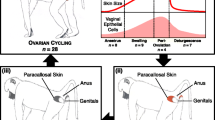
Abstract
The vaginal microbiota of nonhuman primates differs substantially from humans in terms of Lactobacillus abundance, overall taxonomic diversity, and vaginal pH. Given these differences, it remains unclear in what way the nonhuman primate genital microbiota protects against pathogens, in particular sexually transmitted infections. Considering the effect that microbiota variations can have on disease acquisition and outcome, we examined endogenous and exogenous factors that influence the urogenital microbiota of male and female captive rhesus monkeys. The male urethral (n = 37) and vaginal (n = 194) microbiota of 11 breeding groups were examined in a cross-sectional study. During lactation and menstruation, the vaginal microbiota becomes significantly more diverse and more similar to the microbes observed in the male urethra. Group association and cage-mate (sexual partners) relationships were additionally associated with significant differences in the urogenital microbiota. Our results demonstrate that microbiota considerations are necessary in order to make informed selection of nonhuman primates as translational animal models.
Similar content being viewed by others
Introduction
In recent years there has been an increased interest in the microbiota of nonhuman primates (NHPs) for evolutionary, experimental, and conservation purposes. However, microbiota considerations are currently not used to refine and reduce experiments with NHPs, despite increasing evidence that the microbiota in humans can influence disease progression (reviewed by1). Of the NHP animal models, the Asian rhesus monkey (Macaca mulatta) and long-tailed macaque (Macaca fascicularis) are the most extensively utilized species2,3,4. In laboratory settings, rhesus monkeys cycle year-round, have a reproductive cycle that is similar to that of humans and experience similar changes in the hormonal levels during sexual cycle, pregnancy and post-partum5,6,7. Therefore, the vagina of rhesus monkeys has been used to model the human vaginal epithelium and study sexual transmitted infections (STIs)8. For example, rhesus monkeys have been extensively used to study the disease acquisition and outcome of simian-/human immunodeficiency virus (SIV/HIV)3,9. In a study on SIV susceptibility, estrogen treatment in rhesus monkeys protected female rhesus monkeys from the sexually transmitted infection3. Smith et al. proposed that not just the thickening of the vaginal epithelium but also a potential change in vaginal microenvironment may have led to the observed effect under the influence of high estrogen levels3.
Many studies have laid the groundwork in characterizing the genital microenvironment of various species of captive and wild NHPs2,10,11,12. Unlike the vaginal microbiota of humans, which is often dominated by a single Lactobacillus species13, NHPs, including rhesus monkeys, harbor a diverse set of vaginal microbes2,11. In humans, the acidic nature of the vaginal environment (pH ≤ 4.5) protects women against STIs14. The vaginal microbiota of NHPs on the other hand has a low abundance of Lactobacillus (<2% of microbiota), a near neutral vaginal pH and is instead colonized by a diverse set of bacterial genera including Sneathia, Aerococcus, Prevotella, Porphyromonas, Fusobacterium and Atopobium2,11,12,15. Considering these differences, it currently remains unclear in what way the vaginal microenvironment of rhesus monkeys protects against infectious diseases. Additionally, despite increasing evidence that sexual exposures can alter the composition of the human genital microbiota16,17, the urethral microbiota of male NHPs remains largely uncharacterized. A better understanding of factors that influence the rhesus monkey genital microbiota of both male and female animals in health and disease is thus warranted.
In this study, we investigate the genital microbiota of a large breeding colony of rhesus monkeys at the German Primate Center. To identify endogenous and exogenous factors that influence the microbiota, we examined the genital microbiota in the breeding colonies in the context of age, breeding group association, social rank, body mass, and long-term health status. We studied both, the vaginal microbiota of female and the urethral microbiota of male rhesus monkeys which has not been done in previous studies. We are therefore able to compare bacterial composition between male and female animals in a single cohort of rhesus monkeys and found that during menstruation and lactation the vaginal microbiota shifts to be more similar to the male urethral microbiota.
Results
We examined the vaginal microbiota of 194 female rhesus monkeys and the urethral microbiota of 37 male rhesus monkeys housed at the breeding facility of the German Primate Center (data file S1). The mean age, number of breeding groups, and other characteristics of the sampled animals are shown in Table 1. The V4 region of the bacterial 16S rRNA gene was selected as its informative, short-read length from the Illumina MiSeq platform allows full overlap of paired-end reads and thus higher confidence in the sequence data. Sequencing of the V4 region of the rhesus monkey samples generated a total of 14,571,505 unfiltered reads with a mean read count of 48,630 reads per sample ( ± 15,461 SD) after quality filtering. Sequences were rarified to 11,371 sequences per sample and clustered into operational taxonomic units (OTUs) based on the 97% similarity threshold. We first examined the microbiota of the male urethra and vagina separately and then compared composition similarities between male and female animals.
Appropriate control samples and a mock community (Microbial mock community, HM-280, Biodefense and Emerging Infectious Research (BEI) Resources, Manassas, USA) were included in the sequencing run. Using the mock community, the observed error rate for the run was found to be 0.036%. The collected control samples showed that contamination was highest during sample collection procedures, while controls taken during amplification procedures in the laboratory yielded only minimal read counts (Fig. S1a). Taxa plots of control samples that were taken during sampling at two different breeding units, show that the relative abundance of contaminant OTUs was similar (Fig. S1b).
The vaginal microbiota is significantly altered during lactation and menstruation
We investigated the vaginal microbiota (n = 194), vaginal pH (n = 138), and sexual cycle phase (n = 148) of clinically-healthy, reproductively-active rhesus monkeys housed in eleven breeding groups (Table 1). None of the females showed signs of pregnancy, as defined by transabdominal palpation. At the time of sampling 30.4% of the animals were lactating. In addition to lactation, we characterized the sexual cycle phase using exfoliative vaginal cytology (EVC) (see Methods, Table S1). The sexual cycle phase (P1-P3) of non-lactating females were evenly distributed with 35.6% in an ovulatory phase (P1), 41.3% in an intermediate phase (P2) and 23.1% in a menstruation-like phase (P3) (Table S1). For lactating females, 52.3% of the animals were in a menstruation-like phase, 31.8% in an intermediate phase, and 15.9% in the ovulatory phase (Table S1). For the purpose of the microbiota analysis, lactation status and sexual cycle phases were analyzed independently.
