Abstract
Spring migrating sea trout juveniles can be classified as parr, pre-smolt or smolt based on body morphology and osmoregulatory capacity. In this respect, parr are assumed to be less prepared for a marine life and to have lower survival at sea than pre-smolts and smolts. However, the behaviour and survival of these trout phenotypes upon entering the sea is not well known. Using passive integrated transponder telemetry, this study found that the return rate from the sea to the natal river was higher for parr compared to pre-smolts and smolts. Additionally, trout classified as parr generally migrated earlier to the sea and a larger proportion returned to the river after less than one year at sea. The daily mortality rate at sea was comparable among the different phenotypes of trout, suggesting that the higher proportion of returning parr to the river was linked to their shorter duration at sea. These results provide evidence of different life-history strategies for seaward-migrating juvenile sea trout, ultimately affecting their return rate to the natal river. Investigations failing to consider downstream migrating parr and pre-smolts risks neglecting a large part of the anadromous population and may result in inaccurate assessments of sea trout stocks in rivers.
Similar content being viewed by others
Introduction
The life-cycle of anadromous trout (sea trout; Salmo trutta) involves a series of seasonal movements between freshwater and marine habitats1. The shift from freshwater to saltwater is seen as an adaptive strategy allowing migratory individuals to attain larger sizes and greater reproductive potential2,3. However, migration is energetically demanding and usually associated with a high loss of individuals in the first period at sea4,5,6,7,8. Little is known about factors influencing sea survival in wild sea trout, apart from predation9,10. As many wild sea trout populations are declining all over Europe11, there is currently growing interest in understanding factors affecting marine survival to improve the management of wild sea trout populations12.
Juvenile trout typically migrate to the sea in the spring and early summer (e.g.13,14,15). In Denmark, this migration to sea usually takes place from early March to the beginning of June with a peak in number of migrants in the last half of April and early May16,17,18. The transition from freshwater to saltwater involves a series of complex, interrelated processes including physiological, morphological and behavioural changes that optimize performance and survival in the marine environment, known as smoltification19,20. By completion of smoltification, trout can regulate the ion concentration of their body fluid to cope with saltwater21,22. The age of seaward-migrating trout in Denmark range from one to four years16,17, and the average length of trout smolts varies between 120 and 200 mm, with a range from 70 to 300 mm16,23.
The juvenile trout can enter saltwater at different stages during the parr to smolt transformation20,24,25,26,27. Based on their different external morphological features including body shape and coloration20,24, the migrating trout can be classified into three different phenotypes: parr, pre-smolts and smolts (Table 1; Fig. 1). Body colouration is an essential adaptation that allows the fish to blend into the surrounding environment, reducing the risk of being eaten by visual predators (e.g., birds and fish). For instance, dark coats and spotted sides allow parr to blend with bottom rocks and gravel in rivers. Conversely, the light bellies, silvery sides, and dark dorsal surface of smolts help camouflaging fish in a mid-water marine environment. In salmonids, there are also changes in body shape when smolts migrate to the sea in spring, which are likely associated with greater swimming efficiency, with the snout becoming more pointed, the body slimmer and more streamlined, and a lengthening of the caudal peduncle [e.g.24,28,29 Fig. 1]. Thus it could be hypothesised that when parr enter the sea, they are more vulnerable to predation than smolts as being less prepared for a marine life.
The different phenotypes of spring migrating juvenile trout may also differ in their capacity to osmoregulate in saltwater based on their different levels of gill Na+, K+-ATPase activity20,21. Osmoregulation in saltwater has been acknowledged as critical factor for survival of diadromous fish at sea19,20,24,30. Hence, it can also be assumed that parr have lower survival at sea than smolts due to their lower capacity to osmoregulate in saltwater. In addition, migrant parr should stay in low saline water (e.g., fjord systems), whereas the more seawater pre-adapted pre-smolts and smolts should extend their migration beyond the fjord to exploit feeding areas at sea. However, little is known about the behaviour and survival of the various phenotypes of migrating trout once they enter the marine environments.
This study examines whether the different phenotypes of spring migrating juvenile trout (i.e., parr, pre-smolts and smolts) exhibit different life-history strategies (time of seaward migration and time spent at sea) and survival at sea based on the hypothesised differences in their degree of morphological and physiological adaptions for marine life. To test this, a total of 9297 wild juvenile trout were trapped in a Danish river in spring over two consecutive years, PIT-tagged, and categorized as parr, pre-smolts or smolts (based on body shape and coloration) during their downstream migration to the sea. Following tagging, their return from the sea to the river was monitored by a stationary PIT-antenna station, located close to the river mouth.
Materials and Methods
Study site
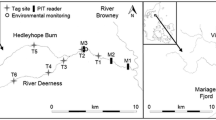
The River Villestrup is located on the east coast of Jutland, Denmark (Fig. 2). The river is about 20 km long and has a catchment area of 126 km2. The river flow is relatively stable due to large groundwater inflows with a mean annual discharge of 1.1 m3 s−1. The river discharges into Mariager Fjord, a sill fjord with relatively low water exchange with the Kattegat Sea31,32. The fjord is about 35 km long and 2 km wide with a total area of 47 km2. The fjord has a tidal range of 20–30 cm and salinity varies between 12 and 25‰ from the inner to the outer fjord.
