Abstract
Carbon monoxide poisoning (COP) may cause injuries to the central nervous and endocrine systems, which might increase the risk of developing hypothyroidism. We wanted to evaluate the association between COP and the risk of developing hypothyroidism because epidemiological data on this potential association are limited. We conducted a nationwide population-based cohort study using the Nationwide Poisoning Database and identified 24,328 COP subjects diagnosed between 1999 and 2012. By matching the index date and age, we selected 72,984 non-COP subjects for comparison. Subjects with thyroid diseases and malignancy before 1999 were excluded. We followed up the two groups of subjects until 2013 and compared the risk of developing hypothyroidism. COP subjects had a significantly higher risk for hypothyroidism than non-COP subjects (adjusted hazard ratio [AHR]: 3.8; 95% confidence interval [CI]: 3.2–4.7) after adjusting for age, sex, underlying comorbidities, and monthly income, and the AHR was particular higher in subjects with diabetes mellitus, hyperlipidemia, and mental disorder. The increased risk was highest in the first month after COP (AHR: 41.0; 95% CI: 5.4–310.6), and the impact remained significant even after 4 years. In conclusion, COP was associated with an increased risk for hypothyroidism. Further studies regarding the underlying mechanisms are warranted.
Similar content being viewed by others
Introduction
Carbon monoxide poisoning (COP) is an important issue in public health because it is one of the leading causes of poisonings worldwide1. There are about 1,000–2,000 accidental deaths due to COP in the US annually, resulting from an estimate of 50,000 exposures each year1. In the past 10 years, suicidal COP has increased greatly in certain countries because carbon monoxide (CO) is odorless and ultimately fatal, which is thought to be a perfect tool for committing suicide2. In Taiwan, the incidence of suicidal COP by charcoal burning increased from 0.22 to 5.4 per 100,000 people between 1999 and 2009, a nearly 25-fold increase2.
Because CO has 250 times higher affinity for hemoglobin than for oxygen, even a small amount of CO can cause severe tissue hypoxia in all the organs, especially the brain and the heart, both of which are organs with the highest oxygen demand3,4,5. In addition to hypoxia, COP induces immunological and inflammatory damages to all the body organs via producing reactive oxygen species, which are longer lasting and cause effects independent of hypoxia6,7. Hypoxia and immunological and inflammatory damages may result in various morbidities and even mortality3,4,5. The most commonly known morbidity is brain injury with neurological sequelae, which include difficulty with higher intellectual functions, short-term memory loss, dementia, amnesia, psychosis, irritability, a strange gait, speech disturbances, Parkinson’s disease-like syndromes, cortical blindness, and a depressed mood6,7,8,9. Thyroid function is regulated by the hypothalamus-pituitary-thyroid (HPT) axis, and therefore an injury to both the brain (i.e. hypothalamus and pituitary gland) and the local organ (i.e. thyroid gland) may contribute to hypothyroidism10,11,12,13,14. Because COP leads to hypoxia, which can affect both the brain and the thyroid gland, it is plausible that COP may lead to hypothyroidism. However, we did not find studies on this issue upon searching the PubMed and Google Scholar using the key words “carbon monoxide,” “poisoning,” “intoxication,” “hypothyroidism,” “thyroid,” and “endocrine.” Therefore, we conducted this study to evaluate the association between COP and the risk of developing hypothyroidism.
Results
The mean age ± standard deviation of subjects in both cohorts was 36.4 ± 15.4 years after matching (Table 1). The age subgroup of 20–34 years had the largest proportion of subjects (39.3%), followed by the 35–49 years subgroup (31.8%). COP subjects had a larger proportion of females (50.6% vs. 49.8%, p = 0.045) and a higher prevalence of underlying comorbidities, including hypertension, diabetes mellitus, hyperlipidemia, rheumatoid arthritis, connective tissue disease, drug abuse, and mental disorder, and higher percentage of lower monthly income than the non-COP subjects.