Overall, a mean of 219.8 ± 160.7 (unless otherwise stated all values are given in mean ± SD) OTUs were observed in the vaginal microbiota of the rhesus monkeys. The most abundant genus was Prevotella with a mean abundance of 20.5 ± 16.4%. Different OTUs were identified as Prevotella, indicating that a diverse set of species from this genus were present (Fig. 1). Porphyromonas (9.5 ± 9.9%), Streptobacillus (9.1 ± 13.4%) and an unclassified genus of the family Ruminococcaceae (9.5 ± 7.3) were the other dominant taxa in the otherwise diverse community (Fig. 1).
Heatmap of the relative abundance of microbial taxa identified in the vaginal microbiota of rhesus monkeys in multiple breeding groups. Ward linkage clustering of samples based on the composition and relative abundance of the 20 most abundant OTUs in the vaginal microbiota. Lactation status (1: lactating and 0: non-lactating), group association (A–K) and EVC (sexual cycle phases) (P1: ovulatory phase, P2: intermediate stage, P3: menstruation-like and NT: not tested) of each sample are shown beside the heatmap. C1 and C2 indicate two main clusters in the ward linkage clustering. See Table 1 for sample size composition.
Lactation status and sexual cycle phase strongly correlated with the OTU richness (identified absolute number of taxa) and evenness (inverse Simpson index; Fig. 2). Lactating females had a significantly higher OTU richness (p ≤ 0.0001 [Mann-Whitney t-test]) and the bacterial taxa were significantly more evenly distributed (p ≤ 0.0001 [Mann-Whitney t-test] than in non-lactating females (Fig. 2a,b). Similarly, animals in a menstruation-like (Phase 3, Table S1) sexual cycle phase had a significantly higher OTU richness (p ≤ 0.0001 [Kruskal-Wallis test]) and were significantly more evenly distributed (p ≤ 0.001 [Kruskal-Wallis test]) than animals in the ovulatory (Phase 1) or intermediate phase (Phase 2; Fig. 2c,d). A heatmap of the relative abundance of the 20 most common OTUs shows that lactating animals and animals in menstruation-like sexual phase clustered separately from other animals (Fig. 1). Vaginal bacterial communities from these animals clustered prominently in cluster 1 (C1) and are characterized by different bacterial taxa than the cluster 2 (C2; Fig. 1). Of the ten most abundant OTUs, Provella, Mobiluncus, Porphyromonas and an unclassified genus of the family Ruminococcaceae were significantly different in the lactating and menstruation-like animals (Fig. S2a,b). These cluster differences were confirmed by significant differences in the unweighted UniFrac distances, which are visualized on the principal coordinates plot along axis 1 (23.1%) (Fig. 3a,b). Pairwise AMOVA confirmed that the differential clustering of lactating and menstruating-like animals resulted in significantly different community structures (p ≤ 0.001).
Alpha diversity measurements for the vaginal microbiota of female rhesus monkeys. Violin plots of the observed OTUs and InvSimpson index clustered based on (a,b) lactation status (Mann-Witney t-test, ***p ≤ 0.0001) and (c,d) sexual cycle phases (P1: ovulatory phase, P2: intermediate stage, P3: menstruation-like) (Kruskal-Wallis test, **p ≤ 0.001, ***p ≤ 0.0001). (e,f) Boxplots (median ± range) of the observed OTUs and InvSimpson index clustered by breeding groups (groups association: A-K). See Table 1 for sample size composition.
The vaginal microbiota of menstruating-like and lactating females clusters separately. Principal coordinates analysis (PCoA) of vaginal samples colored by (a) lactation status (lactating (dark blue, 1) and non-lactating (light blue, 0) and (b) sexual cycle status (P1: ovulatory phase (red), P2: intermediate stage (pink), P3: menstruation-like (dark red) and NT: not tested (gray)). Distances between samples were calculated using the unweighted UniFrac metrics. See Table 1 for sample size composition. Fig. S5 shows the corresponding PCoA plot classified by group association and age classification.
In order to examine an additional functional variable of the vaginal microbiota, we tested the vaginal pH at the time of sampling using pH-indicator paper. The mean overall vaginal pH of the sampled animals was found to be 6.4 ± 0.7 (Table 1). The vaginal pH of lactating females (6.8 ± 0.5) was significantly higher than that of non-lactating females (6.3 ± 0.7) (p ≤ 0.0001 [Mann-Whitney t-test], Fig. S3a). Similarly, animals in menstruation-like sexual phase (7.0 ± 0.5) had a higher vaginal pH compared to individuals in the other sexual cycle phase (P1: 6.0 ± 0.6, P2: 6.3 ± 0.7, p ≤ 0.0001 [Kruskal-Wallis test], Fig. S3b).
Breeding groups influence the vaginal microbiota
Aside from lactation status and cycle phase, we examined if the vaginal microbiota is shaped by age and breeding group. Animals were subdivided into juveniles (<5 years), adults (5–19 years), and geriatric (>19 years). Age was not found to have an influence on alpha diversity (Fig. S4) and beta diversity (p = 0.155 [AMOVA; Fig. S5a). The group association, in contrast, significantly correlate with the OTU richness (p ≤ 0.0001 [Kruskal-Wallis test], Fig. 2e) and evenness (p ≤ 0.0001 [Kruskal-Wallis test], Fig. 2f). The breeding group association of each sample can be seen on the heatmap of the 20 most abundant OTUs (Fig. 1). Additionally, we observed a significant difference in unweighted UniFrac distances when considering all breeding groups (p ≤ 0.001 [AMOVA; Fig. S5b). Pairwise comparisons of alpha and beta diversity measurements between individual breeding groups was, however, not significant for all tested groups.
