-specific differences in 11β-HSD1/11β-HSD2 expression or activity in placentas in the SGA or control group10,24. Others showed the lower activity of 11β-HSD2 in placentas from SGA pregnancies with female fetuses when compared to those with male fetuses41. Also, antenatal betamethasone administration, which usually reduces newborn’s birth weight, is linked with the decreased protein expression and activity of placental 11β-HSD2 only in pregnancies with female fetuses42. We observed that UFF/UFE ratio in normotensive mothers with female SGA fetuses is higher than with male SGA fetuses (Fig. S1). This observation supports the findings of Mericq et al.41 and indicates that the trend towards the lower function of 11β-HSD2 in SGA-NT pregnancies with females concerns both placental and renal 11β-HSD2. A sex-specific difference in the activity of cortisol-metabolizing enzymes was also observed in the pre-eclamptic SGA group. The increased function of 5α-reductase was noted only in the female subgroup (Fig. 2C). According to our best knowledge, there is no report concerning systemic function of 5α-reductase in pregnancies with male vs. female fetuses. Higher activity of 5α-reductase was however noted in placental tissue from male pregnancies43.
Despite plasma or serum F/E ratio is a non-specific parameter that reflects the overall glucocorticoid balance in the body, it expresses more accurately the activity of 11β-HSD2 than 11β-HSD144. It is used to calculate the 11β-HSD2 function in non-pregnant32 and pregnant population17. We assessed the correlation between plasma F/E ratio and urinary UFF/UFE – the best surrogate marker for the activity of renal 11β-HSD218. The significant correlation occurred in AGA-NT group, AGA-PE group and SGA-NT subgroup with female fetuses. These groups were found to have normal function of 5α/β-reductases (Tables S1–S3). A correlation was found neither in SGA-PE group nor in SGA-NT subgroup with male fetuses (Fig. 3) – both characterized by the increased A-ring production (as described above). Pregnant women may exhibit many disturbances in glucocorticoid balance, both physiological (hypercortisolism or the additional source of 11β-HSD2 – the placenta13) and pathological (discussed above). We state that the clear association between plasma F/E and the function of renal 11β-HSD2 exists only in case of the unaffected function of other enzymes involved in the metabolism of F.
The limitation of our study is that the study groups differed in terms of pre-pregnancy BMI. The AGA groups matched to respective SGA groups for BMI (AGA-NT to SGA-NT and AGA-PE to SGA-PE), but women with PE presented higher BMI than normotensives. A similar situation was reported by other authors who studied patients with PE, isolated IUGR, and healthy controls40,45. This finding can be explained by the risk factors of PE (higher maternal BMI37,46,47) and SGA infants (lower BMI48).
Conclusions
Our results indicate that PE in pregnancy is associated with the intensified systemic metabolism of F, further increased in co-existing SGA, especially with female fetuses. We suggest it may result from a compensatory mechanism in response to an excess of proapoptotic F, which reaches the fetus. Such a phenomenon is absent in idiopathic SGA pregnancies with female fetuses, which supports the hypothesis about the different origins of PE and normotensive IUGR. The observed disparities also confirm the existence of various types of PE.
Our main findings include: (i) Compared with healthy controls, the increased function of 5α- and 5β-reductase occurs only in pre-eclamptic SGA pregnancies, while the increased systemic apparent activity of 11β-HSD2 - in all pre-eclamptic pregnancies; the activity of 5α-reductase in SGA-PE is sex-dependent with a much higher function in pregnancies with female than male fetuses; (ii) Idiopathic (normotensive) SGA pregnancies exhibit sex-specific differences in the function of enzymes involved in the metabolism of F: compared with healthy controls, pregnancies with males trend towards higher function of renal 11β-HSD2 and 5β-reductase; normotensive SGA pregnancies with female fetuses lack any systemic glucocorticoid imbalance; (iii) In pregnant women, plasma F/E ratio correlates significantly with UFF/UFE ratio only in cases with the proper function of 5α- and 5β-reductases - when considered as an index of the apparent activity of 11β-HSD2, plasma F/E should be used with a great caution; (iv) Daily production of F changes neither in pre-eclamptic nor in idiopathic SGA pregnancy.