The River Villestrup supports a population of partially anadromous brown trout, where a portion of the individuals smoltify and migrate to sea and the rest remain resident in the river. The proportion of migratory and resident trout in the river system is currently unknown. However, previous studies in Danish rivers typically show that more than two-thirds of the trout migrate to sea33,34. The river has not been stocked with hatchery-reared trout for the last four decades and the fish are therefore considered to be of wild origin23. In 2006, the population of sea trout spawners was estimated to be approximately 1200 fish and the average density of young of the year trout was estimated at 121 individuals per 100 m2 (95% CI: 31–211 individuals per 100 m2) in autumn 200735.
Fish trapping and tracking
A Wolf-type trap36 was used to capture fish during their downstream migration to the fjord. The trap was located 300 m from the river mouth and 100 m downstream from the PIT- antennae station (Fig. 2). The trap surface consisted of a grid of aluminium bars at 8 mm spacing, covering the whole cross-sectional area of the river. This type of trap is suitable for capturing trout larger than 100 mm in total length20 and it redirects fish into a holding chamber where they can be easily accessible. The trap was operated from the 31th March to 2nd June 2008 and from the 20th March to 31st May 2009. No overflow or malfunction occurred during the trapping periods.
All captured fish larger than 120 mm in total length were PIT-tagged (Texas Instruments, RI-TRP-RRHP, half duplex, 134 kHz, length 23.1 mm, diameter 3.85 mm and weight 0.6 g in air). The lower size limit of 120 mm was chosen to minimize possible tagging effects and tag losses37. Hence, it was not possible to include smaller migrating trout of a particular phenotype in this study. Approximately 12% of all trapped trout measured between 100–120 mm but the portion of migrating trout smaller than 100 mm remains unknown as they could swim through the trap. Fish tagging was conducted at the site of capture, the same day or the day following capture, to reduce handling and recovery time. Prior to surgical implantation of the tags, trout were anesthetized with benzocaine (25 mg/L, Sigma Chemical Co., St Louis, USA). Tagging was intraperitoneal via a small ventrolateral incision (3–4 mm long) posterior to the pectoral fins made with a scalpel. The tagged fish were placed in a recovery tank and released in the river downstream of the fish trap about one hour after tagging.
Tagged trout were classified as parr, pre-smolts or smolts based on external phenotypic characteristics (i.e., body shape and coloration) following Tanguy’s24 classification (see Table 1 and Fig. 1). This classification has been shown to be suitable for evaluating the developmental stage and osmoregulatory capacity of juvenile salmonids5,21,22. Additionally, total length to nearest millimetre and the PIT-tag number were registered.
A PIT-antennae station was deployed approximately 400 m upstream from the river mouth to register trout returning from the sea to the river (Fig. 2). This system continuously recorded date, time, and PIT-tag code of tagged fish passing the detection field. The station consisted of two swim-trough antennas located 10 m apart. The antennas were connected to stationary data-logging readers operating at 134 KHz (model TIRIS S- 2000, Texas Instruments). The paired use of antenna allows discrimination between up and downstream movements and increases the detection probability of tagged trout. The in situ detection efficiency of the PIT-station was calculated according to Zydlewski et al.38, and estimated at 98.5%. The PIT-station operated continuously from the beginning of the experiment in March 2008 until November 2012.
At the end of each trapping period in 2008 and 2009, electrofishing surveys were conducted in the river downstream of the trap to Mariager Fjord. None of the tagged trout were caught in this section of the river (i.e., last ~ 300 m of the river), suggesting that all tagged fish migrated to the fjord. Furthermore, the lower reach of the river has a limited number of suitable habitats for juvenile trout; the macrophyte vegetation is sparse and the bottom substrate consists mainly of sand. It is therefore unlikely that trout stayed in this river section as resident individuals. However, it was not possible to conclusively document that all trout entered the fjord system in the present study. In addition, it cannot be excluded that some parr and pre-smolts completed smoltification in the lower reaches of the river or Mariager Fjord. This could potentially make them belong to the smolt group later in the season.
Use of experimental animals in this experiment
Trout were tagged under license (2012-DY-2934-00007) issued by the Danish Experimental Animal Committee. All animal tagging procedures were approved by the Danish Experimental Animal Committee in accordance with the guidelines described in the cited permission.
Statistical analysis
Migration timing from the river to the sea
A GLM (generalized linear model) with a gamma distribution and log link function was used to test whether time of seaward migration, here defined as day of year an individual was captured and PIT-tagged (subsequently referred to as migration time), was related to fish group (parr, pre-smolt and smolt), year of tagging (2008 and 2009), and body length at tagging. The initial model included all two-way interactions:
Return of sea trout to the river
The return rate of sea trout to River Villestrup was calculated as the number of fish that migrated back to the river and was detected by the PIT station relative to the total number of tagged fish. The ultimate fate of trout that did not return to the river could not be determined in the present study. Nonetheless, they may have been associated with any of these factors: fish mortality, tag loss by fish, non-detected fish by the PIT station, and fish straying to other rivers. Despite these limitations, the return rate of sea trout to the river can be used as a proxy for minimum survival at sea.
