Abstract
Association between the amount of enteral nutrition (EN) caloric intake and Glasgow coma scale scores at discharge (GCSdis) in intracranial haemorrhage (ICH) was retrospectively investigated in 230 patients in a single center from 2015 and 2017. GCSdis was used as a dichotomous outcome (≤8 or >8: 56/230 vs. 174/230) and its association with the amount of EN caloric intake within 48 hours was analysed in four logistic models. Model 1 used EN as a continuous variable and showed association with favourable GCSdis (odds ratio [OR], 1.04; 95% confidence interval [CI], 1.01–1.08). Models 2 and 3 categorized EN into two (≤25 and >25 kcal/kg/48 hrs) and three caloric intake levels (≤10, 10~25, and >25 kcal/kg/48 hrs) respectively, and compared them with the lowest level; highest EN level associated with favourable GCSdis in both model 2 (OR, 2.77; 95%CI, 1.25–6.13) and 3 (OR, 4.68; 95%CI, 1.61–13.61). Model 4 transformed EN into four quartiles (Q1-Q4). Compared to Q1, OR increased stepwise from Q2 (OR 1.80, 95%CI 0.59–5.44) to Q4 (OR 4.71, 95%CI 1.49–14.80). Propensity score matching analysis of 69 matched pairs demonstrated consistent findings. In the early stage of ICH, increased EN was associated with favourable GCSdis.
Similar content being viewed by others
Introduction
Intracranial haemorrhage (ICH) remains a significant cause of morbidity and mortality worldwide, and results in significant social burden1,2. Nutrition support plays an important role in the intensive care of ICH patients3, and enteral nutrition (EN) is the preferred feeding method to maintain gastrointestinal integrity and to prevent translocation of enteric bacteria4. Because hypermetabolic responses are almost inevitable following brain injury5, studies have indicated that early initiation of EN in ICH patients improve outcomes6, such as attenuation of the hypercatabolic response and reduced infection risk. However, the appropriate amount of EN calorie intake for patients in the early stage of ICH remains unclear.
Although hypocaloric feeding has been associated with decreased infection rates7 and mortality8 in critically ill patients, contrasting reports state that hypocaloric feeding of critically ill patients in the first 7 days of intensive care was associated with a higher incidence of nosocomial infection9. However, these inconsistencies in findings may be due to the significant heterogeneity among different cohorts, which makes these results inapplicable to ICH patients.
Thus, we performed this retrospective study to investigate the association between the amount of EN caloric intake and neurological outcome in patients with ICH.
Results
Baseline characteristics
Data of 230 patients with ICH were included in this study. In all, 24.3% (56/230) patients had a unfavourable outcome (GCSdis ≤ 8) and the overall in-hospital mortality was 11.3% (26/230). Patients in the high Glasgow coma scale scores at discharge (GCSdis) group (9–15) were significantly younger (59.9 ± 14.4 years vs. 51.6 ± 14.9 years, p < 0.001). Further, the amount of EN caloric intake was significantly higher in patients with high GCSdis scores than in those with low GCSdis scores (15.8 ± 13.4 kcal/kg/48 hrs vs. 22.1 ± 13.8 kcal/kg/48 hrs, p = 0.002). The baseline serum albumin level was comparable between the two groups. Detailed comparisons of demographic characteristics are presented in Table 1. The comparisons between two EN intake groups (≤25 and >25 kcal/kg/48 h) are presented in eTable 1.