References
Sharma, D., Shastri, S. & Sharma, P. Intrauterine growth restriction: antenatal and postnatal aspects. Clin. Med. Insights Pediatr. 10, 67–83 (2016).
Hung, T.-H. et al. Risk factors and perinatal outcomes associated with idiopathic small for gestational age Taiwanese newborns. Int. J. Gynaecol. Obstet. 122, 212–215 (2013).
Suhag, A. & Berghella, V. Intrauterine growth restriction (IUGR): etiology and diagnosis. Curr. Obstet. Gynecol. Rep. 2, 102–111 (2013).
Cosmi, E., Fanelli, T., Visentin, S., Trevisanuto, D. & Zanardo, V. Consequences in infants that were intrauterine growth restricted. J. Pregnancy 2011, 364381 (2011).
Hendrix, N. & Berghella, V. Non-placental causes of intrauterine growth restriction. Semin. Perinatol. 32, 161–165 (2008).
Jeyabalan, A. Epidemiology of preeclampsia: impact of obesity. Nutr. Rev. 71(Suppl 1), S18–S25 (2013).
Aufdenblatten, M. et al. Prematurity is related to high placental cortisol in preeclampsia. Pediatr. Res. 65, 198–202 (2009).
Haram, K., Trovik, J., Sandset, P. M. & Hordnes, K. Severe syndrome of hemolysis, elevated liver enzymes and low platelets (HELLP) in the 18th week of pregnancy associated with the antiphospholipid-antibody syndrome. Acta Obstet. Gynecol. Scand. 82, 679–680 (2003).
Kajantie, E. et al. Placental 11β-hydroxysteroid dehydrogenase-2 and fetal cortisol/cortisone shuttle in small preterm infants. J. Clin. Endocrinol. Metab. 88, 493–500 (2003).
Dy, J., Guan, H., Sampath-Kumar, R., Richardson, B. S. & Yang, K. Placental 11β-hydroxysteroid dehydrogenase type 2 is reduced in pregnancies complicated with idiopathic intrauterine growth restriction: evidence that this is associated with an attenuated ratio of cortisone to cortisol in the umbilical artery. Placenta 29, 193–200 (2008).
Zhu, Z. & Liu, Q. Relationship between 11β-HSD2 mRNA and insulin sensitivity in term small-for-gestational age neonates after birth. Int. J. Clin. Exp. Pathol. 8, 928–932 (2015).
Börzsönyi, B. et al. Gene expression patterns of the 11β-hydroxysteroid dehydrogenase 2 enzyme in human placenta from intrauterine growth restriction: the role of impaired feto-maternal glucocorticoid metabolism. Eur. J. Obstet. Gynecol. Reprod. Biol. 161, 12–17 (2012).
Kosicka, K., Siemiątkowska, A. & Główka, F. 11β-Hydroxysteroid dehydrogenase 2 in preeclampsia. Int. J. Endocrinol. 2016, 5279462 (2016).
Kosicka, K. et al. Glucocorticoid metabolism in hypertensive disorders of pregnancy: analysis of plasma and urinary cortisol and cortisone. PloS One 10, e0144343 (2015).
Kosicka, K. et al. Increased cortisol metabolism in women with pregnancy-related hypertension. Endocrine 61, 125–133 (2018).
Allen, V. M., Joseph, K. S., Murphy, K. E., Magee, L. A. & Ohlsson, A. The effect of hypertensive disorders in pregnancy on small for gestational age and stillbirth: a population based study. BMC Pregnancy Childbirth 4, 17 (2004).
Vasku, M. et al. Apparent systemic 11β-hydroxysteroid dehydrogenase 2 activity is increased in preeclampsia but not in intrauterine growth restriction. Pregnancy Hypertens. 11, 7–11 (2018).