A GLM with a Bernoulli distribution and logit link function was used to assess the probability of fish return (yes/no) from the sea to River Villestrup in relation to fish group (parr, pre-smolt and smolt), year of tagging (2008 and 2009), length at tagging and migration time (day of year). All two-way interactions were included in the initial model:
Marine residence time
The marine residence time could only be determined for trout that returned to River Villestrup and was calculated as the number of days between tagging until first detection at the PIT-antenna station. The returning sea trout were divided into two different sea-age classes according to time spent at sea: 0SW (less than 365 days at sea) and 1 + SW (more than 365 days at sea).
We used a GLM with Bernoulli distribution and logit link function to investigate whether fish group (parr, pre-smolt and smolt), year of tagging (2008 and 2009), length at tagging, and migration time (day of year) were associated with sea-age class (0SW and 1 + SW) as a measure for marine residence time. All two-way interactions were included in the initial model:
Instantaneous mortality rate
The instantaneous mortality rate at sea (Z; day−1) was estimated for the three fish groups (parr, pre-smolt and smolt) in 2008 and 2009 as:
where N0 is the number of trout tagged, Nt is the number of trout returning from the sea to the river, and t is the average number of days spent at sea. This allowed us to compare the time-based mortality rate at sea among the different phenotypes of trout. The equation assumes an exponential decline in the number of trout over time at sea.
General statistical notes
All statistical analyses were performed using R version 3.1.139. Before applying the statistical models, data exploration was performed following the protocol in Zuur et al.40. Collinearity between covariates was assessed using generalized variance inflation factors prior to applying the models. All variance inflation factors were <2, suggesting negligible multicollinearity41. Model selection was conducted by backwards elimination using p = 0.05 as threshold for elimination. Model validation was performed by visual inspection of the residuals and no violations were encountered.
Results
In 2008, a total of 4304 migrating juvenile trout were tagged and 4993 fish were tagged in 2009. The majority of the migrating trout were characterized as smolts (66.5%), followed by pre-smolts (29.6%) and parr (3.9%) (Table 2).
Migration timing from the river to the sea
Step-wise single term elimination of non-significant (p > 0.05) predictors resulted in the following final model:
Summary statistics of the final model are presented in Table 3. Trout were trapped migrating downstream from the end of March until end of May in both years and most fish (70%) migrated in April (Fig. 3). This period coincides with the normal time for the juvenile trout spring migration in the River Villestrup16,17,18.
Number of PIT-tagged juvenile trout during the spring migration in 2008 (n = 4304) and 2009 (n = 4993). The solid lines represent the cumulative percentage of the migrating trout classified as parr (black line), pre-smolt (grey line) and smolt (blue line). The bars represent the daily number of migrating parr (black bars), pre-smolt (grey bars) and smolt (blue bars). Note the different scales on the y-axis.
All phenotypes of trout were captured in the river throughout the trapping period in 2008 and 2009 (Fig. 3). However, parr generally migrated earlier compared to pre-smolt and smolts in both years, and smolts tended to migrate later than pre-smolts (GLM: Group × Year, F = 34.90, df = 2, p < 0.0001; Figs 3 and 4, Table 2). It should be stressed that the interaction between Group and Year is barely noticeable on Fig. 4, despite being statistically significant (Table 3). Therefore, the interactive effect between Group and Year on the migration timing should be interpreted with great care.
Relationship between migration time (day of year) from River Villestrup to the sea and body length (mm) of trout classified as parr, pre-smolt and smolt in 2008 and 2009. Solid lines represent the model fit and shaded areas represent 95% confidence intervals. Open circles indicate observed values. See Table 2 for sample sizes.
In addition, time of seaward migration for the different phenotypes of trout was associated with body length at tagging (GLM: Group × Length, F = 57.50, df = 2, p < 0.0001; Fig. 4). While body length was positively correlated with migration time for parr, a negative relationship was found for smolts. For pre-smolts, there was no clear relationship between body length and timing of migration (Fig. 4).
Return of sea trout to the river
Step-wise single term elimination of non-significant (p > 0.05) predictors resulted in the following final model:
Summary statistics of the final model are presented in Table 4. The probability of return for sea trout to River Villestrup differed significantly between years: individuals tagged in 2008 had lower return rate than those tagged in 2009 (GLM: Year, LRT = 10.15, df = 1, p = 0.001). The overall return of sea trout was 8.3% (n = 358 out of 4304) and 10.0% (n = 498 out of 4993) in 2008 and 2009, respectively. The model also showed that the probability of return was higher for trout that migrated later in the season (GLM: Migration time, LRT = 50.53, df = 1, p < 0.0001; Fig. 5). Lastly, body length at tagging had a significant effect on the probability of return among the fish groups (GLM: Group × Length, LRT = 12.30, p = 0.002; Fig. 6). In the groups of smolt and pre-smolt, the probability of return was greater for smaller individuals, while no conclusive effect was observed for parr (see Fig. 6). Notably, the probability of return was higher for parr (the return rate was 14.2% and 21.2% in 2008 and 2009, respectively) than that of pre-smolt (10.7% and 10.4%) and smolt (6.9% and 9.3%; Table 2; Fig. 6).