Influence of age in adult male rhesus monkeys
We characterized the urethral microbiota of clinically-healthy, reproducing male rhesus monkeys housed in ten different breeding groups (Table 1). Breeding groups are composed of a single adult male (breeding male) and juvenile off-spring males (≤5 years) which are removed from the breeding groups upon reaching adulthood. Overall, the urethral microbiota of male rhesus monkeys is composed of a diverse community of microbes, with a mean of 481.3 ± 127.0 OTUs observed. On a phylum level, Firmicutes (54.1 ± 8.3%), Bacteroidetes (25.5 ± 9.3%), Proteobacteria (9.0 ± 6.7%) and Actinobacteria (6.6 ± 3.2%) made up 95% of the identified sequences. The four dominant phyla were present in all 37 samples. On the genus level, the bacterial community is diverse with no single dominating OTU (Fig. 4). The most abundant genus in the male rhesus monkey urethra was Prevotella with a mean abundance of 14.4 ± 9.7% followed by Porphyromonas (7.5 ± 6.6%) and Ezakiella (7.3 ± 6.8%).
The male urethral microbiota of adult and juvenile rhesus monkeys does not differ significantly. Violin plots of (a) the number of OTUs and (b) the inverse Simpson index for adult and juvenile males (Mann-Witney t-test). (c) UPGMA clustering on unweighted UniFrac including taxa plots showing the relative abundance of the 25 most abundant OTUs in percentage of reads. Genus-level bacterial classification of OTUs shown legend with the percent of sequences that classified with each genus. Group are shown accordingly and adult (breeding male within each group) is indicated. See Table 1 for sample size composition.
We examined if the urethral microbiota of the adult, breeding male differed from the microbiota of juvenile males. Each breeding group had a single adult breeding male with the exception of group H (n = 4) and I (n = 3), which were further divided into subgroups within a single housing unit. Adult males neither differed from other males in the OTU richness (p = 0.145, [Mann-Whitney test], Fig. 4a) nor the evenness (p = 0.453 [Mann-Whitney test], Fig. 4b). Pairwise AMOVA of unweighted UniFrac distances found that being the breeding male in a group had no effect on community structure (p = 0.123). A dendrogram of unweighted UniFrac distances shows that the adult animals did not cluster separately from other animals (parsimony analysis, p = 0.768; Fig. 4c). OTU richness and evenness measurements of each breeding group are shown in Fig. S6. We note here, that the sample size of male animals in each breeding group were low (n = 1 to 4 animals). Therefore, statistical analysis was not performed to examine breeding group differences.
Lactating and menstruating female have a more similar microbiota to the male urethra
On the phylum level, Firmicutes and Bacteroidetes dominated both microbiotas, making up 80.1 ± 6.9% in the male urethra and 69.9 ± 17.8% in the vagina. Yet, Fusobacteria, the third most abundant phylum in the vagina (14.4 ± 16.1%), only made up 1.9 ± 3.3% in the male urethra. The most abundant genus across the dataset for both, male and female genital microbiota was Prevotella. Several OTUs cluster into this bacterial genus and the most abundant Prevotella OTUs (mean abundance of 6.0 ± 8.7%) was found in 226 out of 231 animals. Other Prevotella OTUs were less abundant and only dominant in some samples (Fig. 1: vaginal microbiota and Fig. 4: male urethral microbiota).
To further examine similarities between the vagina and male urethra, overall OTU richness and evenness was compared. As we previously observed a significant difference in alpha and beta diversity of the vaginal microbiota based on lactation status and sexual cycle phase, these variables were plotted separately (Fig. 5). The male urethra had a significantly higher OTU abundance compared to non-lactating and non-menstruation-like (ovulatory and intermediate phase) animals (p ≤ 0.0001 [Kruskal-Wallis test], Fig. 5a,b). Contrary, menstruation-like (P3) and lactating female rhesus monkeys showed no significant difference in the number of OTUs compared to the male urethra microbiota (p ≤ 0.05 [Kruskal-Wallis test], Fig. 5a,b). Similarly, inverse Simpson index measurements were significantly different between males and non-lactating and non-menstruation-like (ovulatory (P1) and intermediate phase (P2)) animals (p ≤ 0.0001 [Kruskal-Wallis test], Fig. 5c,d). Inverse Simpson index measurements were not significantly different between males and lactating and menstruation-like animals (p ≤ 0.05 [Kruskal-Wallis test], Fig. 5c,d). To examine, if the trend in the alpha diversity could be observed in the overall bacterial composition, pairwise unweighted UniFrac distances were calculated between the male urethral microbiota and the vaginal microbiota. More similar microbiotas resulted in a smaller calculated UniFrac distances and vise versa. The UniFrac distances were grouped in violin plots based on either lactation status (Fig. 5e) or sexual cycle phase (Fig. 5f). We found that the bacterial composition of the vaginal microbiota of lactating and menstruating-like animals (P3) was significantly more similar to that of the male urethra microbiota (Fig. 5e,f).
The vaginal microbiota of lactating and menstruating females is more similar to the urethral microbiota. Violin plots of the number of OTUs (a) in the male urethra and vaginal microbiota of lactating and non-lactating females and (b) in the male urethra and vaginal microbiota of females in three sexual cycle phases (P1: ovulatory phase, P2: intermediate stage, P3: menstruation-like) (Kruskal-Wallis test, ***p ≤ 0.0001). Violin plots of the inverse Simpson index for (c) male urethra and vaginal microbiota of lactating and non-lactating females and (d) male urethra and vaginal microbiota of females in three sexual cycle phases (Kruskal-Wallis test, ***p ≤ 0.0001). (e,f) Violin representations showing unweight UniFrac distance of each adult female to the adult male in each group. Data is plotted by (e) lactation status (Kruskal-Wallis test, ***p ≤ 0.0001) and (f) sexual cycle phase (Mann-Witney t-test, ***p ≤ 0.0001). See Table 1 for sample size composition.
