Abstract
Cross-reactivity of classical chlamydial antigens compromises Chlamydia (C.) pneumoniae serology. By testing with 185 human antisera, we expanded 18 previously discovered C. pneumoniae-specific B-cell epitopes to 48 peptide antigens from 12 C. pneumoniae immunodominant proteins. For specific detection of antibodies against C. pneumoniae, we developed novel ELISAs with strongly reactive individual peptide antigens and mixtures of these peptides. By comparison to a composite reference standard (CRS) for anti-C. pneumoniae antibody status of human sera, the top-performing CpnMixF12 peptide assay showed 91% sensitivity at 95% specificity, significantly higher than 4 commercial anti-C. pneumoniae IgG ELISAs (36-12% sensitivity at 95% specificity). Human C. pneumoniae (Cpn) and C. trachomatis (Ctr) seroreactivity was 54% biased towards co-positivity in commercial Cpn and Ctr ELISAs, but unbiased in Cpn and Ctr peptide antibody assays, suggesting severe cross-reactivity of commercial ELISAs. Using hyperimmune mouse sera against each of 11 Chlamydia spp., we confirm that commercial Cpn and Ctr ELISA antigens are cross-reactive among all Chlamydia spp., but Cpn and Ctr peptide antigens react only with antisera against the cognate chlamydial species. With simultaneously high specificity and sensitivity, and convenient use for non-specialized laboratories, these ELISAs have the potential to improve serodiagnosis of C. pneumoniae infection.
Similar content being viewed by others
Introduction
Intracellular Chlamydia (C.) spp. bacteria infect virtually all vertebrates and cause largely chronic and asymptomatic diseases. The principal human chlamydial pathogens are C. pneumoniae and C. trachomatis1,2,3,4. The single human serovar of C. pneumoniae is a common cause of respiratory infection, leading to pharyngitis, bronchitis, and community-acquired pneumonia1,2. C. trachomatis serovars cause ocular and sexually transmitted genitourinary tract infections, and lymphogranuloma venereum3,4. C. psittaci sporadically causes severe zoonotic pneumonia5,6.
Most respiratory C. pneumoniae infections are mild or asymptomatic7,8, similar to Mycoplasma infections, although severe pneumonia can develop in elderly patients and those with coexisting cardiopulmonary diseases9,10. Infection with C. pneumoniae occurs worldwide, resulting in 40–90% prevalence of serum antibodies to classical C. pneumoniae antigens11,12,13,14. C. pneumoniae has been associated with both epidemic and endemic occurrences of acute respiratory disease, and with 6–20% of all community-acquired pneumonias and 5% of bronchitis and sinusitis cases in adults and children9,10,15,16,17,18.
Diagnosis of C. pneumoniae infection is preferably based on the isolation of the organism or its detection by PCR, the preferred method of diagnostic testing recommended by CDC for acute C. pneumoniae infection2. However, appropriate specimens require invasive sampling, and for that reason serology is currently the convenient tool most often applied for the routine diagnosis of C. pneumoniae infections2,19,20,21,22,23,24. In addition, serological assays indicate the history of exposure to C. pneumoniae and are preferable over antigen detection for epidemiologic or retrospective analyses.
Available serological tests for detection of anti-C. pneumoniae antibodies include enzyme-linked immunosorbent assays (ELISA) and the micro-immuno-fluorescence (MIF) test25,26,27,28,29,30. The high prevalence of C. trachomatis infection28,29,30 complicates results of testing for C. pneumoniae antibodies due to the possibility of false seropositivity arising from C. trachomatis infections31,32,33,34,35,36,37,38,39,40. ELISAs based on C. pneumoniae elementary bodies (EB) or outer membrane complex (OMC) suffer from lack of specificity due to cross-reactivity of Chlamydia genus-specific antigens. Similarly, the majority of immundominant protein candidate antigens for anti-C. pneumoniae ELISAs (OmpA, Omp2, PorB, or Hsp60) is highly conserved within Chlamydia spp.2,32,33,39,40, and thus poorly suited for C. pneumoniae-specific ELISAs. The MIF test was initially developed for species/serovar-specific detection of anti-C. trachomatis antibodies25,26,27, and later adopted for C. pneumoniae serology9,10,11,12,13,14,15. The MIF test has remained the gold standard in C. pneumoniae serological testing because of higher specificity and sensitivity than ELISAs16,19.
Purified EBs, the antigenically complex infective forms of Chlamydia, are used as MIF antigens25,26,27. Outer membrane protein A (OmpA), the serovar-determining most immunodominant protein of C. trachomatis, is the main constituent antigen of the MIF EB antigens. For C. trachomatis MIF serology, the OmpA antigen produces strong reactivity with anti-C. trachomatis antibodies during microscopic observation of MIF slides. Consequently, skilled personnel can identify a pattern of specific versus non-specific reactivity in the C. trachomatis MIF test. However, this microscopic observation is a painstaking technique, requiring extensive expertise and subjective interpretation of EB reactivity with anti-Chlamydia spp. antibodies, imposing a risk of high inter-laboratory variation in results19. Nevertheless, the C. trachomatis serovar EB antigens can still provide a good degree of species- and serovar-specificity in the MIF test.
Several studies suggest that the C. pneumoniae MIF test is less sensitive and specific than its general perception2,22,23,36,37. For C. pneumoniae serology, the MIF test is problematic due to the much lower immunogenicity of the C. pneumoniae OmpA antigen32. For example, the C. pneumoniae MIF test failed to detect anti-C. pneumoniae antibodies from sera of C. pneumoniae PCR/culture-positive children, underscoring the poor sensitivity of the C. pneumoniae MIF test2,7,8,22. Additionally, C. pneumoniae, C. trachomatis, and C. psittaci EB MIF antigens detected anti-C. pneumoniae antibodies without marked difference in the MIF antibody titers36,37. This serious cross-reactivity and poor sensitivity of the C. pneumoniae MIF test, together with cumbersome procedures inherently associated with the MIF technique and high inter-laboratory variation in MIF titers, stress the need to identify C. pneumoniae-specific antigens2,7,20,21,22,23,24. Therefore, development of novel specific and sensitive assays, particularly in simple format such as enzyme-linked immunosorbent assay (ELISA), is urgently needed for human chlamydial serology.
Previously, we have identified highly reactive and specific B cell epitopes41,42,43,44,45,46,47 of immunodominant proteins of all Chlamydia species48,49,50,51. In extensive evaluation46,47, we also showed that the C. trachomatis-specific peptide antigens provide superior assays with high sensitivity and specificity. Importantly, the high sensitivity of the peptide assays was mainly achieved by the use of multiple B-cell epitopes of several C. trachomatis immunodominant proteins46,47. Since antibody responses to individual B cell epitopes are stochastic41,42,43,44,45,46, only the combined use of multiple peptide antigens reliably measured host antibodies produced in response to C. trachomatis infection46, similar to the quantitative results obtained with complex antigens. In the present study, we developed and validated peptide assays for detection of anti-C. pneumoniae antibodies. Starting from 18 previously identified peptides41,45, we expanded the repertoire to 48 human sero-reactive C. pneumoniae peptide antigens by testing with human sera. Using optimal subsets of these 48 peptide antigens, we established simple, yet highly specific and sensitive peptide ELISAs for detection of anti-C. pneumoniae antibodies.