Association between EN and GCSdis
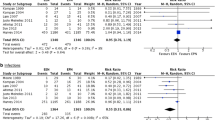
To test the robustness of the association between EN and GCSdis, four logistic models were developed using different methods to stratify the amount of EN caloric intake (Fig. 1, Four logistic models). Model 1 used EN as a continuous variable, and increased EN (1 kcal/kg/48 h) associated significantly with favourable GCSdis (odds ratio [OR], 1.04; 95% confidence interval [CI], 1.01–1.08; p = 0.003). Model 2 classified EN into two levels (≤25 and > 25 kcal/kg/48 h) and high EN levels associated significantly with favourable GCSdis scores (OR, 2.77; 95% CI, 1.25–6.13; p = 0.012). Model 3 classified EN into three levels (≤10, 10–25, >25 kcal/kg/48 h). After adjusting for confounders, only the OR of the highest EN level showed significant association (OR 4.68, 95% CI 1.61–13.61, p = 0.005), compared to the lowest level. Model 4 categorized EN into four quartiles (Q1-Q4). Compared with Q1, OR increased stepwise from Q2 (OR, 1.80; 95% CI, 0.59–5.44; p = 0.294) to Q4 (OR, 4.71; 95% CI, 1.49–14.80; p = 0.008).
Crude (panel A) and adjusted (panel B) odds ratio of the EN caloric intake in four logistic models. In model 1, EN was used as a continuous variable (1 kcal/kg/48hrs). In model 2, model 3 and model 4, EN was divided into two levels (≤25 and >25 kcal/kg/48 hrs), three levels (≤10, 10~25, and >25 kcal/kg/48 hrs) and four quartiles, respectively, and the lowest level was used as reference level. Abbreviation: EN enteral nutrition.
Outcomes after propensity score matching (PSM)
Patients were divided into two groups according to the amount of EN caloric intake (≤25 and >25 kcal/kg/48 h), and PSM was performed to minimize the imbalance between the groups. After PSM, 69 matched pairs were obtained. Comparisons of the standardized difference of the means, ratio of variances (Table 2), and propensity scores for the two groups (Fig. 2) showed excellent matching among all pairs. All ten variables used for PSM were comparable between the two groups, including the GCS score at intensive care unit (ICU) admission (8 [5–11] vs. 7 [6–10], p = 0.649) and disease severity (Acute Physiology and Chronic Health Evaluation II [APACHE II] score, 18.4 ± 6.1 vs. 18.9 ± 5.2; p = 0.666). Among the matched pairs, the proportion of patients with GCS score >8 was significantly higher in the high EN caloric intake group (60/69 vs. 48/69, p = 0.013), and moreover, the duration of hospital stay was longer (21.5 ± 8.7 vs. 17.8 ± 7.2, p = 0.008), compared to the low EN caloric intake group. Furthermore, hospital-acquired pneumonia tended to be less frequent in the low EN caloric group (27/69 vs. 36/69, p = 0.124).
Discussion
In this retrospective study, we found that in ICH patient with relatively low EN caloric intake, an increase in the amount of EN caloric intake was significantly associated with favourable GCS score at discharge (≤25 vs. >25 kcal/kg/48 hrs). The robustness of this finding was verified using different EN grouping methods and PSM analysis. However, because of the retrospective study design, the causal relationship could not be inferred. Therefore, further randomized controlled trials are needed to validate our findings.
Hypermetabolic response5 and gastrointestinal dysfunction are common risks factors for malnutrition in ICH patients, which, consequently, is associated with high morbidity and mortality10. According to the current consensus11, EN is the preferred method to maintain lean body tissue and to preserve the intestinal integrity and reduce the translocation of enteric bacteria12. However, the optimal amount of EN caloric intake needed to achieve these outcomes remains unclear.
Several studies have indicated that even with adequate nutrition supply, an acute hypercatabolic status cannot been completely prevented13,14. Thus, although malnutrition should be prevented in critically ill patients, evidence supporting a high calorie regimen for ICH patients remains weak.
In the last few decades, studies have investigated the effect of low EN calorie regimen in many diseases. A randomized trial8 comprising 240 critically ill patients demonstrated that hypocaloric regimen (60% VS. 90% of the targeted goal), as compared with target feeding, was associated with lower mortality. Yaseen et al.15, in an observational study of 523 patients, found that a near target caloric intake was associated with significantly higher hospital mortality, ICU-acquired infections, and longer durations of mechanical ventilation and hospital stay. Several underlying causes have been proposed for these findings, such as reduction in the metabolic rate and oxidative stress16, generation of mitochondrial free radical17, and activation of the plasma membrane redox system18.