Probability of return to River Villestrup for sea trout in relation to migration time (day of year) for the seaward-migrating trout classified as parr, pre-smolt and smolt in 2008 and 2009. Solid lines represent the predicted probability of return and shaded areas indicate 95% confidence intervals. Body length was set to the average value of 150.1 mm.
Probability of return to River Villestrup for sea trout in relation to body length (mm) at tagging for the seaward-migrating trout classified as parr, pre-smolt or smolt in 2008 and 2009. Solid lines represent the predicted probability of return and shaded areas indicate 95% confidence intervals. Migration timing was set to the average value of 112.7 days.
Marine residence time
Step-wise single term elimination of non-significant (p > 0.05) predictors resulted in the following final model:
Summary statistics of the final model are presented in Table 5. The time spent at sea differed significantly among fish groups (GLM: Group, LRT = 11.13, df = 2, p = 0.004; Table 2, Fig. 7). Tukey contrast test using the “glht” function in the “multcomp” package42 showed that parr had higher probability of staying less than one year at sea than pre-smolts (p = 0.043) and smolts (p = 0.005). In fact, 77% of the tagged parr that returned to the river did so within the first year following tagging, while 54% and 45% of the returning pre-smolts and smolts spent less than one year at sea, respectively (Table 2). In addition, smaller trout tagged in 2009 had higher probability of staying more than one year at sea compared to larger individuals, whereas no effect of body length on marine residence time was observed among trout tagged in 2008 (GLM: Year × Length, LRT = 8.75, df = 1, p = 0.003; Fig. 7). The model also showed that time of seaward migration affected time spent at sea, with trout migrating later having greater propensity to stay longer at sea (GLM: Migration time, LRT = 5.38, df = 1, p = 0.020, Fig. 8).
Probability that sea trout spent more than one year at sea before returning to River Villestrup in relation to body length (mm) at tagging for the seaward-migrating trout classified as parr, pre-smolt and smolt in 2008 and 2009. Solid lines represent the predicted probability of return and shaded areas indicate 95% confidence intervals. Migration time was set to the average value of 114.9 days.
Probability that sea trout spent more than one year at sea before returning to River Villestrup in relation to migration time (day of year) for the seaward-migrating trout classified as parr, pre-smolt and smolt in 2008 and 2009. Solid lines represent the predicted probability of return and shaded areas indicate 95% confidence intervals. Body length was set to the average value of 146.6 mm.
Instantaneous mortality rate
The instantaneous mortality rate at sea was 6.72 × 10−3, 6.83 × 10−3 and 6.82 × 10−3 day−1 for parr, pre-smolts and smolts tagged in 2008, respectively (Table 2). For parr, pre-smolts and smolts tagged in 2009 the instantaneous mortality rate at sea was respectively 6.20 × 10−3, 6.15 × 10−3 and 6.42 × 10−3 day−1.
Discussion
Migration timing from the river to the sea
During the period from March to June in 2008 and 2009, wild sea trout juveniles migrated from the river and likely entered the sea at different developmental stages during their parr-smolt transformation.
The majority of the spring migrants were characterized as smolts (66.5%) followed by pre-smolts (29.6%) and parr (3.9%). Trout classified as parr generally migrated earlier to the sea than pre-smolt and smolts. This suggests that parr phenotypes were functionally adapted to life at sea and migrated downstream without having completed smoltification, at least with regard to changes in body morphology and coloration (i.e. silvery body, darkened fins and slim body shape). This phenomenon has previously been observed in other investigations26,43. Movement of parr to marine environments is also known to occur in autumn and winter in this river and others44,45,46. The migration of parr to the sea may reflect a distinctive life history strategy by which fish achieve better growth in hypotonic estuaries and coastal areas like Mariager Fjord than in their home river. This assumption has also been proposed in other studies involving salmonids (reviewed in47). However, an important limitation of the present study is that the sea trout were not recaptured in the river (e.g. by electrofishing) once they returned. Furthermore, juvenile trout that remained resident in the river were not included in this study. Without such data the costs and benefits of migrating to the sea versus staying in the river remain speculative.
The relationship between individual’s initial body length and the time they migrated to the sea showed different patterns among the phenotypes. Smolts followed the general trend with larger individuals migrating earlier in the season compared to smaller ones48,49. This behaviour is often explained by the lower predation risk associated with larger fish sizes as well as their reduced energetic costs during the seaward migration50,51. The opposite pattern was found for parr with smaller fish migrating earlier than larger individuals and body length of pre-smolts did not influence time of seaward migration. The mechanisms underlying these migratory differences remain unknown and highlight the need for additional studies on anadromous trout populations.