Results
Reactivities of C. pneumoniae-specific peptide antigens with human serum pools
For identification of C. pneumoniae-specific peptide antigens, a panel of 153 C. pneumoniae peptide antigens with high predicted score for B-cell epitopes41,42,43 was initially tested with 4 human serum sub-pools of 185 donors. With combined signal intensities (average of 4 OD values) with the 4 human serum pools, all 153 C. pneumoniae peptides were initially ranked and a set of 48 top ranked peptides from 12 C. pneumoniae immunodominant proteins was selected for further evaluation (Table 1). Peptide antigens with the highest-ranked-reactivities were identified from C. pneumoniae IncA/IncCT119, followed by Pmp6G/I, Pmp21D, OmpA/MOMP, YopC/GspD, CT618/IncCT618, Pmp11G/I, CT529/IncCT529, Pmp2G/I, YwbM/CPn0677, CrpA/CT442, and PdhC (Table 1 and see Supplementary Table S1).
In confirming these 48 Cpn peptide antigens with mouse anti-C. pneumoniae antisera41,45, only a limited set of 14 C. pneumoniae peptides from 5 proteins (IncA, Pmp21D, OmpA, CT618, and CT529; Table 1) showed strong reactivities. Overall, the natural human-hosts of C. pneumoniae produced antibody responses against a wide range of proteins as well as multiple protein regions of single proteins (Table 1), compared to the much more limited response of the non-natural murine-host (Table 1). This finding is in agreement with our previous report that human-hosts produce antibody responses against wide-spectrum proteins of C. trachomatis than murine-hosts45,46.
Sequence conservation of the Cpn B-cell epitopes within Chlamydia spp. and C. pneumoniae strains
The majority of these 48 peptide antigens, except for CpnOmpA (≤81% sequence identity), are highly divergent from other Chlamydia spp. (≤40% identity, Table 1), indicating C. pneumoniae specificity41,43. Importantly, anti-C. trachomatis or C. psittaci antisera did not cross-react with these peptide antigens, with exception of CpnOmpA_309–324 peptide that produced very low cross-reactivities with mouse anti-C. trachomatis and C. psittaci antisera (Table 1). Additionally, these peptide antigen sequences were highly conserved within 6 strains of C. pneumoniae (94–100% sequence identity among CWL029, TW-183, AR39, J138, B21, and LPCoLN; Table 1), with exception of the CpnCrpA (≥71% identity), IncA (≥80%), and OmpA (≥88%). These results indicate that a combination of these Cpn peptide antigens will detect host antibody responses against any of the C. pneumoniae strains.
Specificity confirmation of C. pneumoniae peptide mixes
In an approach to simplify serological testing by use of mixed rather than multiple individual peptide antigens, we tested 13 mixes of subsets of the 48 Cpn peptides (5–48 peptides/mix; see Supplementary Fig. S2) with pools of anti-Chlamydia mouse sera and confirmed highly specific reactivities (see Supplementary Table S3). Out of 13 Cpn peptide mixes, 12 mixes were reactive with mouse anti-C. pneumoniae antisera, and none of them showed cross-reactivity with anti-C. trachomatis and anti-C. psittaci antisera (Table S3). Only one mix (CpnMix B10) was completely non-reactive with anti-C. pneumoniae mouse antisera (Table S3), which was expected given that all of the component Pmp6G/I peptides were not reactive with mouse anti-C. pneumoniae sera (Table 1). As expected, the reactivity magnitudes of these Cpn mixes were largely influenced by the number and signal strength of individual component peptide antigens that were reactive with mouse anti-C. pneumoniae sera (Tables 1, S3). Overall, the Cpn peptide mixes showed high specificity similar to single peptide antigens, albeit the signal magnitude of mixed peptide assays was lower than that of the strongest component individual peptides, analogous to the earlier reported reactivities of C. trachomatis peptide mixes47.
Anti-C. pneumoniae IgG antibodies detected by 29 different assays
To identify anti-C. pneumoniae antibody-positive and -negative sera, a panel of 185 human blood donor sera was tested with C. pneumoniae IgG antibody detection assays of three categories (Fig. 1, and see Supplementary Fig. S4): (i) 13 Cpn peptide mixes prepared by mixing 5–48 Cpn peptide antigens (A5-M48; Fig. S2, Table S3); (ii) 12 individual strongly reactive Cpn peptide antigens from 8 C. pneumoniae proteins (Table 1, Fig. S4); and (iii) 4 commercial C. pneumoniae IgG antibody ELISAs based on (A) Cpn EB antigen (Savyon), (B) Cpn OMC antigen (Serion), (C) lysate antigen of cell-culture propagated Cpn (Euroimmun), and (D) chlamydial LPS-free proprietary purified Cpn antigen (Medac). In the absence of a definitive standard for anti-C. pneumoniae antibodies, the initial composite reference standard 1 (CRS1) was constructed from the sum of the squared OD values of each serum (Figs 1. S4). The equally weighted consensus of the three assay categories resulted in 144 positive and 41 negative sera (77.8% anti-C. pneumoniae antibody prevalence; Figs 1, S4).
Anti-C. pneumoniae IgG antibodies detected by 29 assays. To identify sets of anti-C. pneumoniae antibody-positive and -negative sera, a panel of 185 blood donor sera was analyzed with 13 mixed and 12 individual Cpn peptide antigen assays, and 4 commercial C. pneumoniae ELISAs (details shown in Fig. S4). Analyses in the first three columns (C1-C3) are input for the preliminary composite reference standard 1 (CRS1) in C4. For each serum, the sum of the squared OD values of the individual assays in each category was determined. Grey indicates antibody positivity among the 95 highest reactive sera in combined scoring of the assay categories. Cutoff-independently, the 95 top-ranked reactive sera were specified as positive for anti-C. pneumoniae antibodies, matching the mean 51.4% anti-C. pneumoniae IgG antibody prevalence in the study population determined by 4 commercial ELISAs. Using a 80.5% specificity cutoff based on CRS1, quantitative reactivity scores for assay categories C5-C7 were determined. These scores were derived from the sum of quartile positive reactivities of all component assays of each category (Fig. S4). The total score of all 29 individual assays for each serum, shown in the right bar graph, was derived from the sum of all individual assay scores. For assay performance evaluation, CRS2, CRS3, and CRS4 (C8-C10) were derived. For CRS2, 154 sera with the highest combined score in the 29 assays were considered antibody-positive (red & grey), and 31 sera with the lowest scores were considered antibody-negative (green). CRS3 was derived from CRS2 after exclusion of 59 borderline -positive sera (grey). CRS4 was constructed as peptide assay-based standard from the combined scores of 13 mixed and 12 individual peptide assays (without the 4 commercial ELISAs). The 95 sera with the highest score were considered antibody-positive (red), 40 sera with the lowest scores were considered antibody-negative (green), and 50 weakly-positive sera were excluded.
Average assay sensitivities relative to composite reference standards for human anti-C. pneumoniae IgG status
Two composite reference standards (CRS2 and CRS3) derived from the 29 individual assays were used to evaluate assay performances. CRS2 was constructed from the total score in the 29 component assays (Figs 1, S4), resulting in 154 positive and 31 negative sera for anti-C. pneumoniae antibodies (83.2% prevalence). At 98%, 95%, 90%, 85%, and 80% specificity cutoffs, Cpn Mix F12 achieved the highest average sensitivity (74%), followed by Cpn Mix K24 and J20 (73–72% sensitivity; Table 2). Compared to the mixed peptide assays, sensitivities with single peptide antigens were lower, with 55–21% average sensitivities for the 8 top-ranked individual peptides (Table 2). Combining the results of the 8 peptides substantially improved assay performance to an average of 72% sensitivity at the same 98–80% specificity cutoffs (Table 2). Thus, the commercial ELISAs achieved lower sensitivity (56-68% sensitivity; Table 2) than top-performing Cpn Mixes (F12, K24, or J20) or the combined 8 individual peptide antigens.