Cage-mates are more similar in their genital microbiota
In order to assess if sexual contact shapes the genital microbiota of the captive rhesus monkeys, pairwise unweighted UniFrac distances were calculated between the male urethral microbiota of the adult male in each group and the vaginal microbiota of adult females. Females and males of the same breeding group were considered cage-mates and thus potential sexual partners. UniFrac distances were grouped into violin plots as ‘cage-mates’ or from ‘other breeding groups’ (no sexual contact possible) (Fig. 6). Cage-mates were found to be significantly more similar in the bacterial composition compared to non-cage-mates (p ≤ 0.0001 [Mann-Whitney test], Fig. 6). As we observed a significant difference in lactation status and sexual cycle phase, these variables were additionally plotted in separate paired-violin plots to examine cage-mate differences for each group (Fig. S7a,b). Cage-mates were found to be significantly more similar in the bacterial composition for lactation, menstruation-like and ovulatory phase animals (Fig. S7a,b). Cage-mate similarity was not observed for the non-lactating group or for animals in an intermediate sexual phase (P2; Fig. S7a,b).
Comparison between the male urethral microbiota and female vaginal microbiota of cage-mates. Violin plots showing unweight UniFrac distance of the adult vaginal microbiota to the urethral microbiota of the adult males. Data is plotted separately for female animals in the same breeding group (cage-mates) and females of other breeding groups (other) (Mann-Witney t-test ***p ≤ 0.0001). For further subdivision by lactation status and sexual cycle phase see Fig. S7.
Discussion
Considering the effect that microbiota variation can have on disease acquisition and outcome1, we examined endogenous and exogenous factors that influence the urogenital microbiota of captive rhesus monkeys. The inclusion of optimal negative controls (Fig. S1) and the relatively large sample size strengthen the study. However, based on our cross-sectional study design we were limited in drawing causal relationships between factors and variations in the genital microbiota. Nevertheless, our results urge for the inclusion of microbiota analysis in the selection and experimental use of rhesus monkeys as indicated by the differences between the vaginal microbiota during lactation and sexual cycles phases.
We showed that during lactation and menstruation the bacterial composition shifts towards a more diverse community (Figs. 1–3). As reported previously, we confirmed that the mean vaginal pH of rhesus monkeys (6.4 ± 0.7) is significantly higher than that found in humans (Fig. S3)2. Instead of the Lactobacillus-dominance observed in women (reviewed by18), the vaginal microbiota of captive and wild NHPs harbor a more diverse set of bacteria (Fig. 1)2,11,12,15. Our study shows that in captive rhesus monkeys the already diverse bacterial community shifts to an even more diverse and significantly different bacterial composition during lactation and the menstruation-like phase (Figs. 2–3). While a previous study on captive baboons (Papio anubis) found no difference in the vaginal microbiota of cycling females15, a recent study in wild baboons (Papio cynocephalus) reported that the ovarian cycle phase and the reproductive state shaped the vaginal microbiota12. Both of these studies used visual assessment of perivulvar swellings to determine the sexual cycle phase12,15. Inconsistent classification of these phases in the two studies in combination with low sample sizes may explain the difference in the outcome of both studies. Instead of using perivulvar swellings, we performed vaginal exfoliative cytology to classify the animals into three sexual cycle phases (Table S1)19,20,21. Vaginal exfoliative cytology reflects the current state of the vaginal epithelium and therefore serves as a reliable marker for the sexual cycle phase19,20,21. The even distribution of all three sexual cycle phases in non-lactating rhesus monkeys (Table S1) is indicative of a healthy reproductive community. Using cytology as a marker of sexual cycle phase, this study supports Miller et al.’s finding that ovarian cycle phase (menstruation-like) and reproductive state (lactation) shifts the vaginal microbiota in NHPs12. Similar changes have been reported in temporal and cross-section studies in women during menstruation and post-partum13,22, where it has been demonstrated that the vaginal microbiota shifted from a Lactobacillus-dominant state towards a more diverse bacterial composition13,22. Despite the remarkable differences in bacterial species composition of the rhesus monkeys and human vaginal microbiota, it is interesting that similar factors (e.g. hormonal changes) seem to influence the vaginal microbiota. This is supported by our finding that the observed changes in bacterial vaginal diversity in the rhesus monkeys coincide with changes in the pH, a functional measurement of the vaginal ecosystem. Whether the observed variance in the bacterial diversity and the difference in pH is physiologically relevant cannot be determined in this study. However, none of the animals had any clinical manifestations of vaginitis, which supports our notion that the bacterial variation and the pH differences observed in this study is within physiologically range.
It has been proposed that hormonal fluctuations during the sexual cycle, pregnancy and post-partum shape the vaginal microbiota (reviewed by18). Both lactation and menstruation are marked by hormonal changes in the vagina, which may be indirect or direct driving factors for the shift in vaginal microbiota observed in this study (Figs. 1–3). Studies on SIV susceptibility in rhesus monkeys have shown that hormone treatment can lead to an altered susceptibility3,9. During high levels of estrogen, changes in the vaginal epithelium, including changes in vaginal microenvironment, may have a protective effect3. Further investigations are necessary to examine the causal relationship between hormone levels, changes in the NHP vaginal microbiota, and susceptibility to pathogens. However, it has become clear, that a more holistic understanding on host-pathogens interactions is required for the interpretation of animal experiments as host factors can influence the microbiota and vice versa (reviewed by1).