In the overall comparison, COP subjects had a significantly higher risk of developing hypothyroidism than non-COP subjects (adjusted hazard ratio [AHR]: 3.8; 95% confidence interval [CI]: 3.2–4.7). The AHR was particularly higher in subjects with diabetes mellitus (AHR: 9.5; 95% CI: 4.2–21.1), hyperlipidemia (AHR: 5.2; 95% CI: 2.9–9.2), and mental disorder (AHR: 5.5; 95% CI: 3.8–8.1) (Table 2). In the subjects without mental disorder, the AHR was 3.2 (95% CI: 2.5−4.1) (Supplemental Table 1). COP subjects also had a higher risk of developing hypothyroidism in the subgroups of rheumatoid arthritis and drug abuse (AHR: 8.2; 95% CI: 0.9–78.5 and AHR: 5.2; 95% CI: 0.7–39.6, respectively), but the increases did not reach statistical significance. The risk of developing hypothyroidism associated with COP was similar between the two sexes (female: AHR: 3.9, 95% CI: 3.1–4.9 vs. male: AHR: 3.2, 95% CI: 2.0–5.1). COP subjects had a significantly higher risks of developing hypothyroidism during the whole follow-up period (Fig. 1; p < 0.001 for log-rank tests), even after 4 years (AHR: 2.8; 95% CI: 2.1–3.8). The highest risk was observed in the first month (AHR: 41.0; 95% CI: 5.4–310.6). We evaluated the proportional hazard assumption of the Cox model separately from early to the end of the duration and found the proportional hazard assumption was not violated in every time period. The adjusted competing risks hazard ratio showed the similar results.
Cox proportional hazards regression analysis showed that COP, older age, female sex, hyperlipidemia, mental disorder, and higher monthly income ( ≥ 40,000 New Taiwan Dollars) were independent predictors for hypothyroidism after adjusting for all the other variables in the full model, including age, sex, hypertension, diabetes mellitus, hyperlipidemia, rheumatoid arthritis, connective tissue disease, psoriasis, drug abuse, mental disorder, and monthly income (Table 3). The reduced model revealed that age, sex, hyperlipidemia, and mental disorder were independent predictors for hypothyroidism. In the COP subjects, those who had received hyperbaric oxygen therapy (HBOT) had a significantly higher risk of developing hypothyroidism than non-COP subjects (AHR: 4.0; 95% CI: 2.9−5.5 in the reduced model). While the risk was also higher in the COP subjects with acute respiratory failure (ARF), the increase did not reach statistical significance (AHR: 3.2; 95% CI: 0.4−22.9).
Discussion
This nationwide population-based cohort study showed that COP subjects had a significantly higher risk of developing hypothyroidism, especially in those with diabetes mellitus, hyperlipidemia, and mental disorder. The impact of COP on the increased risk of developing hypothyroidism was highest in the first month after COP and remained significant even after 4 years. Older age, female sex, hyperlipidemia, mental disorder, and higher monthly income were also independent predictors for hypothyroidism, in addition to COP.
Injuries to the HPT axis due to COP-related hypoxia and immunological and inflammatory damages may be the causes for hypothyroidism. Hypothyroidism is a common thyroid disorder in the adult population and is more prevalent in older women15. The common etiology is through an autoimmune mechanism, such as primary atrophic hypothyroidism, Hashimoto’s thyroiditis, radioactive iodine therapy, or thyroid surgery15. The HPT axis is a part of the neuroendocrine system responsible for the regulation of metabolism. While earlier studies in rats reported that exposure to 100 ppm CO induced a decrease in the levels of hypothalamic noradrenaline, brain serotonin, and serum thyroxine16,17, human studies on the effects of CO on the hypothalamus, pituitary gland, or thyroid are very limited. In addition to exogenous CO due to poisoning, endogenous CO itself is a neuromodulator that influences the physiological and pathological processes in the central and peripheral nervous systems18, which suggests that COP may cause morbidities by affecting hormone regulation in the human body.