In CRS2 evaluation, the performance of peptide assays for anti-C. pneumoniae antibodies was lower than those of the previously reported C. trachomatis peptide antigen assays46. This discrepancy may be due to poorly defined antibody positivity in CRS2 that has a high 83.2% prevalence of anti-C. pneumoniae IgG (Figs 1, S4). In the commercial ELISAs, we determined in average a 51.4% anti-C. pneumoniae IgG prevalence. This translates into 95 positives out of 185 sera. To improve the quality of the reference standard CRS2, we constructed CRS3 from the 95 strongest reactive sera (positive) and the same set of 31 weakest reactive (negative) sera, but excluded ambiguous 59 weakly reactive sera (borderline) that were included in CRS2 (Figs 1, S4). Comparison of average sensitivities demonstrated that relative to CRS3 all assays performed substantially better than to CRS2 (Table 2). This suggests that CRS3 evaluation may represent a realistic epidemiological situation in which individuals with high anti-C. pneumoniae antibody levels are contrasted with individuals with baseline serum reactivity (antibody-negative).
Distribution of anti-C. trachomatis antibodies in anti-C. pneumoniae antibody-positive and -negative sera
Cross-reactivity for detection of Chlamydia species-specific antibodies has been widely reported in serological assays with classical chlamydial antigens (such as EB, EB lysate, OMC, or OmpA, OmcB/Omp2 recombinant proteins) that are used in commercial ELISAs or in the gold standard microimmunofluorescence (MIF) tests19,20,21,22,23,24,25,26,27,31,32,33,34,35,36,37,38,39,40. To determine potential cross-reactivities of Cpn and Ctr antigens, we analyzed the distribution of anti-C. trachomatis and anti-C. pneumoniae antibody positivity in the 185 human sera for peptide assays and commercial ELISAs (Fig. 2, and see Supplementary Fig. S5). Instead of using a single assay for each test category, we constructed a consensus for anti-C. trachomatis and anti-C. pneumoniae antibody status of 4–6 assays each in the two categories of mixed peptide assays and commercial ELISAs (Figs 2, S5). An assays category-dependent distribution bias of anti-C. trachomatis antibodies among Cpn-positive and -negative sera would identify and quantify potential Ctr-Cpn cross-reactivity.
Distribution of anti-C. pneumoniae and anti-C. trachomatis antibodies in 185 human sera. To evaluate bias in distribution of anti-C. trachomatis antibodies among anti-C. pneumoniae antibody-positive and -negative sera, the 185 blood donor sera were binomially separated by antibody status (Pos or Neg) in Cpn- and Ctr Mix peptide assays and commercial ELISAs. To derive this antibody status (C1-C4), the consensus of 4–6 component assay results for each of the 4 assay categories was used (details in Fig. S5). For this consensus, any serum that was positive in any component assay of a category was considered antibody-positive (black), and antibody-negative if all component assays were negative (white). Column C1 indicates the consensus of 6 mixed peptide anti-C. pneumoniae antibody assays (Fig. S5). C2 shows the consensus antibody status of the four commercial anti-Cpn IgG ELISAs, with manufacturer defined cutoffs. The consensus antibody status of Ctr mixed peptide assays at previously described cutoffs47 is shown in C3, and in C4 the Ctr commercial ELISAs with manufacturer defined cutoffs. In C5, red or green indicates double positive or double-negative sera, respectively, for anti-C. pneumoniae antibodies in Cpn mixed peptide assays as well as Cpn cELISAs. Single-positive sera in the Cpn mixed peptide assays are shown by pink, and single-positive sera in cELISAs by yellow (C5). The final column (C6) indicates CRS2 status as shown in Figs 1 and S4.
With Ctr and Cpn mixed peptide assays, Ctr-positive reactivities were 16% lower in Cpn antibody-positive sera than in -negative sera (P = 0.187, two-tailed Fisher Exact test; Table 3). With commercial Ctr and Cpn ELISAs, Ctr seroreactivity was 54% higher in Cpn-positive than in Cpn-negative sera in Cpn commercial ELISAs (P = 0.005; Table 3). Thus, compared to the mixed peptide assays, commercial ELISAs showed 70% (16% + 54%) increased anti-C. trachomatis antibody-positivity in anti-C. pneumoniae antibody-positive over -negative sera (Table 3). This indicates a profound specificity (cross-reactivity) problem of commercial Cpn and Ctr ELISAs, but not of mixed peptide assays. In comparison to seroreactivity against any Chlamydia spp. (determined by a Chlamydia LPS ELISA; Figs 2, S5, and see Supplementary Table S6), the Cpn commercial ELISAs showed 47% (42% + 5%) excess positive reactivity over the Cpn mixed peptide assays in anti-chlamydial LPS antibody-positive over -negative sera (P = 0.005). Thus, differentiation of human anti-C. pneumoniae from anti-C. trachomatis or anti-Chlamydia spp. antibodies is severely compromised by cross-reactivity of commercial ELISAs, but high specificity is achieved by Cpn and Ctr mixed peptide assays.
Cross-reactivities of Cpn and Ctr commercial ELISAs
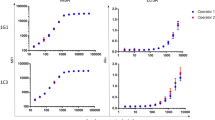
To further evaluate cross-reactivities of Cpn and Ctr commercial ELISA antigens, a panel of Chlamydia species-specific pooled and individual mouse antisera were tested with two Cpn and two Ctr commercial ELISAs in which the anti-human IgG conjugate was substituted by an anti-mouse IgG conjugate (Fig. 3). The Savyon (Cpn EB) and Serion (Cpn OMC) ELISA antigens showed very high cross-reactivities with mouse pooled sera raised against each of the 11 Chlamydia spp. (Fig. 3). Additional testing of these Cpn ELISAs with individual anti-C. psittaci and anti-C. trachomatis sera showed high cross-reactivity, with ~25-fold higher OD over reactivities with naïve mouse sera (Fig. 3). These results conclusively confirm very high cross-reactivity of Cpn commercial ELISA antigens (Savyon and Serion).
Reactivities of C. pneumoniae and C. trachomatis commercial ELISA antigens with anti-Chlamydia species-specific mouse sera. The anti-human IgG conjugates of the ELISA kits were substituted by an anti-mouse IgG conjugate. Reactivities of C. pneumoniae and C. trachomatis commercial ELISA antigens with homologous antisera that are consistent with expected reactivities are shown by green bars, and cross-reactivities with non-homologous antisera are shown by blue or red bars. Assay backgrounds determined with naïve sera are shown by black bars. Mice were immunized against each of 11 Chlamydia spp.41,45, and used as individual or pools of individual sera against a single Chlamydia spp. Cab indicates a serum pool raised against C. abortus; Cps, C. psittaci; Cca, C. caviae; Cfe, C. felis; Cav, C. avium; Cga, C. gallinacea; Cpe, C. pecorum; Cpn, C. pneumoniae; Cmu, C. muridarum; Csu, C. suis; and Ctr, C. trachomatis.
In evaluation of potential cross-reactivity of two Ctr commercial ELISAs, the GenWay (Ctr EB) antigen also showed very high cross-reactivities with 15 anti-C. pneumoniae and 8 anti-C. psittaci individual sera, similar to the Cpn EB antigen-based Savyon ELISA (Fig. 3). However, the Serion Ctr-specific OmpA fragment antigen did not show cross-reactivity, indicating that this C. trachomatis-specific antigen captured only anti-C. trachomatis antibodies.