We examined the male urethral microbiota of the rhesus monkeys to further compare the genital microbiota of females and males in a single breeding unit. To our knowledge, there has been no studies on the urethral microbiota of wild or captive NHPs to date. Four bacterial phyla, Firmicutes, Bacteroidetes, Proteobacteria, and Actinobacteria, compose the majority of identified sequences in the urethra. On the phylum level, the urethral microbiota of the male rhesus monkeys were similar to that reported in humans with Firmicutes making up the largest proportion23. In our male animals, notable urethral taxa include Prevotella, Porphyromonas, and Ezakiella, have all been previously associated with the urinary tract microbiota of adult men23,24,25. Prevotella has been previously detected in the genital microbiota of healthy female rhesus monkeys indicating that this genus plays a residential role in the rhesus monkeys’ genital microbiota10. In humans, some species of Prevotella have been associated with disease states (e.g., bacterial vaginosis26) while other species can be found in clinically healthy women (e.g., post-partum22). Identifying the specific species of Prevotella and their functional role in NHPs may be important to further understand the vaginal microbiota of NHPs. For the urethral microbiota, it is difficult to compare the prevalence of Prevotella in the male rhesus monkey to other studies, as there is currently no consensus on the core urethral microbiota, even in humans25. As a result, large scale investigations need to be performed to study the male urogenital microbiota including factors that influence this unique ecosystem in health and disease25.
It has been hypothesized that sexual exposures can alter the composition of the genital microbiota13,16,25. A recent study on sexual partners with bacterial vaginosis (BV), showed that women with BV were significantly more similar to the urogenital microbiota of their partner17. To test if sexual contact affected the genital microbiota of NHPs, we first examined if adult, breeding males in captive rhesus monkeys had different urethral microbiota from juvenile males. Breeding groups in this study contained a single adult male, who monopolized the cage-mates in estrus. We found that the age of male rhesus macaques did not shape the urethral microbiota (Fig. 4). This may be due to the fact that juvenile rhesus monkeys already engage in socio-sexual mounting as a form of play27. Sexual history in healthy adolescent men has been reported to be a possible determinant of the urogenital microbiota16. Known sexually transmitted bacteria and taxa associated with the urethral tract of adult men23, were observed rarely in adolescent men16. To further study the effect of sexual contact, we examined the similarity of the genital microbiota in cage-mates (adult male to adult females in the same breeding group). We found that overall, cage-mates were significantly more similar to each other compared to non-cage-mates (Fig. 6). When subdividing cage-mate by lactation status and sexual cycle, the observed cage-mate effect was not seen for non-lactation and intermediate sexual phase animals (Fig. S7a,b). This may be due to an inappropriate subsampling of these two groups. For example, the intermediate sexual phase classification used in the EVC may represent both, proliferative phase and secretory phase, and is therefore an oversimplification. This highlights the limitation of this cross-sectional study in assessing cage-mate similarities. A controlled temporal study is necessary to examine the effect of sexual contract in NHP breeding groups. NHPs can be an advantages model to further examine microbiota similarities in sexual partners (in health and disease) as sexual contact is easily observed and controlled.
A surprising finding of our study was that independent of breeding group association, the bacterial composition of lactating monkeys and/or those in the menstruation-like sexual phase were more similar to the urethral microbiota than the non-lactating/non-menstruating animals (Fig. 5). As females in a menstruation-like sexual phase are less attractive to male rhesus monkeys, we presume that the similarity is not caused by recent sexual contact. A possible explanation for this finding is that the altered hormonal state allows otherwise more-suppressed bacteria to dominate the microbiota. To understand the cause of the vaginal microbiota shift towards the male urethra microbiota, controlled temporal experiments in NHPs would be necessary. Interestingly, a temporal study in humans has shown that the vaginal microbiota post-partum shifts towards the gut microbiota22. The study was able to show that the shift towards the gut microbiota persisted for multiple months and was independent of delivery method (vaginal vs. caesarean). These findings support the notion that during changes in the genital ecosystem (e.g., shifts in hormones or delivery), the vagina is more susceptible to ‘foreign’ bacteria. This potentially altered susceptibility should be carefully considered when performing vaginal inoculations in NHPs for future experiments (e.g., HIV).
We found that breeding groups can have an effect on the vaginal microbiota (Fig. 2). Breeding group similarities could be influenced by various factors including host genetics28, differences in group size or cage effects29. Many of these factors could not be properly examined in this study and require planned and controlled animal experiments. In mice, it has been shown that animals kept in the same cage become more similar in microbiota composition over time29. This effect could be studied in captive NHPs by examining microbiota changes in various ecological niches (genital, skin, fecal) during cage transfers. A cage effect in NHPs could have major implications for the use of NHPs as translational animal models. A better understanding of the NHP microbiota could therefore refine animal selection for animal experiments where a higher standardization can lead to reduced animal numbers3,9. The inclusion of appropriate controls in microbiota studies cannot be stressed enough30. Especially low abundance microbiotas like the urethral microbiota are vulnerable to contaminations during sampling and laboratory analysis31. The inclusion of blank control samples, especially at the site of sample collection, is essential and should be understood as Good Laboratory and Scientific Practice (Fig. S1). Only well-planned and controlled microbiota studies on NHPs will provide a better understanding of factors that influence microbiotas of NHPs.
Methods
Ethical statement
All samples included into this study were obtained from clinically healthy rhesus monkeys that underwent the mandatory annual health check at the German Primate Center between June 2016 and May 2017. Animals were not purposely immobilized to collect samples for this study. Swabs were taken as part of a routine annual health monitoring and tuberculosis screening. Animal were short-term immobilized by trained veterinarians who checked and documented the general health condition of each individual. Sampling included the collection of blood, oral and genital swab samples. The use of the samples was reviewed and approved by the animal welfare and ethics committee of the German Primate Center (EC No. 1–16). All work steps involving the handling of live animals followed the rules of ‘Good Veterinary Practice’.
Study design and animals
Urethral swabs of 37 male and vaginal swabs of 194 female rhesus monkeys were collected. A cross-sectional study design was applied. Samples from apparently pregnant individuals, clinically diseased animals, or animals that received medical treatment within the last 6 months were excluded from analysis. Moreover, we excluded samples from animals below the age of three. Data file S1 provides a detailed overview on the samples analyzed in this study as well as the respective NCBI Sequence Read Archive numbers. Lot numbers for consumables were kept consistent and are reported in the Supplementary Material (Table S2).