We found that the impact of COP on the increased risk of developing hypothyroidism was larger in the subgroups of subjects with diabetes mellitus, hyperlipidemia, and mental disorder. In other words, in the populations with diabetes mellitus, hyperlipidemia, or mental disorder, people with COP had a higher risk for hypothyroidism than those without COP. In the population without mental disorder, the risk for hypothyroidism was still high (AHR: 3.2), which indicates that mental disorder might not have a strong interaction with COP. Because these are novel findings of this study, mechanistic evidences of the associations are limited. Diabetic patients are known to be more vulnerable to hypothyroidism than the general population (6% vs. 3%)15. One of the reasons is that the presence of one autoimmune disease may increase the risk for another autoimmune disease15. For example, previous studies have reported that up to 30% of females with type 1 diabetes have thyroid disease15 and that young patients with type 1 diabetes have a higher risk of developing thyroid disorders19. Type 2 diabetes also increases the risk of developing thyroid dysfunction due to insulin resistance, which is caused by perturbed genetic expression of a constellation of genes along with physiological aberrations leading to impaired glucose utilization and disposal in muscles, overproduction of hepatic glucose output, and enhanced absorption of splanchnic glucose20. Thyroid function significantly affects lipoprotein metabolism21 and mental status22. Hypothyroidism is associated with increases in total cholesterol, low-density lipoprotein cholesterol, and triglycerides as well as a decrease in the high-density lipoprotein cholesterol level21,23. Thyroid hormones have important actions in the brain, and overt hypothyroidism has major effects on neuropsychiatric function, which may result in mental disorders22,24. Treatment of hypothyroidism tends to improve related mental disorders24. However, the reasons why patients with hyperlipidemia or mental disorders are vulnerable to hypothyroidism are unclear.
In our study, older age and female sex were independent predictors for hypothyroidism, which is consistent with previous studies25,26,27,28. A national survey in the US reported that both the thyroid-stimulating hormone level and the presence of antithyroid antibodies are greater in women and increase with age27. The Framingham study reported a 4.4% prevalence of hypothyroidism in older participants (age > 60 years) and found that women exhibited hypothyroidism (5.9%) more often than men (2.3%)26.
Although this study had the major strengths of nationwide data and casted some light on an unanswered question, there were some limitations. First, details of some variables related to hypothyroidism such as family history were not available in the database. However, we had excluded potential subjects with thyroid diseases or malignancy and included variables of several underlying comorbidities, which may serve as surrogates for the unavailable confounders, and therefore the confounding effect may be minimized. Second, there is no information about the amount, duration, severity, and intent of CO exposure in the Taiwan National Health Insurance Research Database, and therefore the dose-response relationship could not be assessed in this study. Even though the exposure dose is an important factor affecting the prognosis of COP, however, it is hard to determine in clinical settings. In general, except for investigation or research purposes, reconstruction of the exposure dose is rarely done for patients. Therefore, the patient is likely to be the only source of information in many cases, but information such as the concentration and duration of exposure is usually hard to validate, even if the patient is able to provide it. Although COHb is an objective biomarker of exposure, it is hard to estimate the exposure dose accurately on the basis of COHb without accurate information on the duration of exposure, which is often unavailable. Third, this study revealed that COP subjects who received HBOT also had a higher risk for hypothyroidism, with an AHR similar to the overall COP cohort in comparison with the non-COP cohort. Whereas this might imply that HBOT does not have a beneficial effect on the occurrence of hypothyroidism, it needs further studies that make direct comparison between COP subjects with and without HBOT (i.e., 100% normobaric oxygen only) to provide more specific and accurate answers. Fourth, we did not investigate whether or not those who developed hypothyroidism within a few weeks following exposure remained hypothyroid subsequently. Further study is needed to clarify this issue. Fifth, the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) coding made by the emergency physicians might be incorrect and might need validation. However, in our recent hospital-based study29, after a chart review we found that all the ICD-9-CM diagnostic coding of COP made in the emergency department were correct. In addition, we used at least one hospitalization or at least three ambulatory care claims to define hypothyroidism and underlying comorbities, which could more confirm the correct diagnosis and avoid possible up-coding. Sixth, although this was a nationwide study, whether it could be generalized to other nations, because of differences in race, culture, and disease treatments, needs to be validated.
Conclusions
This nationwide population-based cohort study showed that COP significantly increased the risk of developing hypothyroidism, especially in subjects with diabetes mellitus, hyperlipidemia, and mental disorders. The highest risk was observed in the first month after COP, and the increased risk persisted even after 4 years of COP. Possible mechanisms include direct damages by COP to the central nervous system and the thyroid gland via hypoxia and indirect immunological and inflammatory reactions resulted from COP, which result in thyroid dysfunction and then hypothyroidism. Further studies on the detailed mechanisms are needed to validate findings in this epidemiological study.
Methods
Data source
The National Poisoning Database (NPD) and the Longitudinal Health Insurance Database 2000 (LHID2000), two sub-databases of the Taiwan National Health Insurance Research Database, were used for this study. Because the Taiwan National Health Insurance program covers nearly 100% of Taiwan’s population, the NPD represents all the poisonings including COP in Taiwan between 1999 and 201330. The National Health Insurance Research Database contains registration files and original claim data for reimbursement30. Large, computerized databases derived from this system by the National Health Insurance Administration (the former Bureau of National Health Insurance, BNHI), Ministry of Health and Welfare (the former Department of Health, DOH), Taiwan, and maintained by the National Health Research Institutes, Taiwan, are provided to scientists in Taiwan for research purposes30.