High specificity of Cpn and Ctr peptide antigens
To evaluate potential cross-reactivities of Cpn and Ctr peptide antigens with the panel of individual mouse sera raised against C. pneumoniae and C. trachomatis, we similarly tested with Cpn and Ctr peptide antigens (see Supplementary Fig. S7). Cpn peptide assays showed strong reactivity with all 15 mouse sera raised against C. pneumoniae and did not cross-react with any of the 15 mouse sera raised against anti-C. trachomatis (Fig. S7). Additionally, C. trachomatis-specific peptide mixture (CtrMix1) reacted with anti-C. trachomatis sera but did not react with any of the 15 mouse anti-C. pneumoniae sera (Fig. S7). Taken together, these results confirm highly specific reactivity of Cpn or Ctr peptide antigens when they are tested either as single antigens or as mixture of multiple peptide antigens.
Assay sensitivities for detection of anti-C. pneumoniae IgG in comparison to a peptide antigen-based standard
Given the high cross-reactivities of commercial Cpn ELISAs (Fig. 3 and Table 3), inclusion of these assays in a combined reference standards, such as in CRS2 and CRS3 is inadvisable. To obtain a more reliable standard, CRS4 was derived only from the 13 mixed and 12 individual peptide assays, without the 4 commercial ELISAs (Figs 1, S4). A total of 95 sera with the highest sum score in these 25 peptide assays was considered positive and 40 sera with the lowest score were considered negative (135 sera in total), and the remaining 50 weakly positive borderline sera were excluded.
Using CRS4 for anti-C. pneumoniae IgG status (see Supplementary Table S8), we evaluated assay sensitivities at 98%, 95%, 90%, 85%, and 80% specificity cutoffs. At 80% specificity cutoff, the top performing mixed peptide assay with Cpn Mix F12 (Table S3) achieved 96% sensitivity, but even at high stringency 98% specificity still achieved 87% sensitivity. The combined result of all 8 top-ranked individual peptide antigens achieved 90-71% sensitivity at these 80–98% specificity cutoffs. In contrast, even the top performing Medac ELISA among the commercial Cpn ELISAs achieved only 71-35% sensitivity, and the poorly performing Euroimmun ELISA only 56-12% sensitivity.
Averaging sensitivity data at the 5 specificity cutoffs, Cpn Mix F12 achieved the highest average sensitivity (93%), followed by Cpn Mix K24 and Cpn Mix J20 (Table 4). Combining the results of the 8 top-ranked individual peptides achieved 82% average sensitivity (Table 4). In contrast, the commercial ELISAs performed significantly lower (56-35% sensitivity) than even the lowest performing peptide assay (P ≤ 0.001, two-tailed Fisher exact test, Table 4).
Demographic distribution of anti-C. pneumoniae and anti-C. trachomatis antibodies
To evaluate assay performance and the consequences of different cross-reactivity in sero-epidemiology of human chlamydial infection, we analyzed the demographic distribution of anti-C. pneumoniae and anti-C. trachomatis antibodies in the 185 human sera, determined by both mixed peptide assays or commercial ELISAs (Figs 2, S5, Table 5). To obtain a reliable status of each serum for anti-C. trachomatis and anti-C. pneumoniae antibodies, we used the consensus of 4–6 assays for both peptide assay and commercial ELISA categories as described in Figs 2 and S5. Compared to male donors, female donors had only a 4.9% higher anti-C. pneumoniae antibody frequency in the Cpn mixed peptide assays (P = 0.53, Fisher Exact test), and 7.9% higher in the Cpn commercial ELISAs (P = 0.24). However, females had an 18% higher frequency of anti-C. trachomatis antibodies in the Ctr mixed peptide assays compared to male donors (P = 0.013; Table 5), but only 4.5% higher frequency in the Ctr commercial ELISAs (P = 0.55). In both mixed peptide assays as well as commercial ELISAs, Caucasian males had lower frequencies anti-C. pneumoniae and anti-C. trachomatis antibodies compared to African American and Mixed ethnic origin males (Table 5). Overall, the results in Table 5 show that the commercial Ctr ELISAs underestimated anti-C. trachomatis antibody prevalence, particularly in female individuals (Figs 2, S5; 46). In contrast, the commercial Cpn ELISAs slightly overestimated the prevalence of anti-C. pneumoniae antibodies, but frequently misclassified the anti-C. pneumoniae antibody status (Figs 2, S5, and Table 5).
Discussion
In this study, we established simple, yet highly specific and sensitive peptide ELISA methods for detection of anti-C. pneumoniae antibodies. Assays using mixtures of 12–48 strongly reactive C. pneumoniae-specific peptide antigens (Cpn Mix F12, K24, J20, M48, or G12) achieved on average 93-86% sensitivity at 80–98% specificity (Tables 4, S8). This result from a single well is better than the 82% sensitivity obtained by combining the results of the individually tested 8 most reactive peptides (Table 4). Highest assay sensitivity is achieved by mixing C. pneumoniae peptide antigens from several strongly immunodominant proteins rather than multiple antigens from the same protein or from weakly immunodominant proteins (Cpn Mix F12 vs B10; Figs 1, S2, S4, Table 4). High specificity (non-cross reactivity) of the assays is the direct consequence of using peptide antigens that are highly specific for C. pneumoniae, and not conserved in other Chlamydia spp. (usually ≤ 40% sequence identity; Table 1). For most accurate measurement of the anti-C. pneumoniae antibody status, detection of reactivity to multiple B-cell epitopes in the peptide mixtures mimics reactivity with a complex chlamydial antigen, but with a profound specificity and sensitivity advantage (Tables 4, S8). Thus, these assays simultaneously determine anti-C. pneumoniae antibodies produced against a wide-spectrum of C. pneumoniae antigens (Tables 1, S1), including EB outer-membrane structural proteins (OmpA, CrpA, PmpD, Pmp6G/I, Pmp11G/I, Pmp2G/I) as well as inclusion membrane proteins (IncA, IncCT618, IncCT529) and other proteins (YopC, YwbM, PdhC). Importantly, given the severe cross-reactivity problem of current immunoassays (31–40; Table 3 and Fig. 3), these assays fill the void of immunoassays for specific detection of anti-C. pneumoniae antibodies. For specific and sensitive detection of anti-C. pneumoniae antibodies, we recommend use of the Cpn Mix F12 peptide antigens that achieved the highest performance (e.g. 91% sensitivity at 95% specificity; Table S8). This antigen mixture for single-well ELISA is composed of 12 highly specific and strongly reactive peptide antigens from 8 immunodominant C. pneumoniae proteins.
Cross-reactivity between antigens used in detection of anti-chlamydial antibodies has plagued the field since inception19,20,21,22,23,24,25,26,27,31,32,33,34,35,36,37,38,39,40. In agreement with these reports, Cpn EB or OMC antigen-based commercial ELISAs exhibited profound cross-reactivity with other Chlamydia spp. when tested with mono-specific anti-chlamydial mouse sera (Fig. 3). Similarly, C. trachomatis EB antigen, but not recombinant C. trachomatis-specific OmpA antigen, showed cross-reactivity (Fig. 3). In addition, anti-C. pneumoniae and anti-C. trachomatis antibodies in the 185 human sera are not randomly distributed when detected by commercial ELISAs. Rather, Cpn antibody-positive sera show a highly significant 54% excess Ctr reactivity over Cpn antibody-negative sera (P = 0.005; Table 4). In contrast, anti-C. pneumoniae and anti-C. trachomatis antibodies determined by peptide assays are equally distributed (Table 4). These results strongly suggest that the increased Ctr-Cpn co-reactivities in commercial ELISAs are due to the high cross-reactivity of the antigens.