However, other studies have reported contrasting results. Recent multicentre trials that compared normal EN caloric support showed that the mortality19,20 or incidence of infectious complication20 were not reduced in the low EN caloric group in critically ill patients. Petros et al.9 reported that as compared with normal caloric feeding (100% of daily energy expenditure), low caloric feeding (50% of the daily energy expenditure) of critically ill patients during the first 7 days was associated with more incidences of nosocomial infections. In an observational study of 58 patients with poor-grade subarachnoid haemorrhage (SAH), Badjatia et al.21 found that negative energy balance during the first 7 days results in a potential risk of infections. The inconsistencies among findings may be attributed to both heterogeneity among the different cohorts and different regimens of low caloric intake. In the current study, we found that non-SAH ICH patients who received a high amount of EN caloric intake (>25 kcal/kg/48 hrs) demonstrated better neurological outcomes at discharge, and this finding remained stable both after adjusting for confounders, including GCS scores at emergency admission, and in PSM analysis. In 2015, Badjatia et al.22 also revealed that in SAH patients, lower caloric intake (<11.3 kcal/kg/day) was associated with a higher risk of infection and negative nitrogen balance, which resulted in a significant risk for a poor neurological outcome. Furthermore, Young et al.23 showed that compared with patients receiving inadequate gastric feeding, those receiving adequate parenteral nutrition support had a better neurologic outcome at 3 months (mean cumulative caloric balance: 75.6% vs. 59%). However, the early application of parenteral nutrition makes these conclusions inapplicable to ICH patients according to the current guideline11. Furthermore, despite named the “high EN caloric group” in the current study, the amount of EN caloric intake in our study was much lower than that in Young et al.’s study, which compared to an intervention trial, may be more in line with clinical practice. On the contrary, negative energy balance is associated with increasing number of complications, particularly infections24. However, in contrast to previous findings9,25 including those in ASH21,22, the incidence of hospital acquired pneumonia, a critical complication in ICH patients, was similar in low and high EN caloric groups in the current study. We believe that low EN caloric intake may be a reason for this non-significant finding. The fact that only 32.6% of the included patients received more than 50% of the target EN goal (50 kcal/kg/48 h) in the current study is concerning. Based on our experience, the low EN caloric intake may be attributable to gastrointestinal dysfunction, such as delayed gastric emptying, increased resting energy expenditure, catabolism, immobilization, and delayed and inadequate nutritional supply11,26. However, if our findings are validated in future rigorous randomized trials, strategies aiming to improve EN support, such as those involving jejunal feeding and gastrointestinal motility drugs, should be more actively applied for patients in the early stage of ICH.
Our study has several limitations. First, the definition of a high EN calorie regimen was inaccurate as the overall EN caloric intake was relatively low in our cohort. Thus, in contrast to other studies8,9, patients receiving>25 kcal/kg/48 h were assigned to the high EN caloric group in the current study. The comparison between the actual low and high EN caloric regimen (for instance, 20% vs. 90% of the target goal) needs further investigation. Furthermore, caloric intake from parenteral nutrition was not included in the current study. In our hospital, the basic fluid protocol for ICH patients is the same, and thus, 5% dextrose and propofol are the main resources of parenteral nutrition. However, the use of both dextrose and propofol is often suspended owing to different conditions, such as fluid overload and sedative status. Thus, calories from parenteral intake were excluded in this analysis. Third, the reason for choosing 2 days as the cut-off point was empirical and somewhat arbitrary. According to clinical experience, the volume of EN intake was largely different in the early stage of ICH patients, while similar in the late stage, and similar finding was also reported in an observational study3. Fourth, information on EN-related complications such as diarrhoea, abdominal distension, and vomiting were not available in the electronic medical record system, and thus were not evaluated in the current study. Fifth, although the use of PSM supports our hypotheses, the limitations inherent to the retrospective nature of this study cannot be excluded. Thus, the causal relationship and underlying mechanisms for accelerating neurologic recovery could not be inferred.