Identification of COP and non-COP subjects
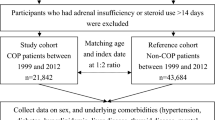
We used the NPD to identify all the COP subjects in Taiwan diagnosed between 1999 and 2012 and non-COP subjects from the LHID2000 by matching (1:3) by index date (i.e. date of admission or ambulatory care) and age (Fig. 2). The index date in the non-COP subjects was determined by the index date of the matched COP subjects. We adopted the matching by 1:3 according to previous studies31,32. Potential subjects with thyroid diseases and malignancy before the index date were excluded because these two conditions may affect the incidence of hypothyroidism. Thyroid diseases were defined as ICD-9-CM 240–246 or 193. Malignancy was defined as ICD-9-CM 140–208.
Definitions of included variables
COP was defined as ICD-9-CM codes of 986, E868, E952, or E982 in either hospitalization or emergency department care as one of the main diagnoses31,32,33,34. Hypothyroidism was defined as having the ICD-9-CM code 244 (i.e., acquired hypothyroidism) as one of the main diagnoses in at least one hospitalization or at least three ambulatory care claims. By this definition, we included only cases of acquired hypothyroidism, and the etiology is either a thyroid disease (primary hypothyroidism) or a hypothalamic-pituitary disease (central hypothyroidism, as in cases with low thyroid stimulating hormone [TSH] and thyroid hormone)35. Primary hypothyroidism may be either subclinical (high serum TSH and normal serum free thyroxine [T4] concentrations) or overt (high serum TSH and low serum free T4 concentrations)35. For example, patients with low T3 but normal T4 and TSH could present non thyroidal illness syndrome instead of hypothyroidism, and such cases would not be included in our analyses. Likewise, autoimmune thyroid disease (i.e., ICD-9-CM 245) and euthyroid sick syndrome (i.e., ICD-9-CM 790.94) were not included, neither. However, a middle elevated TSH with normal thyroid hormones could indicate resistance to TSH, and the patient would fit our case definition. Age subgroups were classified as < 20, 20–34, 35–49, 50–64, and ≥65 years. Underlying comorbidities were defined as hypertension (ICD-9-CM 401–405), diabetes mellitus (ICD-9-CM 250), hyperlipidemia (ICD-9-CM 272), rheumatoid arthritis (ICD-9-CM 714), connective tissue disease (ICD-9-CM 710), vitiligo (ICD-9-CM 709.01), scleroderma (ICD-9-CM 701.0), psoriasis (ICD-9-CM 696), drug abuse (ICD-9-CM 303–305), and mental disorder (ICD-9-CM 290–302, 306–319). To ensure the accuracy of diagnoses, we included only the cases with the same diagnoses in at least one hospitalization or at least three ambulatory care claims. We included these underlying comorbidities because they were possible confounding factors. ARF was defined as ICD-9-CM: 518.81 or 518.84 or management codes 960, 9601, 9602, 9603, 9604, 9605, 9390, 9391, or 311. HBOT was defined as management codes 47054C, 9395, 59003B, 59004B, 59003A, or 59004A. We included ARF and HBOT in order to investigate the effects of COP severity (ARF stands for a more severe COP) and HBOT on the risk of developing hypothyroidism. Although we did not review the diagnoses in the claims, the National Health Insurance randomly samples claims for reviews by appointed experts on a regular basis.
Ethics statement
This study was conducted strictly according to the Declaration of Helsinki and approved by the Institutional Review Board at Chi-Mei Medical Center. Informed consent from the subjects is waived because the NPD contains de-identified information, which does not affect the right and welfare of the subjects.