In the absence of any reliable standard for anti-C. pneumoniae antibody status, we evaluated the Cpn peptide assays by comparison to three composite reference standards (CRS2, CRS3, and CRS4) derived from 25–29 individual assays in 2–3 assay categories. The advantage of such CRS derived from multiple tests is relatively accurate assignment of antibody-negative status. Selection of reliably anti-C. pneumoniae antibody-negative sera is a critical requirement for assay performance evaluation, given that the ubiquitous nature of human C. pneumoniae infection has exposed virtually any adult person to C. pneumoniae. Results of the 29 individual assays (Figs 1, S4) show that almost all donors have been exposed to C. pneumoniae in their life-time. However, donor sera with low anti-C. pneumoniae reactivity are classified as antibody-negative for CRS2, CRS3, and CRS4 (Figs 1, S4).
A disadvantage of any CRS is the potential accumulation of false positives if one or more of component tests lack specificity, resulting in poor quality for the positive dataset. To minimize such artifacts, we did not use consensus-based standards CRS2 and CRS3. Rather, we derived CRS2 and CRS3 from the total score of all 29 assays for each serum (Figs 1, S4), by consideration of (i) frequency of positive tests, and (ii) reaction strength. Using CRS2 as reference, Cpn mixed and combined individual peptide assays showed higher assay performance than the 4 commercial Cpn IgG ELISAs (Table 2). The performance of all 29 assays improved when they were referenced against the more reliable CRS3, in which borderline reactive sera from CRS2 were excluded (Figs 1, S4).
Given the high cross-reactivity of commercial Cpn ELISAs with anti-C. trachomatis antibodies, the commercial Cpn IgG ELISAs rendered CRS2 and CRS3 unreliable for anti-C. pneumoniae antibody status. Therefore, results of the 4 commercial Cpn ELISAs were not included in a more reliable reference standard, CRS4, which was derived only from 13 mixed and 12 individual peptide assays. Compared to CRS4, the top-performing mixed peptide assays (Cpn Mix F12, K24, J20) achieved 88–84% sensitivity at 98% specificity. In contrast, the 4 commercial ELISAs achieved only 36-12% sensitivity. Acceptable assay specificity for these commercial Cpn ELISAs required a very high OD cutoff to avoid cross-reactivity, while this was not required for the highly specific peptide assay. When the assay cutoff was lowered to 80% specificity, commercial ELISAs achieved 71-56% sensitivities, still 25–40% lower than the 96% sensitivity of Cpn Mix F12 at this cutoff. These results confirm that the severe specificity problem of commercial Cpn ELISAs profoundly compromises sensitivity when the assay cutoff is set for acceptable specificity.
We observed a significantly higher prevalence of IgG antibodies against C. pneumoniae (83% in CRS2; Figs 1, S4) than against C. trachomatis (66% in Ctr peptide consensus; P = 0.0002, Fisher Exact test; Figs 2, S5). However, serum levels of these IgG antibodies were much higher against C. trachomatis, particularly in females, than against C. pneumoniae. In addition, short-lived IgG3 and IgA1&IgA2 antibodies against C. trachomatis were frequently detected, but not against C. pneumoniae (Figs 2, S5). Such dominance of short-lived antibody isotypes, indicative of recent C. trachomatis infection, is a reflection of the endemic and frequent C. trachomatis infections in the young and sexually active blood donor demographic (mean 22 year-old, range 18–38 years). In contrast, the dominance of highly prevalent, but low level long-lived anti-C. pneumoniae IgG antibodies suggests infrequent epidemic spread of C. pneumoniae infection at low-level endemic maintenance.
Ctr peptide assays, but not Cpn peptide assays, correlated with the Chlamydia LPS ELISA (Table S6). Given that anti-chlamydial LPS antibodies usually indicate recent chlamydial infection47, these results suggest that the Chlamydia LPS ELISA detected anti-LPS antibodies that had been elicited mainly by C. trachomatis, but not by C. pneumoniae. In contrast, both commercial Ctr and Cpn ELISAs correlated highly significantly with the Chlamydia LPS ELISA (Table S6). We attribute this Cpn positivity concomitant with LPS positivity to cross-reactivity of commercial Cpn ELISAs that detected not only anti-C. pneumoniae antibodies, but also anti-C. trachomatis antibodies (Fig. 3).
We observed for commercial Cpn ELISAs frequent, mainly false-positive misclassification of anti-C. pneumoniae antibody status (Figs 2, S5). We attribute this problem to the high cross-reactivity of the complex antigens that are used in commercial Cpn ELISAs. Conversely, we observed many false-negatives for commercial Ctr ELISAs (Figs 2, S5). In the case of OmpA antigen-based Ctr ELISAs, we attribute this to the inherently low-sensitivity of single-epitope ELISAs46, because such ELISAs fail to detect antibodies produced against non-OmpA proteins such as Pmps or Incs. In the case of EB-based complex antigens, we attribute the false-negatives to cross-reactivity that forces a high assay cutoff, resulting in low sensitivity. As overall consequence, in our test population of sexually active young adults the performance characteristics of commercial chlamydial ELISAs result in slightly overestimated C. pneumoniae prevalence due to false-positive misclassifications, and severely underestimated C. trachomatis prevalence due to frequent false-negatives (Table 5). More broadly, antibody ELISAs using complex antigens of any Chlamydia spp. will be relatively specific for the endemic chlamydial species in a given host (i.e. C. trachomatis in sexually active humans). In turn, the high frequency of antibodies against the most prevalent chlamydial species will strongly compromise antibody detection against low-prevalence Chlamydia spp. infection (e.g., C. pneumoniae or C. psittaci).
In terms of antigen specificity of anti-chlamydial antibodies, we observed an immunodominance of reticulate body antigens (IncA) for C. pneumoniae, in contrast to the immundominance of elementary body antigens (OmpA) for C. trachomatis46. This may explain the low sensitivity and cross-reactivity of the EB-based MIF assay for C. pneumoniae compared to C. trachomatis19,20,32. Thus, inherent C. pneumoniae antigenic properties (immunodominance of IncA over OmpA, cross-reactivity of complex Cpn antigens) may have prevented accurate C. pneumoniae serology. Hence, identification of 48 highly reactive peptide antigens from 12 immunodominant C. pneumoniae proteins is an important discovery that will enable specific detection of anti-C. pneumoniae antibodies, particularly when used in highly parallel multi-antigen microarray format52,53,54.
With this study, we conclude a series of investigations to establish robust peptide ELISA methodology for Chlamydia species-specific human serology. After defining sets of highly, but specifically reactive peptide antigens41,45, we have validated assays using individual and mixed peptides for detection of antibodies against both C. pneumoniae and C. trachomatis46,47. With the use of fully synthetic peptide antigens in simple ELISA formats, these serological assays will now be within reach of any laboratory. Compared to commercially available Cpn and Ctr ELISAs, these peptide assays can provide vastly improved assay sensitivity with unprecedented specificity for simultaneous detection and differentiation of anti-C. pneumoniae and anti-C. trachomatis antibodies, and thus improved serodiagnosis of human Chlamydia spp. infections.