Palermo, M., Delitala, G., Mantero, F., Stewart, P. M. & Shackleton, C. H. L. Congenital deficiency of 11β-hydroxysteroid dehydrogenase (apparent mineralocorticoid excess syndrome): Diagnostic value of urinary free cortisol and cortisone. J. Endocrinol. Invest. 24, 17–23 (2001).
Tomlinson, J. W. et al. 11β-Hydroxysteroid dehydrogenase type 1: a tissue-specific regulator of glucocorticoid response. Endocr. Rev. 25, 831–866 (2004).
Campino, C. et al. 11β-Hydroxysteroid dehydrogenase type-2 and type-1 (11β-HSD2 and 11β-HSD1) and 5β-reductase activities in the pathogenia of essential hypertension. Endocrine 37, 106–114 (2010).
Steen, N. E. et al. Altered systemic cortisol metabolism in bipolar disorder and schizophrenia spectrum disorders. J. Psychiatr. Res. 52, 57–62 (2014).
Finken, M. J. J., Andrews, R. C., Andrew, R. & Walker, B. R. Cortisol metabolism in healthy young adults: sexual dimorphism in activities of A-ring reductases, but not 11β-hydroxysteroid dehydrogenases. J. Clin. Endocrinol. Metab. 84, 3316–3321 (1999).
Wächter, R., Masarik, L., Bürzle, M., Mallik, A. & von Mandach, U. Differential expression and activity of 11β-hydroxysteroid dehydrogenase in human placenta and fetal membranes from pregnancies with intrauterine growth restriction. Fetal Diagn. Ther. 25, 328–335 (2009).
Struwe, E. et al. Simultaneously reduced gene expression of cortisol-activating and cortisol-inactivating enzymes in placentas of small-for-gestational-age neonates. Am. J. Obstet. Gynecol. 197, 43.e1–43.e6 (2007).
Funghi, L. et al. Expression and regulation of 11β-hydroxysteroid dehydrogenase type 1 in first trimester human decidua cells: Implication in preeclampsia. Mol. Cell. Endocrinol. 437, 163–170 (2016).
American College of Obstetricians and Gynecologists. ACOG Practice bulletin no. 134: fetal growth restriction. Obstet. Gynecol. 121, 1122–1133 (2013).
Gadzinowski, J., Kaliszewska-Drozdowska, M. D., Kosińska, M., Mazela, J. & Stoińska, B. Birth weight and gestational age of newborns from Wielkopolski and Lubuski regions. Ginekol. Pol. 74, 186–192 (2003).
Roberts, J. M. et al. Report of the American College of Obstetricians and Gynecologists’ Task Force on hypertension in pregnancy (2013).
Kosicka, K. et al. Detailed analysis of cortisol, cortisone and their tetrahydro- and allo-tetrahydrometabolites in human urine by LC-MS/MS. J. Pharm. Biomed. Anal. 140, 174–181 (2017).
Stirrat, L. I. et al. Decreased maternal hypothalamic-pituitary-adrenal axis activity in very severely obese pregnancy: Associations with birthweight and gestation at delivery. Psychoneuroendocrinology 63, 135–143 (2016).
Dovio, A. et al. Intense physical exercise increases systemic 11β-hydroxysteroid dehydrogenase type 1 activity in healthy adult subjects. Eur. J. Appl. Physiol. 108, 681–687 (2010).
Mondok, A. et al. 11β-Hydroxysteroid dehydrogenase activity in acromegalic patients with normal or impaired carbohydrate metabolism. Steroids 74, 725–729 (2009).
Jayasuriya, N. A. et al. A lower maternal cortisol-to-cortisone ratio precedes clinical diagnosis of preterm and term preeclampsia by many weeks. J. Clin. Endocrinol. Metab. 104, 2355–2366 (2019).
Ho, J. T. et al. Reduced maternal corticosteroid-binding globulin and cortisol levels in pre-eclampsia and gamete recipient pregnancies. Clin. Endocrinol. (Oxf.) 66, 869–877 (2007).
Giurgescu, C. et al. Relationships among psychosocial factors, biomarkers, preeclampsia, and preterm birth in African American women: a pilot. Appl. Nurs. Res. ANR 28, e1–6 (2015).