Data analysis
We compared the risk of developing hypothyroidism between COP and non-COP groups by following up until 2013 (Fig. 1). Independent t-test was used for continuous variables and chi-square test was used for categorical variables in the comparison of demographic data, underlying comorbidities, and monthly income between COP and non-COP subjects. Cox proportional hazards regression analysis and adjusted competing risks hazard ratio were used for comparing the risk of developing hypothyroidism between COP and non-COP subjects, with adjustment for age, sex, selected comorbidities, and monthly income. Because age, sex, and underlying comorbidities may affect the association between COP and hypothyroidism, we performed stratified analyses to evaluate the possible effect modifications. Stratified analyses by follow-up period were also performed to evaluate whether the effects of COP change over time. Kaplan–Meier method and the log-rank test were used to compare the risk for hypothyroidism between COP and non-COP subjects during the follow-up period. We treated death as a censoring event. In addition, subjects who were out of the National Health Insurance Database during study duration were defined as lost to follow up censoring. Nonetheless, as the insurance is compulsory with a long grace period for premium payment, almost all such cases are deaths with delayed reporting, which are extremely rare.
Multi-variate Cox proportional hazards models were constructed to identify the independent predictors for hypothyroidism and evaluate their effects. A full model was first constructed to provide a comprehensive assessment, which included all the variables selected from uni-variate analyses that are supported by the literature as risk factors for hypothyroidism. To obtain a reduced model that is more useful in clinical setting, we first excluded comorbidities with prevalence less than 1% and then exclude variables that did not reach statistical significance. We used SAS 9.4 for Windows (SAS Institute, Cary, NC, USA) for all analyses. The significance level was set at 0.05 (two-tailed).
References
Buckley, N. A., Juurlink, D. N., Isbister, G., Bennett, M. H. & Lavonas, E. J. Hyperbaric oxygen for carbon monoxide poisoning. Cochrane Database Syst Rev. 4, CD002041 (2011).
Pan, Y. J., Liao, S. C. & Lee, M. B. Suicide by charcoal burning in Taiwan, 1995-2006. J Affect Disord. 120, 254–257 (2010).
Ernst, A. & Zibrak, J. D. Carbon monoxide poisoning. N Engl J Med. 339, 1603–1608 (1998).
Huang, C. C. et al. Long-term prognosis of patients with carbon monoxide poisoning: a nationwide cohort study. PLoS One. 9, e105503 (2014).
Hampson, N. B., Hauff, N. M. & Rudd, R. A. Increased long-term mortality among survivors of acute carbon monoxide poisoning. Crit Care Med. 37, 1941–1947 (2009).
Weaver, L. K. Clinical practice. Carbon monoxide poisoning. N Engl J Med. 360, 1217–1225 (2009).
Hampson, N. B., Piantadosi, C. A., Thom, S. R. & Weaver, L. K. Practice recommendations in the diagnosis, management, and prevention of carbon monoxide poisoning. Am J Respir Crit Care Med. 186, 1095–1101 (2012).
Zou, J. F. et al. Lack of pupil reflex and loss of consciousness predict 30-day neurological sequelae in patients with carbon monoxide poisoning. PLoS One. 10, e0119126 (2015).
Zou, J. F. et al. A positive Babinski reflex predicts delayed neuropsychiatric sequelae in Chinese patients with carbon monoxide poisoning. Biomed Res Int. 2014, 814736 (2014).
Marieb, E. N. & Hoehn, K. N. Anatomy & physiology. Glenview, IL: Pearson Education, Inc. ISBN 978-0321861580 (2014).
Boron, W. F. & Boulapep, E. L. Medical Physiology (2nd ed.). Philadelphia: Saunders. p. 1052. ISBN 978-1-4377-1753-2 (2012).
Yr, B. S. & G, U. Illuminating gas poisoning and hyperthyroidism. Rev Clin Esp. 55, 113–117 (1954).
Kangogaku, Z. Clinical description of dementia. 13. Various types of dementia. (3). Subdural hematoma, hypothyroidism, anterior pituitary hypofunction, carbon monoxide poisoning, and pseudodementia. Kangogaku Zasshi 50, 574–577 (1986).
Almgren, S. Carbon monoxide poisoning and thyrotoxicosis. Acta Med Scand. 141, 36–42 (1951).
Wu, P. Thyroid Disease and Diabetes. Clinical Diabetes. 18, 38 (2000).
Vyskocil, A., Tusl, M. & Zaydlar, K. The effect of carbon monoxide on hormone levels and organ weights in rats. J Appl Toxicol. 6, 443–446 (1986).
Vyskocil, A., Tusl, M. & Zaydlar, K. The effect of chronic exposure to 100 ppm carbon monoxide on brain biomines, serum corticosterone and organ weights in rats. J Appl Toxicol. 3, 307–309 (1983).