Materials and Methods
C. pneumoniae-specific peptide antigens
Suitable B cell epitope regions for identification of C. pneumoniae species-specific peptide antigens had been identified before within polymorphic regions in Chlamydia spp. protein alignments41,45. These were further subjected to in silico B cell epitope identification42,43, and a total of 176 C. pneumoniae species-specific peptide antigens of 20 immunodominant proteins were selected for screening that were highly divergent from other Chlamydia spp., but conserved within C. pneumoniae41,45. Peptide antigens were chemically synthesized with N-terminal biotin followed by a serine-glycine-serine-glycine spacer41.
Random peptides as negative controls
For determination of assay background with non-specific peptide antigens, 4 random peptides (23AA long) were generated with the RandSeq tool of ExPASy. A Blast-search confirmed that these AA sequences were not conserved (≤38% sequence identity) with any chlamydial or non-chlamydial protein in the NCBI database.
C. pneumoniae-specific peptide antigen mixtures
A total of 13 mixtures of C. pneumoniae peptide antigens (A5-M48; Fig. S2, Table S3) were prepared. The letter in the alphanumeric CpnMix designation indicates the sequential order and the number indicates the total number of constituent individual peptide antigens.
Human sera
For selection of anti-C. pneumoniae antibody-positive and -negative sera (Figs 1, S4), we screened the sera of 95 women and 90 men47. The sera originated from healthy blood donors of African American, Caucasian, Hispanic, Asian, or mixed race, and the age of all study subjects ranged from 18–38 years, with an average of 22 years. These sera were collected in the US from blood donors in FDA-licensed and registered collection facilities (Commercial supplier: BioIVT North America & Asia Pacific, Westbury, NY; https://www.bioivt.com/about/quality-assurance/). Sample collection at all donation centers was approved by the Institutional and Review Boards of BioIVT and collaborators. In accordance with the relevant guidelines and regulations, informed consent was obtained and all sera were anonymized.
Mouse sera
Chlamydia monospecies-specific mouse sera for each of the 11 Chlamydia spp. were used to confirm specificity of peptide antigens for detection of species-specific antibodies41,45. Preparation and pooling of these sera have been described in detail by Rahman et al.41,45. All animal experimental protocols were approved by the Institutional Animal Care and Use Committee at Auburn University under protocol numbers 2011-1901 and 2014–2468 and performed in accordance with the relevant guidelines and regulations.
Determination of anti-C. pneumoniae IgG with four commercial ELISAs
All 185 study sera were tested for anti-C. pneumoniae IgG with four commercial ELISAs according to manufacturers’ instructions: (i) Savyon C. pneumoniae elementary body (EB) antigen (Savyon Diagnostics Ltd., Ashdod, Israel), (ii) Serion C. pneumoniae outer membrane complex (OMC) antigen (Serion Immunologics, Würzburg, Germany), (iii) Euroimmun lysate antigen of cell-culture propagated C. pneumoniae (Euroimmun AG, Lübeck, Germany), and (iv) Medac chlamydial LPS-free proprietary purified Cpn antigen (Medac GmbH, Wedel, Germany). In addition, study sera were also ELISA-tested for IgG against Chlamydia lipopolysaccharide (LPS) by using recombinant chlamydial LPS antigen (Medac GmbH, Wedel, Germany) that detects antibodies against all Chlamydia spp. due to genus-wide conservation of chlamydial LPS (Chl LPS).
Pooling of human sera
Individual sera were first ranked by reactivity with 4 commercial Cpn ELISAs, and 4 serum sub-pools were prepared of (i) 40 high-reactivity male donor sera, (ii) 45 low-reactivity male donor sera, (iii) 45 high-reactivity female donor sera, and (iv) 50 low-reactivity female donor sera.
Selection of 48 C. pneumoniae-specific peptide antigens
Out of the 176 tested C. pneumoniae-specific predicted B-cell epitopes, a total of 48 peptide antigens from 12 immunodominant proteins of C. pneumoniae were initially selected based on reactivity rank with human serum pools (Table 1). Importantly, the sequences of these C. pneumoniae peptides are highly evolutionarily divergent from C. trachomatis (<45% sequence identity; Table 1) and have only a marginal probability (~0.02) of cross-reactivity with antibodies raised against non-C. pneumoniae Chlamydia spp.41,43. In addition, the sequences of these C. pneumoniae peptide antigens are highly conserved within C. pneumoniae strains (80–100% sequence identity). Previously, we have determined that identities of 45%, 60%, 75%, and 90% between two peptides translate into 0.02, 0.12, 0.46, and 0.84, respectively, probabilities of antibody cross-reactivity between these peptides41,43. Sequences with identities below 40% typically cannot be aligned correctly and the probability of cross-reactivity is less than 1%41,43.
Chemiluminescent and colorimetric ELISAs with C. pneumoniae peptide antigens
Primary human and mouse antibodies were detected with horseradish peroxidase-conjugated secondary antibodies in ELISAs as described before41,42,43,44,45,46,47. Polyclonal rabbit anti-mouse or anti-human IgG-h + l cross-adsorbed antibody-HRP conjugates were obtained from Bethyl Laboratories, Inc., Montgomery, TX, USA (Cat# A90-217P and A80-218P). Monoclonal mouse anti-human antibody conjugates were obtained from Southern Biotech, Birmingham, AL, USA: IgG3-HRP (9210-05), IgA1-HRP (B3506B4) and IgA2-HRP (9140-05). For determination of anti-C. pneumoniae antibody status, all 185 human sera were screened by use of 13 mixed and 12 individual C. pneumoniae peptide antigens, in addition to the 4 commercial Cpn ELISAs (Figs 1, S4). For cross-reactivity testing of commercial Cpn and Ctr ELISA antigens within Chlamydia spp, by use of anti-Chlamydia spp. mouse sera, original anti-human IgG conjugates were replaced with polyclonal anti-mouse IgG(h + l) conjugate.
Composite reference standards (CRS) for anti-C. pneumoniae antibody status
In the absence of any reliable standard for anti-C. pneumoniae antibody status, for assay cutoff selection we used CRS1 derived from 29 individual assays (13 mixed and 12 individual Cpn peptide antigen assays, and 4 commercial C. pneumoniae ELISAs; Figs 1, S4). For chemiluminescence peptide ELISAs, inter-assay coefficient of variation (CV) was approximately ~13%, and intra-assay CV ~9%. For colorimetric peptide ELISAs, inter-assay CV was ~6% and intra-assay CV ~4%. Background-corrected signals (RLU-CV or OD - CV) were used for mixed and individual peptide antigen assays47, but OD data for standard ELISAs were processed as described by the manufacturers. The mean OD value of each individual test for the 185 sera was adjusted to the mean OD value of all 29 individual tests. In each assay category, a total of 95 sera was considered antibody-positive based on reactivity rank determined by the sum of the squared OD values of the constituent assays in each category. These 95/185 positive sera matched the average 51.4% anti-C. pneumoniae antibody prevalence determined by the 4 commercial ELISAs. CRS1 was derived as the consensus of the 3 equally weighted assay categories (Figs 1, S4).
For assay performance evaluation, we used 3 additional composite reference standards (CRS2, CRS3, and CRS4). CRS2 was derived from reactivity scores in all 29 individual assays. Relative to CRS1, an 80.5% specificity cutoff for each assay was chosen for determination of anti-C. pneumoniae positive and negative sera (Figs 1, S4). Depending on reactivity strength, positive sera were scored from +4, +3, +2, to +1, and negative sera were scored 0. The total score of all 29 individual assays for each serum, shown in the right bar graph in Figs 1 and S4, was derived from the sum of all individual assay scores. For CRS2, a total of 154 sera with the highest combined score in the 29 assays was considered antibody-positive, and 31 sera with the lowest scores were considered antibody-negative. CRS3 was derived from CRS2 after exclusion of 59 weakly-positive borderline sera. CRS4 was derived solely from reactivities in all peptide assays without the 4 commercial ELISAs. The 95 sera with the highest score were considered antibody-positive, 40 sera with the lowest scores were considered antibody-negative, and 50 weakly-positive sera were excluded.