Swab sample collection
Immobilized female rhesus monkeys were placed in dorsal recumbency and the area around the vulva was cleaned using 70% ethanol. To facilitate sampling, a sterile silicon tube, 15 mm diameter and 40 mm length, was used to avoid swab contamination with skin or fecal material. A flocked swab (FLOQSwabs, Copan Improve Diagnostics, Brescia, Italy) was moistened using a single drop of sterile physiological saline solution (WDT eG, Garbsen, Germany) and was subsequently inserted midway into the vaginal canal. Subsequently the swab was rotated 20-times on the dorsal wall before it was gently removed and transferred into 500 µl of custom-made lysis buffer (10 mM Tris, pH 8.0, 0.1 M EDTA, pH 8.0 and 0.5% SDS). Samples were kept on ice until transported to the inhouse laboratory facilities where they were stored at −80 °C30.
An additional swab was collected to perform an EVC. Briefly, the swab was rolled onto a microscope glass slide after which it was allowed to air-dry. Slides were then stained with a Romanowski stain (Diff-Quik) and subsequently examined under the microscope by two independent investigators19. Cytological scoring was performed as previously described by McLennan et al.20. The maturation index was calculated by counting 100 representative epithelial cells, which were scored according to their cell type. Briefly, parabasal cells were assigned a value of 0, intermediate cells a value of 0.5, and superficial cells a value of 1. Based on the cumulative maturation score, the animals were categorized into three stages (ovulatory phase (P1), intermediate phase (P2), and menstruation-like phase (P3); see Supplementary Table 2).
The vaginal pH was measured using a swab which was inserted midway into the vagina and then rolled onto a pH-indicator paper (Merck & Co., Kenilworth, New Jersey). The vaginal pH was scored by two independent researchers following the manufacturer’s instructions using a scale ranging from 5.5 to 9.0.
Immobilized male rhesus monkeys were placed in ventral recumbency and sampled for urethral swabs. A minitip FLOQ swabs (Copan Improve Diagnostics) was moistened using sterile physiological saline solution and subsequently inserted 1–2 cm into the urethra of the animal. Subsequent handling of the samples was identical to the procedure described for vaginal swab samples.
Suitable precautions were taken during sample collection to avoid microbial contamination. As a sample collection control, a FLOQ swab with a single drop of sterile physiological saline solution was immediately transferred into a 500 µl custom-made lysis buffer at the breeding facility at the time of sampling.
DNA extraction
We used the QIAamp DNA Mini Kit (Qiagen GmbH, Hilden, Germany) to extract bacterial DNA. This kit was previously validated for microbial analysis of swab material30. Briefly, proteinase K (50 mg/μl) was added and the samples were incubated overnight at 56 °C at 600 rpm (Thermomix comfort, Eppendorf, Hamburg, Germany). Appropriate amounts of AL buffer (Qiagen GmbH) and ethanol were added. The DNA was subsequently purified from the lysate using the spin columns provided in the kit. Extracted DNA was eluted in 75μl Microbial DNA-Free water (Qiagen GmbH). Suitable precautions were taken during sample handling and processing in the laboratory to limit microbial contamination and maintain consistency during all procedures. The order of sample processing was randomized to avoid handling bias. As a laboratory analysis collection control, a FLOQ swab was transferred into a 500 µl custom-made lysis buffer under the DNA extraction bench at the time the rhesus monkey samples were handled.
16S ribosomal RNA gene sequencing
The universal primers 515 F and 806 R, which were adapted with linker regions and barcode sequences, were used to amplify the V4 region of the 16S ribosomal RNA (16S rRNA) gene32. Phusion Hot Start II High-Fidelity DNA Polymerase (Thermo Fisher Scientific, Waltham, Massachusetts), which has been previously validated for the use in microbiota studies30, was used to amplify each sample in triplets. PCR reactions consisted of 12.5μl of 2x PCR master mix, 8μl of Microbial DNA-Free water (Qiagen GmbH), 1.25μl of each primer (0.5 mM each, Metabion, Steinkirchen, Germany) and 2μl of template in a total reaction volume of 25μl. PCR cycling conditions comprised of a pre-denaturation step of 30 s at 98 °C, followed by 30 cycles of 98 °C for 10 s, 55 °C for 15 s and 72 °C for 60 s, as well as a final 10 min extension step at 72 °C. A blank control (Microbial DNA-Free water) and a mock control sample (Microbial mock community, HM-280, Biodefense and Emerging Infectious Research (BEI) Resources, Manassas, Virginia) were included in 16S rRNA gene amplification. The amplicon triplets were pooled, purified using 0.7x AMPure XP beads (Beckman Coulter, Brea, California), and quantified using the Qubit 3.0 Fluorometer (Thermo Fisher Scientific). Subsequently, we verified the amplicon integrity for a representative number of eleven samples using the BioAnalyzer 2000 (Agilent, Santa Clara, California). Equimolar amounts (10 nM) of sample amplicon and maximum volume of control samples (5μl) were pooled prior to sequencing. Illumina MiSeq. 2 × 250 bp paired-end sequencing (V2 chemistry, Illumina, San Diego, California) was performed in the Transcriptome and Genome Analysis Laboratory at the University of Goettingen in accordance with published guidelines32.
Data processing and analysis
The sequencing reads were processed using the mothur software package (v.1.39.5)33. According to the MiSeq SOP33, contigs were assembled, sequences were quality filtered, and PCR artifacts were removed. The SILVA bacterial reference database34 was used to align the sequences and OTUs were assigned based on 97% sequence similarity. Cross-sample singletons and poorly aligned sequences were removed. The seq.error command was used to determine the error rate and the mock community was eliminated from the dataset. Due to low read numbers, control sample reads were excluded from the dataset and analyzed separately.