Conclusion
In the early stage of ICH, increased amount of EN caloric intake is associated with improved neurological outcome at hospital discharge, with no significant increase in pneumonia incidence. Future larger randomized clinical trials are required to confirm and validate this association.
Materials and Methods
Study location and population
Between June 2015 and September 2017, all patients admitted to the ICU after intracerebral surgery due to ICH in Dongyang People’s Hospital who met the following inclusion criteria were enrolled: (1) Glasgow coma scale (GCS) score ≥4 and ≤12 on admission; (2) Hospital stay duration of more than 3 days; and (3) stable haemodynamics. Patients were excluded if any of the following criteria were met: (1) Age lower than 18 years; (2) Pregnancy; (3) A diagnosis of aneurysmal subarachnoid haemorrhage; (4) Without severe kidney or liver dysfunction; (5) Without uncontrolled hyperglycaemia; and (6) Severe abdominal injury. The general surgery procedures were provided in the Supplementary File. No specific interventions were made, and all patients received standard treatment according to the current guidelines10,11. The requirement for informed consent was waived by the ethics committee due to the retrospective nature of the study. The ethics committee of Dongyang People’s Hospital approved this study.
Data source and primary outcome
All data were extracted from electronic medical records. All demographic characteristics, such as age, sex, weight, and comorbidities, were recorded. Biochemical indexes at ICU admission, including findings from routine blood examinations and clinical scores such as the Acute Physiology and Chronic Health Evaluation II (APACHE II) score, were also extracted. Caloric intake through enteral nutrition within 2 days after admission was calculated. The GCS score at hospital discharge (GCSdis) was used as the primary outcome and as a dichotomous outcome in the logistic models: level one: 3–8 and level two: 9–15. For patients who died during the hospital stay, the GCSdis was recorded as 3. The duration of ICU and hospital stay and the occurrence of hospital-acquired pneumonia were recorded as secondary outcomes.
Grouping methods for EN in logistic regression models
EN was used as a continuous variable in the initial analysis. To test the robustness of the conclusion, EN was further divided into two (≤25 and >25 kcal/kg/48 h) and three levels (≤10, 10–25 and >25 kcal/kg/48 h). For better interpretation, quartile grouping was also applied, and EN quartile 1 was used as the reference in the multivariate logistic regression models.
Propensity score matching (PSM)
Patients were divided into two groups according to the EN caloric intake (≤25 and >25 kcal/kg/48 h). To minimize the effect of confounding factors such as GCS score at admission, PSM12 was applied using a one-to-one nearest neighbour matching algorithm and a calliper of 0.05. The following variables were selected to generate the propensity score: age, body weight, blood loss, hypertension, GCS score at ICU admission, APACHE II score, white blood cell and platelet counts, and serum creatinine and albumin levels. Kernel density plots of the p-score were used to examine the degree of PSM. Matching quality was evaluated by comparing the standardized difference of the means and the ratio of the variances as well as by graphically inspecting the propensity scores for the two groups. Finally, 69 matched pairs were obtained and analysed further.
Statistical analysis
Continuous variables are expressed as the mean ± standard deviation or median (interquartile range), as appropriate. The student’s t-test or Wilcoxon rank-sum test was used, as appropriate. Categorical data are expressed as proportions, and were compared using the χ2 test or Fisher’s exact test. Fourteen confounders with a p value <0.20 in the univariate analyses were included in multivariate logistic regression analyses: age, alcohol consumption, hypertension, frontal lobe and epidural bleeding, thalamencephalon bleeding, platelet and white blood cell count, serum sodium level, lung and liver disease, fluid balance, GCS at emergency department and APACHE II score. A stepwise backward method with p < 0.1 was used to build the model and seven confounders were excluded, including alcohol consumption, hypertension, frontal lobe and epidural bleeding, thalamencephalon bleeding, lung disease, and fluid balance. Multicollinearity was tested using the variance inflation factor (VIF) method, and platelet count, serum sodium level and APACHE II score were excluded for VIF ≥ 5. Four models were included in the final model: age, liver disease, white blood cell count, and GCS at emergency department. PSM was used to minimize between-group imbalances. Two-tailed tests were performed, and p < 0.05 was considered statistically significant. All statistical analyses were performed using Stata 11.2 (Stata Corp., College Station, TX, USA).