Return of sea trout to the river
Sea trout classified as parr at time of tagging had a higher return rate to the river compared with pre-smolts and smolts, suggesting that the degree of pre-adaptation to saltwater is not of overriding importance for the survival of trout in Mariager Fjord. These results add to the growing body of evidence that trout may not need to complete smoltification to survive in marine environments for extended periods of time, at least not in low saline areas25,26,27,43,52. This possibility is critical for studies investigating the migratory behaviour and survival of trout at sea. For instance, considering merely individuals with silvery body coloration and absence of parr marks as true sea trout smolts risks excluding the contribution made by migrant parr and pre-smolt to the anadromous population (up to 34% in the present study; Table 2). Potentially, this might bias estimates of sea trout population sizes in the rivers and marine survival, for instance, when evaluating sea survival as the number of returning adults in relation to the number of seaward migrating smolts. However, it should be stressed that parr phenotypes only constituted 3.9% of the total numbers of tagged fish in the present study, and consequently their importance for the whole trout population might be limited. Lastly, it cannot be ruled out that parr tolerated handling and tagging better than fully smoltified trout, which may have contributed to the higher return rate of parr to the river.
The return from the sea to the river was lower for larger fish among pre-smolts and smolts, but no clear pattern was found for parr. Since predation has been recognized as one of the primary factors affecting survival of juvenile trout in similar fjord systems around Denmark9,10, this result may suggest a size selective predation biased towards larger fish sizes. This interpretation however, is in contrast to several other studies showing reduced predation risk as body size increases (reviewed in50). Hence, other confounding factors could account for the generally lower marine survival of larger individuals. Numerous studies have documented how habitats providing faster growth often have a higher predation risk than habitats supporting slower growth (e.g.51,53). This requires fish to make a behavioural trade-off between rapid growth and protection from predators. Thus, for sea trout populations distributed across a mosaic of habitats, as is the case for trout in the current system54, bigger might be better within a single habitat, but not necessarily across the population when considering that many fast growing individuals reside in risky habitats.
The return rate to the river was higher for trout that migrated later to the sea. This is in agreement with the findings of several previous studies on sea trout smolts [e.g.48,49,54,55,56] and numerous mechanisms could explain this pattern. For instance, food availability and predation pressure often fluctuate in fjord systems during the smolt season and have been recognised as important factors influencing the survival of trout in the early marine phase14,57. It is therefore possible that the early migrating trout experienced higher predation rates (e.g., from birds) and/or lower food availability compared to conspecifics migrating later to the sea. Parasitism is another potential factor influencing trout survival e.g.58, and if early migrants were more exposed or susceptible to parasites this could result in increased mortality. Furthermore, recent research has shown that juvenile trout can shift stream system through marine environments59. Hence, the lower return rate of early migrants could also be due to a higher migration rate of these individuals into non-natal streams in the fjord system. Additional telemetry studies are required to validate these assumptions.
Marine residence time
77% of the returning parr spent less than one year at sea. This differs from the other phenotypes whereby 54% of pre-smolts and 45% of smolts returned from the sea after less than one year. The greater proportion of parr returning to the river after less than a year may suggest they remained in the fjord close to the river of origin. Conversely, the lower proportion of pre-smolts and smolts returning after one year may reflect a migration out of the fjord to the sea for these phenotypes, possibly to explore other feeding habitats (e.g. in the Kattegat Sea). This scenario is supported by a parallel study demonstrating that sea trout show different migratory strategies in Mariager Fjord (i.e. ranging from fjord residents to individuals migrating out of the fjord)54. Indeed, recent evidence suggests that sea trout from the same population can display high variation in habitat utilization and migration behaviour in fjord systems15,57,60. The PIT-telemetry methods used in this study did not allow us to assess the extent of migration out of the fjord system. Other methods, such as acoustic telemetry or otolith microchemical analysis, would be useful in addressing this question and further exploring the marine behaviour and habitat use of different trout phenotypes.
As previously mentioned, parr phenotypes were found to have a relatively higher return rate to the River Villestrup than the other phenotypes. In an attempt to clarify the mechanism underlying the greater return rate of parr to the river, instantaneous mortality rates at sea were calculated for each of the phenotypes. The results indicated similar daily mortality rates for each phenotype, suggesting that parr, pre-smolts and smolts performed equally at sea. Hence, the higher return rate of parr compared to pre-smolts and smolts seems to be related to their shorter duration at sea.
Trout that migrated earlier to the sea had a higher probability of staying less than one year at sea than conspecifics migrating later in the season. Early downstream migration prolongs the first growing season at sea and could potentially increase an individual’s chances of reaching sexual maturity at the end of the season. Thus, it is possible that a higher proportion of the early migrating trout reached sexual maturity at the end of their first marine season in comparison to fish migrating later and therefore returned earlier from the sea to the river to spawn. However, more research is needed to confirm this possibility. The relationship between time of seaward migration and marine residence time deserves more attention in future studies.
Lastly, the body length of trout tagged in 2009 was negatively correlated with time spent at sea. Although the explanation for this relationship remains unknown, it is possible that smaller trout can achieve a greater fitness by staying longer at sea relative to larger individuals as also seen in other salmonid species61,62. Again, recapture of adult trout in the river with known time at sea and size at tagging is needed to validate this possibility. The marine residence time was not influenced by body length of the trout tagged in 2008, emphasising that conclusions of behavioural studies on sea trout should ideally be drawn on data collected over multiple years.