To examine differences in the microbial community structure, alpha (species richness within a single sample) and beta diversities (microbial community diversity between samples) were calculated. As alpha diversity measurements, we determined the number of observed OTUs and calculated the inverse Simpson Metrix using the summary.single command in mothur. Beta diversity was determined using unweighted UniFrac metrics35. The dissimilarity matrix was visualized using Principal Coordinates Analysis (PCoA) and a Newick formatted dendrogram (visualized in FigTree v.1.4.2, http://tree.bio.ed.ac.uk/software/figtree/). ClustVis tool (https://biit.cs.ut.ee/clustvis/) was used to create a heatmap of the relative abundance of bacterial taxa36. Violin plots (R package plot.ly) and box plots (GraphPad Prism 6) were used to visualize data points for different variables.
Statistical analysis
The statistical significance of the pooled data was analyzed in GraphPad Prism 6 (GraphPad software) and in R (v3.4.3;37) using the package vegan (version 2.5;38). Whenever appropriate, we tested for normality distribution of the data using the Kolmogorov-Smirnov normality test. The significance in alpha diversity and pair-wise beta diversity between two or more groups was tested using the non-parametric Mann-Whitney-U or Kruskal-Wallis tests including correction for multiple testing using Dunn’s post hoc tests. Differences in community structure based on age of animals, group association, and lactation status was tested using analysis of molecular variance (AMOVA, 1,000 permutations) in mothur39. PCoA plots of unweighted UniFrac metrics and UPGMA-clustered dendrograms (unweighted UniFrac metrics) were used to visualize data points. Differences in the ten most abundant OTUs in vaginal samples were assessed using the metastats command in mothur40. p-values for differences in individual OTUs were corrected for multiple comparisons using Bonferroni correction. Values of p ≤ 0.05 were considered statistically significant.
Data availability
All generated read files have been deposited in the NCBI Sequence Read Archive under the accession number SRP184988. Detailed information about the samples is provided in Data file S1 in the supplemental material.
References
Wang, B., Yao, M., Lv, L., Ling, Z. & Li, L. The human microbiota in health and disease. Engineering 3, 71–82 (2017).
Spear, G. T. et al. Identification of rhesus macaque genital microbiota by 16S pyrosequencing shows similarities to human bacterial vaginosis: implications for use as an animal model for HIV vaginal infection. AIDS Res. Hum. Retroviruses 26, 193–200, https://doi.org/10.1089/aid.2009.0166 (2010).
Smith, S. M., Baskin, G. B. & Marx, P. A. Estrogen protects against vaginal transmission of simian immunodeficiency virus. J. Infect. Dis. 182, 708–715 (2000).
Haus, T. et al. Genome typing of nonhuman primate models: implications for biomedical research. Trends Genet. 30, 482–487, https://doi.org/10.1016/j.tig.2014.05.004 (2014).
Bielert, C. et al. Mating in the rhesus monkey (Macaca mulatta) after conception and its relationship to oestradiol and progesterone levels throughout pregnancy. J. Reprod. Infertil. 46, 179–187 (1976).
Gordon, T. P. Reproductive behavior in the rhesus monkey: social and endocrine variables. Am. Zool. 21, 185–195 (1981).
Wilson, M., Walker, M., Pope, N. & Gordon, T. Prolonged lactational infertility in adolescent rhesus monkeys. Biol. Reprod. 38, 163–174 (1988).
King, B. F. Ultrastructure of the nonhuman primate vaginal mucosa: epithelial changes during the menstrual cycle and pregnancy. J. Ultrastruct. Res. 82, 1–18 (1983).
Marx, P. A. et al. Progesterone implants enhance SIV vaginal transmission and early virus load. Nat. Med. 2, 1084–1089 (1996).
Chen, Z. et al. Diversity of macaque microbiota compared to the human counterparts. Sci. Rep. 8, 15573, https://doi.org/10.1038/s41598-018-33950-6 (2018).
Yildirim, S. et al. Primate vaginal microbiomes exhibit species specificity without universal Lactobacillus dominance. ISME J. 8, 2431–2444, https://doi.org/10.1038/ismej.2014.90 (2014).
Miller, E. A., Livermore, J. A., Alberts, S. C., Tung, J. & Archie, E. A. Ovarian cycling and reproductive state shape the vaginal microbiota in wild baboons. Microbiome 5, 8, https://doi.org/10.1186/s40168-017-0228-z (2017).
Gajer, P. et al. Temporal dynamics of the human vaginal microbiota. Sci. Transl. Med 4, 132ra52, https://doi.org/10.1126/scitranslmed.3003605 (2012).
Breshears, L. M., Edwards, V. L., Ravel, J. & Peterson, M. L. Lactobacillus crispatus inhibits growth of Gardnerella vaginalis and Neisseria gonorrhoeae on a porcine vaginal mucosa model. BMC Microbiol. 15, 276, https://doi.org/10.1186/s12866-015-0608-0 (2015).
Uchihashi, M. et al. Influence of age, reproductive cycling status, and menstruation on the vaginal microbiome in baboons (Papio anubis). Am. J. Primatol. 77, 563–578, https://doi.org/10.1002/ajp.22378 (2015).
Nelson, D. E. et al. Bacterial communities of the coronal sulcus and distal urethra of adolescent males. PloS One 7, e36298, https://doi.org/10.1371/journal.pone.0036298 (2012).
Zozaya, M. et al. Bacterial communities in penile skin, male urethra, and vaginas of heterosexual couples with and without bacterial vaginosis. Microbiome 4, 16, https://doi.org/10.1186/s40168-016-0161-6 (2016).
Farage, M. A., Miller, K. W. & Sobel, J. D. Dynamics of the vaginal ecosystem—hormonal influences. Infect. Dis. Res. Treat. 3, 1–15, https://doi.org/10.4137/IDRT.S3903 (2010).
Knauf, S., Batamuzi, E., Mätz-Rensing, K., Leendertz, F. & Wehrend, A. Exfoliative vaginal cytology: a diagnostic tool for sexual cycle stages in nonhuman primates. Tanzania Veterinary Journal 26, 1–6 (2009).