Data availability
All the data are available from the corresponding author on reasonable request.
References
van Asch, C. J. et al. Incidence, case fatality, and functional outcome of intracerebral haemorrhage over time, according to age, sex, and ethnic origin: a systematic review and meta-analysis. Lancet Neurol 9, 167–176, https://doi.org/10.1016/S1474-4422(09)70340-0 (2010).
Feigin, V. L., Lawes, C. M., Bennett, D. A., Barker-Collo, S. L. & Parag, V. Worldwide stroke incidence and early case fatality reported in 56 population-based studies: a systematic review. Lancet Neurol 8, 355–369, https://doi.org/10.1016/S1474-4422(09)70025-0 (2009).
Zarbock, S. D. et al. Successful enteral nutritional support in the neurocritical care unit. Neurocritical care 9, 210–216, https://doi.org/10.1007/s12028-008-9120-9 (2008).
Ojo, O. Enteral feeding for nutritional support in critically ill patients. British journal of nursing (Mark Allen Publishing) 26, 666–669, https://doi.org/10.12968/bjon.2017.26.12.666 (2017).
Young, B., Ott, L., Yingling, B. & McClain, C. Nutrition and brain injury. Journal of neurotrauma 9(Suppl 1), S375–383 (1992).
Seule, M., Brunner, T., Mack, A., Hildebrandt, G. & Fournier, J. Y. Neurosurgical and Intensive Care Management of Traumatic Brain Injury. Facial plastic surgery: FPS 31, 325–331, https://doi.org/10.1055/s-0035-1562884 (2015).
Casaer, M. P. et al. Role of disease and macronutrient dose in the randomized controlled EPaNIC trial: a post hoc analysis. American journal of respiratory and critical care medicine 187, 247–255, https://doi.org/10.1164/rccm.201206-0999OC (2013).
Arabi, Y. M. et al. Permissive underfeeding and intensive insulin therapy in critically ill patients: a randomized controlled trial. The American journal of clinical nutrition 93, 569–577, https://doi.org/10.3945/ajcn.110.005074 (2011).
Petros, S., Horbach, M., Seidel, F. & Weidhase, L. Hypocaloric vs Normocaloric Nutrition in Critically Ill Patients: A Prospective Randomized Pilot Trial. JPEN. Journal of parenteral and enteral nutrition 40, 242–249, https://doi.org/10.1177/0148607114528980 (2016).
Goiburu, M. E. et al. The impact of malnutrition on morbidity, mortality and length of hospital stay in trauma patients. Nutr Hosp 21, 604–610 (2006).
McClave, S. A. et al. Guidelines for the Provision and Assessment of Nutrition Support Therapy in the Adult Critically Ill Patient: Society of Critical Care Medicine (SCCM) and American Society for Parenteral and Enteral Nutrition (A.S.P.E.N.). JPEN. Journal of parenteral and enteral nutrition 33, 277–316, https://doi.org/10.1177/0148607109335234 (2009).
MacFie, J. et al. Bacterial translocation studied in 927 patients over 13 years. Br J Surg 93, 87–93, https://doi.org/10.1002/bjs.5184 (2006).
Wolfe, R. R. Regulation of skeletal muscle protein metabolism in catabolic states. Curr Opin Clin Nutr Metab Care 8, 61–65 (2005).
Shaw, J. H., Wildbore, M. & Wolfe, R. R. Whole body protein kinetics in severely septic patients. The response to glucose infusion and total parenteral nutrition. Ann Surg 205, 288–294 (1987).