Sea trout display a wide range of life-histories and after feeding in marine environments they can return to the natal river as immature or mature individuals for wintering and/or spawning23,24,43,62,63. It is important to note that the return rate of immature and mature trout from the sea to the riverine environment is not directly comparable, at least not in the context of survival until first sexual reproduction. Unfortunately, the proportion of immature and mature trout returning from the sea could not be determined in the present study as the river was not resampled during the spawning season. However, the return rate estimates for the different phenotypes likely included both immature and mature trout. Keeping in mind that 77% of the returning parr spent less than one year at sea, it is possible that a higher proportion of the parr used the fjord as summer habitat and returned to the river as immature trout for wintering compared to the other phenotypes. Conversely, the longer duration at sea for pre-smolts and smolts may suggest that a higher proportion returned to the river as mature trout for spawning. Thus, while parr had surprisingly high return rate to the river, their marine survival might not be directly comparable with the survival of pre-smolts and smolt at the time of first return to the river. The return rates of the phenotypes should therefore be compared with caution.
Conclusion
In accordance with previous studies, the results of the present study suggest that the various phenotypes of seaward-migrating juvenile trout not only differ in morphology, but also display different life-history strategies in terms of migration time to the sea, marine residence time and potentially habitat use. It is suggested that these differences in life-history strategies can influence survival at sea. In particular, the relative higher return rate to the river for parr compared to pre-smolts and smolts seems to be related to their shorter duration at sea and/or potential difference in habitat utilization.
In the present study, trout were divided into the three phenotypic groups based on visual assessment of body shape and coloration. Although this approach has also been used in previous studies, image analysis of body morphometric and coloration provides unbiased and quantitative data with high reproducibility. Quantitative imaging techniques should therefore be considered in future studies as a tool for grouping trout with distinct phenotypic traits. Furthermore, development of a continuous classification system, potentially including physiological measurements coupled with fine scale telemetry or otolith microchemistry, might more fully resolve how different levels of seawater preparedness during the downstream spring migration affects behaviour and survival at sea. Future studies should also attempt to compare fitness-oriented endpoints (e.g., growth and fecundity) among the different phenotypes of seaward-migrating trout once they return to the river. This would allow an in-depth assessment of the costs and benefits of long versus short time at sea and migrating earlier versus later to the sea.
References
Elliott, J. M. Quantitative ecology and the brown trout. Oxford University Press (1994).
Lucas, M. C, Baras, E., Thom, T. J., Duncan, A. & Slavík, O. Migration of freshwater fishes. Wiley Online Library (2001).
Gross, M. R. Evolution of diadromy in fishes. American Fisheries Society Symposium, 1,14–25 (1987).
Boel, M., Aarestrup, K., Baktoft, H., Larsen, T. & Søndergaard, S. M. The physiological basis of the migration continuum in brown trout (Salmo trutta). Physiological and Biochemical Zoology. 87, 334–345 (2014).
Forseth, T., Nesje, T. F., Jonsson, B. & Hårsaker, K. Juvenile migration in brown trout: a consequence of energetic state. Journal of Animal Ecology 68, 783–793 (1999).
Elliott, J. The critical-period concept for juvenile survival and its relevance for population regulation in young sea trout, Salmo trutta. Journal of Fish Biology 35, 91–98 (1989).
Armstrong, J., Kemp, P., Kennedy, G., Ladle, M. & Milner, N. Habitat requirements of Atlantic salmon and brown trout in rivers and streams. Fisheries Research 62, 143–170 (2003).
Thorstad, E. B., Todd, C. D., Uglem, I., Bjørn, P. A. & Gargan, P. G. Marine life of the sea trout. Marine Biology 163, 1–19 (2016).
Jepsen, N., Holthe, E. & Økland, F. Observations of predation on salmon and trout smolts in a river mouth. Fisheries Management and Ecology 13, 341–344 (2006).
Koed, A., Baktoft, H. & Bak, B. D. Causes of mortality of Atlantic salmon (Salmo salar) and brown trout (Salmo trutta) smolts in a restored river and its estuary. River Research and Applications 22, 69–78 (2006).
ICES 2018, Report of the Baltic Salmon and Trout Assessment Working Group (WGBAST). ICES CM 2018/ACOM:10 (2018).
Drenner, S. M., Clark, T. D., Whitney, C. K., Martins, E. G. & Cooke, S. J. A synthesis of tagging studies examining the behaviour and survival of anadromous salmonids in marine environments. PLoS One 7, e31311 (2012).
Jonsson, B. & Jonsson, N. Ecology of Atlantic salmon and brown trout: habitat as a template for life histories. Springer (2011).
ICES 1994. Report of the Baltic Salmon and Sea Trout Assessment Working Group (WGBAST). ICES CM 1994/Assess: 15 (1994).
Aarestrup, K., Jepsen, N. & Thorstad, E. B. Brown Trout on the Move: migration ecology and methodology. Brown Trout: Biology, Ecology and Management, Wiley 17, 401–444 (2017).
Rasmussen, G. The population dynamics of brown trout (Salmo trutta L.) in relation to year-class size. Polskie Archiwum Hydrobiologii 33, 489–508 (1986).
Christensen, O., Pedersen S. & Rasmussen, G. Review of the Danish Stocks of Sea Trout (Salmo trutta). International Council for Exploration of the Sea, C.M.1993/M: 22 (1993).