Consensus of anti-C. pneumoniae or anti-C. trachomatis antibody status
For determination of the distribution bias of anti-C. trachomatis antibodies in dependence of anti-C. pneumoniae antibodies in the sera, most reliable consensus antibody status in Cpn and Ctr peptide assays and commercial ELISAs were determined. Any serum that was positive in any component assay of a consensus category was considered antibody-positive, and antibody-negative if all component assays were negative (Figs 2, S5). The component assays for these 4 consensus are as follows (Figs 2, S5): (i) 6 Cpn mixed peptide assays with the 4 top-performing Cpn mixes (IgG reactivities of Cpn Mix F12, K24, J20, G12, and IgG3 and IgA reactivities of J20; Table 3); (ii) 4 Cpn commercial IgG ELISAs; (iii) 6 Ctr mixed peptide assays with 2 top-performing Ctr mixes previously reported (IgG, IgG3, and IgA reactivities of CtrMix1 and Ctr Mix2; 47); and (iv) 4 Ctr commercial IgG ELISAs46.
Statistical analyses and receiver operating characteristic (ROC) curves for assay performance
Statistical analyses were performed and graphical outputs generated by the software packages Microsoft Excel 2016 (Microsoft Corporation, Redmond, Washington) or Statistica 7.1 (Statsoft, Tulsa, Oklahoma, USA). ROC curves (Tables 2, 4 and S8) were plotted and area under the curve (AUC) determined as described before46,47,55. Sensitivities at 98%, 95%, 90%, 85%, and 80% specificities were calculated from ROC curves. Antibody detection frequencies were compared by two-tailed Fisher exact test.
Data availability
All data of this study are included in this published article and its Supplementary Information File.
References
Kuo, C. C., Stephens, R. S., Bavoil, P. M., Kaltenboeck, B. & Genus I. Chlamydia Jones, Rake and Stearns 1945, 55AL, 4, 846-65. In: Bergey’s Manual of Systematic Bacteriology, 2nd edition. (Springer, NY, 2011).
Hammerschlag, M. R. Pneumonia due to Chlamydia pneumoniae in children: epidemiology, diagnosis, and treatment. Pediatr. Pulmonol. 36, 384–90 (2003).
Pedersen, L. N., Herrmann, B. & Møller, J. K. Typing Chlamydia trachomatis: from egg yolk to nanotechnology. FEMS Immunol. Med. Microbiol. 55, 120–30 (2009).
Leonard, C. A. & Borel, N. Chronic chlamydial diseases: from atherosclerosis to urogenital infections. Curr. Clin. Microbiol. Reports 1, 1–12 (2014).
Hulin, V. et al. Host preference and zoonotic potential of Chlamydia psittaci and C. gallinacea in poultry. Pathog. Dis. 73, 1–11 (2015).
Rodolakis, A. & Mohamad, K. Y. Zoonotic potential of Chlamydophila. Vet. Microbiol. 140, 382–391 (2010).
Chirgwin, K., Roblin, P. M., Gelling, M., Hammerschla, G. M. R. & Schachter, J. Infection with Chlamydia pneumoniae in Brooklyn. J. Infect. Dis. 163, 757–761 (1991).
Verkooyen, R. P. et al. Evaluation of PCR, culture, and serology for diagnosis of Chlamydia pneumoniae respiratory infections. J. Clin. Microbiol. 36, 2301–2307 (1998).
Pacheco, A. et al. Community acquired pneumonia caused by Chlamydia pneumoniae strain TWAR in chronic cardiopulmonary disease in the elderly. Respiration 58, 316–320 (1991).
Grayston, J. T. et al. Evidence that Chlamydia pneumoniae causes pneumonia and bronchitis. J. Infect. Dis. 168, 1231–1235 (1993).
Marton, A., Károlyi, A. & Szalka, A. Prevalence of Chlamydia pneumoniae antibodies in Hungary. Eur. J. Clin. Microbiol. Infect. Dis. 11, 139–142 (1992).
Freidank, H. M. & Brauer, D. Prevalence of antibodies to Chlamydia pneumoniae TWAR in a group of German medical students. J. Infect. 27, 89–93 (1993).
Wang, J. H. et al. Seroprevalence of Chlamydia pneumoniae in Taiwan. Scand. J. Infect. Dis. 25, 565–568 (1993).
Ben-Yaakov, M. et al. Prevalence of Chlamydia pneumoniae antibodies in patients with acute respiratory infections in Israel. J. Clin. Pathol. 47, 232–235 (1994).
Aldous, M. B., Grayston, J. T., Wang, S. P. & Foy, H. M. Seroepidemiology of Chlamydia pneumoniae TWAR infection in Seattle families, 1966–1979. J. Infect. Dis. 166, 646–649 (1992).
Ekman, M. R. et al. Evaluation of serological methods in the diagnosis of Chlamydia pneumoniae pneumonia during an epidemic in Finland. Eur. J. Clin. Microbiol. Infect. Dis. 12, 756–760 (1993).
Karvonen, M., Tuomilehto, J., Pitkäniemi, J. & Saikku, P. The epidemic cycle of Chlamydia pneumoniae infection in eastern Finland, 1972–1987. Epidemiol. Infect. 110, 349–360 (1993).
Clemmons, N. S. et al. Outbreak of Chlamydia pneumoniae infections and X-ray-confirmed pneumonia in army trainees at Fort Leonard Wood, Missouri, 2014. Mil. Med. 184, e196–e199, https://doi.org/10.1093/milmed/usy402 (2019).
Peeling, R. W. et al. Chlamydia pneumoniae serology: interlaboratory variation in microimmunofluorescence assay results. J. Infect. Dis. 181, S426–29 (2000).
Kutlin, A., Roblin, P. M. & Hammerschlag, M. R. Antibody response to Chlamydia pneumoniae infection in children with respiratory illness. J. Infect. Dis. 177, 720–4 (1998).
Tuuminen, T., Palomäki, P. & Paavonen, J. The use of serologic tests for the diagnosis of chlamydial infections. J. Microbiol. Methods 42, 265–79 (2000).
Goldstein, E. J., Kumar, S. & Hammerschlag, M. R. Acute respiratory infection due to Chlamydia pneumoniae: current status of diagnostic methods. Clin. Infect. Dis. 44, 568–576 (2007).
Villegas, E., Sorlozano, A. & Gutierrez, J. Serological diagnosis of Chlamydia pneumoniae infection: limitations and perspectives. J. Med. Microbiol. 59, 1267–1274 (2010).
Puolakkainen, M. Laboratory diagnosis of persistent human chlamydial infection. Front. Cell. Infect. Microbiol. 3, 99 (2013).
Wang, S. P. & Grayston, J. T. Immunologic relationship between genital TRIC, lymphogranuloma venereum, and related organisms in a new microtiter indirect immunofluorescence test. Am. J. Ophthalmol. 70, 367–74 (1970).
Wang, S. P., Kuo, C. C. & Grayston, J. T. A simplified method for immunological typing of trachoma-inclusion conjunctivitis-lymphogranuloma venereum organisms. Infect. Immun. 7, 356–60 (1973).