Gomes, D. A. et al. Carbon monoxide and nitric oxide modulate hyperosmolality-induced oxytocin secretion by the hypothalamus in vitro. Biosci Rep. 30, 351–357 (2010).
Lu, M. C., Chang, S. C., Huang, K. Y., Koo, M. & Lai, N. S. Higher risk of thyroid disorders in young patients with type 1 diabetes: a 12-year nationwide, population-based, retrospective cohort study. PLoS One. 11, e0152168 (2016).
Wang, C. The relationship between type 2 diabetes mellitus and related thyroid diseases. Diabetes Res. 2013, 390534 (2013).
Duntas, L. H. Thyroid disease and lipids. Thyroid. 12, 287–93 (2002).
Samuels, M. H. Psychiatric and cognitive manifestations of hypothyroidism. Curr Opin Endocrinol Diabetes Obes. 21, 377–383 (2014).
Willard, D. L., Leung, A. M. & Pearce, E. N. Thyroid function testing in patients with newly diagnosed hyperlipidemia. JAMA Intern Med. 174, 287–289 (2014).
Davis, J. D. & Tremont, G. Neuropsychiatric aspects of hypothyroidism and treatment reversibility. Minerva Endocrinol. 32, 49–65 (2007).
Wiener, R., Utiger, R. D., Lew, R. & Emerson, C. H. Age, sex, and serum thyrotropin concentrations in primary hypothyroidism. Acta Endocrinol (Copenh). 124, 364–369 (1991).
Bensenor, I. M., Olmos, R. D. & Lotufo, P. A. Hypothyroidism in the elderly: diagnosis and management. Clin Interv Aging. 7, 97–111 (2012).
Hollowell, J. G. et al. Serum TSH, T4, and thyroid antibodies in the United States population (1988 to 1994): National Health and Nutrition Examination Survey (NHANES III). J Clin Endocrinol Metab. 87, 489–499 (2002).
Teng, W., Shan, Z., Patil-Sisodia, K. & Cooper, D. S. Hypothyroidism in pregnancy. Lancet Diabetes Endocrinol. 1, 228–237 (2013).
Huang, C. C. et al. Exposure duration and history of hypertension predicted neurological sequelae in patients with carbon monoxide poisoning. Epidemiology. 30, S76–S81 (2019).
National Health Insurance Administration, Ministry of Health and Welfare, Taiwan, R.O.C. National Health Insurance Annual Report 2014–2015 (2014).
Huang, C. C. et al. Increased risk for diabetes mellitus in patients with carbon monoxide poisoning. Oncotarget. 8, 63680–63690 (2017).
Huang, C. C. et al. Risk of myocardial infarction after carbon monoxide poisoning: a nationwide population-based cohort study. Cardiovasc Toxicol. 19, 147–155 (2019).
Huang, C. C. et al. Demographic and clinical characteristics of carbon monoxide poisoning: nationwide data between 1999 and 2012 in Taiwan. Scand J Trauma Resusc Emerg Med. 25, 70 (2017).
Huang, C. C. et al. Hyperbaric oxygen therapy is associated with lower short- and long-term mortality in patients with carbon monoxide poisoning. Chest. 152, 943–953 (2017).
LaFranchi, S. Acquired hypothyroidism in childhood and adolescence, https://www.uptodate.com/contents/acquired-hypothyroidism-in-childhood-and-adolescence (2018).
Acknowledgements
This study was supported by grant CMFHR10739 and CMFHR10734 from Chi-Mei Medical Center and Grant MOST 108–2238-B-006-001-MY2 from the Ministry of Science and Technology, Taiwan, R.O.C.
Author information
Authors and Affiliations
Contributions
C.C. Huang and H.R.G. designed and conceived this study and wrote the manuscript. H.C.H. and Y.C.C. performed the statistical analysis and wrote the manuscript. C.C. Hsu, H.J.L., S.B.S. and J.J.W. provided professional suggestions and wrote the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Huang, CC., Ho, CH., Chen, YC. et al. Increased risk for hypothyroidism associated with carbon monoxide poisoning: a nationwide population-based cohort study. Sci Rep 9, 16512 (2019). https://doi.org/10.1038/s41598-019-52844-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-52844-9
- Springer Nature Limited
This article is cited by
-
Association between carbon monoxide poisoning and adrenal insufficiency: a nationwide cohort study
Scientific Reports (2022)