Birnie-Gauvin, K. et al. River connectivity reestablished: Effects and implications of six weir removals on brown trout smolt migration. River Research and Applications 34, 548–554 (2018).
Hoar, W. The Physiology of Smolting Salmonids. Fish physiology 11, 275–343 (1988).
Nielsen, C., Aarestrup, K., Nørum, U. & Madsen, S. Pre‐migratory differentiation of wild brown trout into migrant and resident individuals. Journal of Fish Biology 63, 1184–1196 (2003).
Aarestrup, K., Nielsen, C. & Madsen, S. S. Relationship between gill Na+, K+-ATPase activity and downstream movement in domesticated and first-generation offspring of wild anadromous brown trout (Salmo trutta). Canadian Journal of Fisheries and Aquatic Sciences 57, 2086–2095 (2000).
Nielsen, C., Aarestrup, K., Nørum, U. & Madsen, S. S. Future migratory behaviour predicted from premigratory levels of gill Na+/K+-ATPase activity in individual wild brown trout (Salmo trutta). Journal of Experimental Biology 207, 527–533 (2004).
Pedersen, S. Sea trout and salmon populations and rivers in Denmark: HELCOM assessment of salmon (Salmo salar) and sea trout (Salmo trutta) populations and habitats in rivers flowing to the Baltic Sea. Baltic Sea environment proceedings 126B (2011).
Tanguy, J., Ombredane, D., Bagliniere, J. & Prunet, P. Aspects of parr-smolt transformation in anadromous and resident forms of brown trout (Salmo trutta) in comparison with Atlantic salmon (Salmo salar). Aquaculture 121, 51–63 (1994).
Limburg, K., Landergren, P., Westin, L., Elfman, M. & Kristiansson, P. Flexible modes of anadromy in Baltic sea trout: making the most of marginal spawning streams. Journal of Fish Biology 59, 682–695 (2001).
Landergren, P. Factors affecting early migration of sea trout (Salmo trutta) parr to brackish water. Fisheries Research 67, 283–294 (2004).
Landergreen, P. Survival and growth of sea trout parr in fresh and brackish water. Fish Biology 53, 591–593 (2001).
Riddell, B. E. & Leggett, W. C. Evidence of an adaptive basis for geographic variation in body morphology and time of downstream migration of juvenile Atlantic salmon (Salmo salar). Canadian Journal of Fisheries and Aquatic Sciences 38, 308–320 (1981).
Hard, J. J., Winans, G. A. & Richardson, J. C. Phenotypic and genetic architecture of juvenile morphometry in Chinook salmon. Journal of Heredity 90, 597–606 (1999).
McCormick, S. D., Hansen, L. P., Quinn, T. P. & Saunders, R. L. Movement, migration, and smolting of Atlantic salmon (Salmo salar). Canadian Journal of Fisheries and Aquatic Sciences 55, 77–92 (1998).
Fallesen, G., Andersen, F. & Larsen, B. Life, death and revival of the hypertrophic Mariager Fjord (Denmark). Journal of Marine Systems 25, 313–321 (2000).
Olesen, M. Sedimentation in Mariager Fjord, Denmark: the impact of sinking velocity on system productivity. Ophelia 55, 11–26 (2001).
Midwood, J. D. et al. Does cortisol manipulation influence outmigration behaviour, survival and growth of sea trout? A field test of carryover effects in wild fish. Marine Ecology Progress Series 496, 135–144 (2014).
Birnie-Gauvin, K. et al. Oxidative stress and partial migration in brown trout (Salmo trutta). Canadian Journal of Zoology 95, 829–835 (2017).
Mikkelsen, J. S. & Carøe, M. Plan for fiskepleje i Vandsystemer mellem Mariager Fjord og Limfjorden Distrikt, 16-vandsystem, 01–22a (2017).
Wolf, P. A trap for the capture of fish and other organisms moving downstream. Transactions of the American Fisheries Society 80, 41–45 (1951).
Larsen, M. H., Thorn, A. N., Skov, C. & Aarestrup, K. Effects of passive integrated transponder tags on survival and growth of juvenile Atlantic salmon (Salmo salar). Animal Biotelemetry 1, 19 (2013).
Zydlewski, G. B., Horton, G., Dubreuil, T., Letcher, B. & Casey, S. Remote monitoring of fish in small streams: a unified approach using PIT tags. Fisheries 31, 492–502 (2006).
RCore Team. R: a language and environment for statistical computing. Version 3.1. 1. R Foundation for Statistical Computing, Vienna, Austria (2013).
Zuur, A. F., Leno, E. N. & Elphick, C. S. A protocol for data exploration to avoid common statistical problems. Methods in Ecology and Evolution 1, 3–14 (2010).
Zuur, A. F., Ieno, E. N., Walker, N. J., Saveliev, A. A. & Smith, G. M. GLM and GAM for absence–presence and proportional data. Mixed effects models and extensions in ecology with R. Springer New York, 245–259 (2009).
Hothorn, T., Bretz, F., Westfall, P. & Heiberger, R. M. multcomp: Simultaneous Inference in General Parametric Models, http://CRAN.R-project.org. R package version 1.0-0 (2008).