MacLennan, A. H. & Wynn, R. M. Menstrual cycle of the baboon: I. clinical features, vaginal cytology and endometrial histology. Obstet. Gynecol. 38, 350–358 (1971).
Weinbauer, G. F. et al. Physiology and endocrinology of the ovarian cycle in macaques. Toxicol. Pathol. 36, 7S–23S, https://doi.org/10.1177/0192623308327412 (2008).
DiGiulio, D. B. et al. Temporal and spatial variation of the human microbiota during pregnancy. Proc. Natl. Acad. Sci. USA 112, 11060–11065, https://doi.org/10.1073/pnas.1502875112 (2015).
Nelson, D. E. et al. Characteristic male urine microbiomes associate with asymptomatic sexually transmitted infection. PloS One 5, e14116, https://doi.org/10.1371/journal.pone.0014116 (2010).
Sabat, A. J. et al. Targeted next-generation sequencing of the 16S-23S rRNA region for culture-independent bacterial identification-increased discrimination of closely related species. Sci. Rep. 7, 3434, https://doi.org/10.1038/s41598-017-03458-6 (2017).
Whiteside, S. A., Razvi, H., Dave, S., Reid, G. & Burton, J. P. The microbiome of the urinary tract—a role beyond infection. Nat. Rev. Urol. 12, 81, https://doi.org/10.1038/nrurol.2014.361 (2015).
Pybus, V. & Onderdonk, A. B. Evidence for a commensal, symbiotic relationship between Gardnerella vaginalis and Prevotella bivia involving ammonia: Potential significance for bacterial vaginosis. J. Infect. Dis. 175, 406–413 (1997).
Brown, G. R. & Dixson, A. F. The development of behavioural sex differences in infant rhesus macaques (Macaca mulatta). Primates 41, 63–77 (2000).
Goodrich, J. K. et al. Human genetics shape the gut microbiome. Cell 159, 789–799 (2014).
McCafferty, J. et al. Stochastic changes over time and not founder effects drive cage effects in microbial community assembly in a mouse model. ISME J. 7, 2116–2125, https://doi.org/10.1038/ismej.2013.106 (2013).
Hallmaier-Wacker, L. K., Lueert, S., Roos, C. & Knauf, S. The impact of storage buffer, DNA extraction method, and polymerase on microbial analysis. Sci. Rep. 8, 6292, https://doi.org/10.1038/s41598-018-24573-y (2018).
Salter, S. J. et al. Reagent and laboratory contamination can critically impact sequence-based microbiome analyses. BMC Biol. 12, 87, https://doi.org/10.1186/s12915-014-0087-z (2014).
Kozich, J. J., Westcott, S. L., Baxter, N. T., Highlander, S. K. & Schloss, P. D. Development of a dual-index sequencing strategy and curation pipeline for analyzing amplicon sequence data on the MiSeq Illumina sequencing platform. Appl. Environ. Microbiol. 79, 5112–5120 (2013).
Schloss, P. D. et al. Introducing mothur: Open-source, platform-independent, community- supported software for describing and comparing microbial communities. Appl. Environ. Microbiol. 75, 7537–7541 (2009).
Quast, C. et al. The SILVA ribosomal RNA gene database project: improved data processing and web-based tools. Nucleic Acids Res. 41, D590–D596 (2012).
Lozupone, C. & Knight, R. UniFrac: A new phylogenetic method for comparing microbial communities. Appl. Environ. Microbiol. 71, 8228–8235 (2005).
Metsalu, T. & Vilo, J. ClustVis: a web tool for visualizing clustering of multivariate data using Principal Component Analysis and heatmap. Nucleic Acids Res. 43, W566–W570 (2015).
R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing. Available from, https://www.R-project.org (2018).
Oksanen, J., et al. vegan: Community ecology package. Available from, https://CRAN. R-project.org/package=vegan (2018).
Excoffier, L., Smouse, P. E. & Quattro, J. M. Analysis of molecular variance inferred from metric distances among DNA haplotypes: application to human mitochondrial DNA restriction data. Genetics 131, 479–491 (1992).
White, J. R., Nagarajan, N. & Pop, M. Statistical methods for detecting differentially abundant features in clinical metagenomic samples. PLoS Comput. Biol. 5, e1000352, https://doi.org/10.1371/journal.pcbi.1000352 (2009).
Acknowledgements
The authors thank the Biodefense and Emerging Infectious Research (BEI) Resources, NIAID, NIH for providing the cells from Microbial Mock Community (Even, HM-280) as part of the Human Microbiome Project. We thank Tamara Becker, Annette Schrod, Annette Husung, Melina Urh, and all the animal caretakers for their assistance during sample collection. Additionally, we thank Uwe Schönmann and Dietmar Zinner (Deutsches Primatenzentrum GmbH) for their guidance and general support. We thank the staff of the Transcriptome and Genome Analysis Laboratory at the Georg-August-University of Goettingen for their assistance in optimizing the sequencing run.
Author information
Authors and Affiliations
Contributions
L.H.W., C.R. and S.K. designed the study. Sample collection was performed by L.H.W., S.L. and S.K. Laboratory work was conducted at the German Primate Center and performed by L.H.W. and S.L. Data were analyzed by L.H.W. and S.K. All authors (L.H.W., S.L., C.R. and S.K.) contributed to the manuscript preparation.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Hallmaier-Wacker, L.K., Lüert, S., Roos, C. et al. Lactation and menstruation shift the vaginal microbiota in captive rhesus monkeys to be more similar to the male urethral microbiota. Sci Rep 9, 17399 (2019). https://doi.org/10.1038/s41598-019-53976-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-53976-8
- Springer Nature Limited
This article is cited by
-
Vaginal microbial dynamics and pathogen colonization in a humanized microbiota mouse model
npj Biofilms and Microbiomes (2023)
-
Feeding hempseed cake alters the bovine gut, respiratory and reproductive microbiota
Scientific Reports (2023)
-
Microbiome Studies in Non-human Primates
Current HIV/AIDS Reports (2021)