Carpenter, T., Grecian, S. M. & Reynolds, R. M. Sex differences in early-life programming of the hypothalamic-pituitary-adrenal axis in humans suggest increased vulnerability in females: a systematic review. J. Dev. Orig. Health Dis. 8, 244–255 (2017).
Villar, J. et al. Preeclampsia, gestational hypertension and intrauterine growth restriction, related or independent conditions? Am. J. Obstet. Gynecol. 194, 921–931 (2006).
Newhouse, S. M., Davidge, S. T., Winkler-Lowen, B., Demianczuk, N. & Guilbert, L. J. In vitro differentiation of villous trophoblasts from pregnancies complicated by intrauterine growth restriction with and without pre-eclampsia. Placenta 28, 999–1003 (2007).
Biron-Shental, T. et al. Short telomeres may play a role in placental dysfunction in preeclampsia and intrauterine growth restriction. Am. J. Obstet. Gynecol. 202, 381.e1–381.e7 (2010).
Laskowska, M., Laskowska, K. & Oleszczuk, J. Elevated maternal serum sP-selectin levels in preeclamptic pregnancies with and without intrauterine fetal growth restriction, but not in normotensive pregnancies complicated by isolated IUGR. Med. Sci. Monit. 19, 118–124 (2013).
Mericq, V. et al. Differences in expression and activity of 11β-hydroxysteroid dehydrogenase type 1 and 2 in human placentas of term pregnancies according to birth weight and gender. Eur. J. Endocrinol. 161, 419–425 (2009).
Braun, F. et al. Sex-specific and lasting effects of a single course of antenatal betamethasone treatment on human placental 11β-HSD2. Placenta 69, 9–19 (2018).
Vu, T. T. et al. Changes in human placental 5alpha-reductase isoenzyme expression with advancing gestation: effects of fetal sex and glucocorticoid exposure. Reprod. Fertil. Dev. 21, 599–607 (2009).
Morita, H. et al. Plasma cortisol and cortisone concentrations in normal subjects and patients with adrenocortical disorders. Metabolism. 53, 89–94 (2004).
Hung, T.-H. et al. Increased autophagy in placentas of intrauterine growth-restricted pregnancies. PloS One 7, e40957 (2012).
Duckitt, K. & Harrington, D. Risk factors for pre-eclampsia at antenatal booking: systematic review of controlled studies. BMJ 330, 565 (2005).
Leeners, B. et al. BMI: new aspects of a classical risk factor for hypertensive disorders in pregnancy. Clin. Sci. 111, 81–86 (2006).
Watanabe, H. et al. Risk factors for term small for gestational age infants in women with low prepregnancy body mass index. J. Obstet. Gynaecol. Res. 36, 506–512 (2010).
Acknowledgements
This work was supported by the National Science Center (Narodowe Centrum Nauki) in Poland [Grant Number 2012/05/B/NZ7/02532].
Author information
Authors and Affiliations
Contributions
A.S. performed the analysis, analyzed the data and wrote the first draft of the manuscript with input of K.K.; K.K. designed the study, performed the analysis and analyzed the data; A.S.G. recruited the patients and analyzed the data; M.K. was involved in protocol development and patient recruitment; G.H.B. was involved in protocol development and supervised the clinical aspects of the study; F.K.G. was involved in protocol development and supervised the analytical aspects of the study. All authors discussed the results and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Siemiątkowska, A., Kosicka, K., Szpera-Goździewicz, A. et al. Cortisol metabolism in pregnancies with small for gestational age neonates. Sci Rep 9, 17890 (2019). https://doi.org/10.1038/s41598-019-54362-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-54362-0
- Springer Nature Limited
This article is cited by
-
Maternal Signatures of Cortisol in First Trimester Small-for-Gestational Age
Reproductive Sciences (2022)
-
Associations between maternal awakening salivary cortisol levels in mid-pregnancy and adverse birth outcomes
Archives of Gynecology and Obstetrics (2022)