Järvi, T. et al. Newly - emerged Salmo trutta fry that migrate to the sea - an alternative choice of feeding habit? Nordic Journal of Freshwater Research 72, 52–62 (1996).
Peiman, K. S. et al. If and when: intrinsic differences and environmental stressors influence migration in brown trout (Salmo trutta). Oecologia 184, 375–384 (2017).
Winter, E., Tummers, J., Aarestrup, K., Baktoft, H. & Lucas, M. Investigating the phenology of seaward migration of juvenile brown trout (Salmo trutta) in two European populations. Hydrobiologia 775, 139–151 (2016).
Aarestrup, K., Birnie‐Gauvin, K. & Larsen, M. H. Another paradigm lost? Autumn downstream migration of juvenile brown trout: Evidence for a presmolt migration. Ecology of Freshwater Fish 27, 513–516 (2018).
Enayatmehr, M. & Jamili, S. Effects of Salinity on Growth, Hormonal and Enzymatic Status in Fish: A Review. International Journal of Science and Research 7, 805–813 (2013).
Bohlin, T., Dellefors, C. & Faremo, U. Optimal time and size for smolt migration in wild sea trout (Salmo trutta). Canadian Journal of Fisheries and Aquatic Sciences 50, 224–232 (1993).
Bohlin, T., Dellefors, C. & Faremo, U. Date of smolt migration depends on body‐size but not age in wild sea‐run brown trout. Journal of Fish Biology 49, 157–164 (1996).
Sogard, S. M. Size-selective mortality in the juvenile stage of teleost fishes: a review. Bulletin of Marine Science 60, 1129–1157 (1997).
Lima, S. L. & Dill, L. M. Behavioural decisions made under the risk of predation: a review and prospectus. Canadian Journal of Zoology 68, 619–640 (1990).
Okumus, I., Kurtoglu, I. Z. & Atasaral, S. General overview of Turkish sea trout (Salmo trutta L.) populations. Sea trout: Biology, Conservation and Management, 115–127 (2006).
Milinski, M. Predation risk and feeding behaviour. Behaviour of teleost fishes. Fish and Fisheries (Chapman & Hall) 7, 285–305 (1993).
del Villar-Guerra, D., Aarestrup, K., Skov, C. & Koed, A. Marine migrations in anadromous brown trout (Salmo trutta). Fjord residency as a possible alternative in the continuum of migration to the open sea. Ecology of Freshwater Fish 23, 594–603 (2013).
Jonsson, N. & Jonsson, B. Migration of anadromous brown trout (Salmo trutta) in a Norwegian river. Freshwater Biology 47, 1391–1401 (2002).
Jonsson, B. & Jonsson, N. Migratory timing, marine survival and growth of anadromous brown trout (Salmo trutta) in the River Imsa, Norway. Journal of Fish Biology 74, 621–638 (2009).
Eldøy, S. H. et al. Marine migration and habitat use of anadromous brown trout (Salmo trutta). Canadian Journal of Fisheries and Aquatic Sciences 72, 1366–1378 (2015).
Bristow, G. A. Parasites of Norwegian freshwater salmonids and interactions with farmed salmon - a review. Fisheries Research 17, 219–227 (1993).
Taal, I. et al. Parr dispersal between streams via a marine environment: a novel mechanism behind straying for anadromous brown trout? Ecology of Freshwater Fish 27, 209–215 (2018).
Flaten, A. C. et al. The first months at sea: marine migration and habitat use of sea trout Salmo trutta post-smolts. Journal of Fish Biology 89, 1624–1640 (2016).
Hoar, W. S. Smolt transformation: evolution, behaviour, and physiology. Journal of the Fisheries Board of Canada 33, 1233–1252 (1976).
Hutchings, J. A. Norms of reaction and phenotypic plasticity in salmonid life-histories. Evolution Illuminated: Salmon and Their Relatives. Oxford University Press, 154–174 (2004).
Klemetsen, A. et al. Atlantic salmon Salmo salar L., brown trout Salmo trutta L. and Arctic charr Salvelinus alpinus (L.): a review of aspects of their life histories. Ecology of Freshwater Fish 1, 1–59 (2003).
Acknowledgements
The study was funded by the Living North Sea project under the “Interreg IVB, North Sea Region programme” funding mechanisms. Elements of the research were also funded by the Danish rod and net fish license funds and the strategic project SMOLTPRO, financed by the Swedish Research Council Formas. The authors gratefully acknowledge the assistance of Alan F. Youngson (University of the Highlands and Islands), in editing the manuscript.
Author information
Authors and Affiliations
Contributions
D.d.V.G., K.A., M.H.L. and H.B. conceived the ideas and designed methodology; K.A., H.B. and A.K. collected the data; D.d.V.G., M.H.L., H.B. analysed the data; D.d.V.G. and M.H.L. led the writing of the manuscript. All authors contributed to the manuscript and gave final approval for publication.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
del Villar-Guerra, D., Larsen, M.H., Baktoft, H. et al. The influence of initial developmental status on the life-history of sea trout (Salmo trutta). Sci Rep 9, 13468 (2019). https://doi.org/10.1038/s41598-019-49175-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-49175-0
- Springer Nature Limited