Arabi, Y. M. et al. Near-target caloric intake in critically ill medical-surgical patients is associated with adverse outcomes. JPEN. Journal of parenteral and enteral nutrition 34, 280–288, https://doi.org/10.1177/0148607109353439 (2010).
Dandona, P. et al. The suppressive effect of dietary restriction and weight loss in the obese on the generation of reactive oxygen species by leukocytes, lipid peroxidation, and protein carbonylation. J Clin Endocrinol Metab 86, 355–362, https://doi.org/10.1210/jcem.86.1.7150 (2001).
Gredilla, R., Sanz, A., Lopez-Torres, M. & Barja, G. Caloric restriction decreases mitochondrial free radical generation at complex I and lowers oxidative damage to mitochondrial DNA in the rat heart. FASEB J 15, 1589–1591 (2001).
Hyun, D. H., Emerson, S. S., Jo, D. G., Mattson, M. P. & de Cabo, R. Calorie restriction up-regulates the plasma membrane redox system in brain cells and suppresses oxidative stress during aging. Proc Natl Acad Sci USA 103, 19908–19912, https://doi.org/10.1073/pnas.0608008103 (2006).
Arabi, Y. M. et al. Permissive Underfeeding or Standard Enteral Feeding in Critically Ill Adults. The New England journal of medicine 372, 2398–2408, https://doi.org/10.1056/NEJMoa1502826 (2015).
National Heart, L. et al. Initial trophic vs full enteral feeding in patients with acute lung injury: the EDEN randomized trial. JAMA 307, 795–803, https://doi.org/10.1001/jama.2012.137 (2012).
Badjatia, N. et al. Relationship between energy balance and complications after subarachnoid hemorrhage. JPEN. Journal of parenteral and enteral nutrition 34, 64–69, https://doi.org/10.1177/0148607109348797 (2010).
Badjatia, N. et al. Inflammation, negative nitrogen balance, and outcome after aneurysmal subarachnoid hemorrhage. Neurology 84, 680–687, https://doi.org/10.1212/WNL.0000000000001259 (2015).
Young, B. et al. The effect of nutritional support on outcome from severe head injury. Journal of neurosurgery 67, 668–676, https://doi.org/10.3171/jns.1987.67.5.0668 (1987).
Villet, S. et al. Negative impact of hypocaloric feeding and energy balance on clinical outcome in ICU patients. Clin Nutr 24, 502–509, https://doi.org/10.1016/j.clnu.2005.03.006 (2005).
Rubinson, L., Diette, G. B., Song, X., Brower, R. G. & Krishnan, J. A. Low caloric intake is associated with nosocomial bloodstream infections in patients in the medical intensive care unit. Critical care medicine 32, 350–357, https://doi.org/10.1097/01.CCM.0000089641.06306.68 (2004).
Bratton, S. L. et al. Guidelines for the management of severe traumatic brain injury. XII. Nutrition. Journal of neurotrauma 24(Suppl 1), S77–82, https://doi.org/10.1089/neu.2006.9984 (2007).
Acknowledgements
Yanfei Shen received funding from the Zhejiang medical and health science and technology project (NO. 2018261355).
Author information
Authors and Affiliations
Contributions
Y.S. designed the study and wrote the draft of the manuscript. K.D. and X.J. extracted and verified the data, W.R. and X.C. performed all statistical analyses. Y.H. and W.Z. revised the manuscript for important intellectual content. Y.X. revised the manuscript to the final version. All the authors gave final approval for the manuscript to be published and agreed to be accountable for all aspects of the work, including the accuracy or integrity of any part of the work.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cheng, X., Ru, W., Du, K. et al. Association between enteral nutrition support and neurological outcome in patients with acute intracranial haemorrhage: A retrospective cohort study. Sci Rep 9, 16507 (2019). https://doi.org/10.1038/s41598-019-53100-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-53100-w
- Springer Nature Limited