Wang, S. P. & Grayston, J. T. Human serology in Chlamydia trachomatis infection with microimmunofluorescence. J. Infect. Dis. 130, 388–97 (1974).
Geisler, W. M. et al. Immunoglobulin-specific responses to Chlamydia elementary bodies in individuals with and at risk for genital chlamydial infection. J. Infect. Dis. 206, 1836–43 (2012).
Horner, P. J. et al. Chlamydia trachomatis Pgp3 antibody persists and correlates with self-reported infection and behavioural risks in a blinded cohort study. PloS One 11, e0151497, https://doi.org/10.1371/journal.pone.0151497 (2016).
Woodhall, S. C. et al. Chlamydia trachomatis Pgp3 antibody population seroprevalence before and during an era of widespread opportunistic Chlamydia screening in England (1994–2012). PloS One 12, e0152810, https://doi.org/10.1371/journal.pone.0152810 (2017).
Haralambieva, I. et al. Cross-reaction between the genus-specific lipopolysaccharide antigen of Chlamydia spp. and the lipopolysaccharides of Porphyromonas gingivalis, Escherichia coli O119 and Salmonella newington: Implications for diagnosis. Diagn. Microbiol. Infect. Dis. 41, 99–106 (2001).
Campbell, L. A., Kuo, C. C. & Grayston, J. T. Structural and antigenic analysis of Chlamydia pneumoniae. Infect. Immun. 58, 93–7 (1990).
Bas, S., Muzzin, P. & Vischer, T. L. Chlamydia trachomatis serology: diagnostic value of outer membrane protein 2 compared with that of other antigens. J. Clin. Microbiol. 39, 4082–4085 (2001).
Ozanne, G. & Lefebvre, J. Specificity of the microimmunofluorescence assay for the serodiagnosis of Chlamydia pneumoniae infections. Can. J. Microbiol. 38, 1185–89 (1992).
Wong, Y. K., Sueur, J. M., Fall, C. H., Orfila, J. & Ward, M. E. The species specificity of the microimmunofluorescence antibody test and comparisons with a time resolved fluoroscopic immunoassay for measuring IgG antibodies against Chlamydia pneumoniae. J. Clin. Pathol. 52, 99–102 (1999).
Wagenvoort, J. H., Koumans, D. & van de Cruijs, M. How useful is the Chlamydia micro-immunofluorescence (MIF) test for the gynaecologist? Eur. J. Obstet. Gynecol. Reprod. Biol. 84, 13–15 (1999).
Kern, D. G., Neill, M. A. & Schachter, J. A seroepidemiologic study of Chlamydia pneumoniae in Rhode Island. Evidence of serologic cross-reactivity. Chest J. 104, 208–13 (1993).
Rabenau, H. F., Köhler, E., Peters, M., Doerr, H. W. & Weber, B. Low correlation of serology with detection of Chlamydia trachomatis by ligase chain reaction and antigen EIA. Infection 28, 97–102 (2000).
Bas, S. et al. Chlamydial serology: comparative diagnostic value of immunoblotting, microimmunofluorescence test, and immunoassays using different recombinant proteins as antigens. J. Clin. Microbiol. 39, 1368–1377 (2001).
Bas, S., Genevay, S., Schenkel, M. C. & Vischer, T. L. Importance of species‐specific antigens in the serodiagnosis of Chlamydia trachomatis reactive arthritis. Rheumatol. 41, 1017–20 (2002).
Rahman, K. S. et al. Defining species-specific immunodominant B cell epitopes for molecular serology of Chlamydia species. Clin. Vacc. Immunol. 22, 539–52 (2015).
Rahman, K. S., Chowdhury, E. U., Sachse, K. & Kaltenboeck, B. Inadequate reference datasets biased towards short non-epitopes confound B-cell epitope prediction. J. Biol. Chem. 291, 14585–99 (2016).
Rahman, K. S. In silico prediction and experimental confirmation of B-cell epitopes for molecular serology of Chlamydia spp. Doctoral Dissertation, Auburn University, Auburn, Alabama, USA, http://etd.auburn.edu/handle/10415/5029 (2016).
Sachse, K. et al. A novel synthetic peptide microarray assay detects Chlamydia species-specific antibodies in animal and human sera. Sci. Rep. 8, 4701, https://doi.org/10.1038/s41598-018-23118-7 (2018).
Rahman, K. S. et al. Discovery of human-specific immunodominant Chlamydia trachomatis B cell epitopes. mSphere 3, e00246–18, https://doi.org/10.1128/mSphere.00246-18 (2018).
Rahman, K. S. et al. Comprehensive molecular serology of human Chlamydia trachomatis infections by peptide enzyme-linked immunosorbent assays. mSphere 3, e00253–18, https://doi.org/10.1128/mSphere.00253-18 (2018).
Rahman, K. S. et al. Mixed Chlamydia trachomatis peptide antigens provide a specific and sensitive single-well colorimetric enzyme-linked immunosorbent assay for detection of human anti-C. trachomatis antibodies. mSphere 3, e00484–18, https://doi.org/10.1128/mSphere.00484-18 (2018).
Bunk, S. et al. Immunoproteomic identification and serological responses to novel Chlamydia pneumoniae antigens that are associated with persistent C. pneumoniae infections. J. Immunol. 180, 5490–98 (2008).
Yasui, Y. et al. Genomic screening for Chlamydophila pneumoniae‐specific antigens using serum samples from patients with primary infection. FEMS Microbiol. Lett. 329, 168–76 (2012).
Li, Z. et al. Characterization of fifty putative inclusion membrane proteins encoded in the Chlamydia trachomatis genome. Infect. Immun. 76, 2746–57 (2008).
Wang, J. et al. A genome-wide profiling of the humoral immune response to Chlamydia trachomatis infection reveals vaccine candidate antigens expressed in humans. J. Immunol. 185, 1670–80 (2010).
Carmona, S. J. et al. Towards high-throughput immunomics for infectious diseases: use of next-generation peptide microarrays for rapid discovery and mapping of antigenic determinants. Mol. Cell. Proteomics 14, 1871–84 (2015).
Lu, Y. et al. Chimeric peptide constructs comprising linear B-cell epitopes: application to the serodiagnosis of infectious diseases. Sci. Rep. 5, 13364 (2015).
Hansen, L. B., Buus, S. & Schafer-Nielsen, C. Identification and mapping of linear antibody epitopes in human serum albumin using high-density peptide arrays. PLoS One 8, e68902, https://doi.org/10.1371/journal.pone.0068902 (2013).
Eng, J. ROC analysis: web-based calculator for ROC curves. Baltimore: Johns Hopkins University. Updated March 19; 2014; cited Jan 4, 2017, http://www.jrocfit.org (2014).
Acknowledgements
This work was supported by Grant 106 of the Auburn University Molecular Diagnostics Laboratory.
Author information
Authors and Affiliations
Contributions
K.S.R. and B.K. conceived the experiments, K.S.R. conducted the experiments, K.S.R. and B.K. analyzed the results, and K.S.R. and B.K. wrote and reviewed the manuscript.
Corresponding author
Ethics declarations
Competing interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rahman, K.S., Kaltenboeck, B. Multi-peptide ELISAs overcome cross-reactivity and inadequate sensitivity of conventional Chlamydia pneumoniae serology. Sci Rep 9, 15078 (2019). https://doi.org/10.1038/s41598-019-51501-5
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-51501-5
- Springer Nature Limited