Abstract
Activated sludge from wastewater treatment plants was seeded into a sequencing batch reactor (SBR) in which synthetic wastewater was used as the influent. The sludge was bulked by decreasing the concentration of dissolved oxygen (DO). By adding a 30 min step of anaerobic stirring after the water inflow, the sludge bulking was rapidly inhibited after 10 running cycles, and the sludge volume index (SVI) decreased from 222 to 74 mL·g−1. The results of high-throughput sequencing showed that the relative abundance of bacteria Thiothrix, bacteria norank_o_Sphingobacteriales and fungi Trichosporon was increased by 6.3, 4.3 and 81.2%, after initial SBR stages, but these bacteria were inhibited by the addition of an anaerobic step, as their relative abundances decreased by 0.7, 0.8 and 14.7%, respectively. The proliferation of Thiothrix, norank_o_Sphingobacteriales and Trichosporon was the primary reason for the observed sludge bulking in the reactor. After the anaerobic step was added, the sludge extracellular polymeric substances (EPS) concentration was increased from 84.4 to 104.0 mg·(gMLSS)−1 (grams of mixed liquor suspended solids). Thus, the addition of an anaerobic step can inhibit the growth of filamentous bacteria, increasing the sludge EPS concentration and promoting the precipitation of activated sludge.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Introduction
Activated sludge processing is the most widely used biological wastewater treatment technique1. However, the common occurrence of sludge bulking severely impacts the normal operation of activated sludge biological wastewater treatment systems, affecting the effluent quality and increasing the time required for the system to recover2. There are two primary causes of sludge bulking: the first is the filamentous bulking caused by the excessive proliferation of filamentous bacteria, while the second is the viscous bulking caused by the large amount of extracellular polymeric substances (EPS) produced by zoogloea. The primary cause for the filamentous bulking is the different reactions of microflora to environmental changes, resulting in the disruption of the microflora balance in the sludge system3. The excessive proliferation of some filamentous bacteria4,5 or filamentous fungi6 increases the difficulty of separating the sludge and water. EPS are natural high molecular weight polymers secreted during the growth and metabolism of microorganisms. EPS are primarily composed of polysaccharides and proteins, contain abundant functional groups, and are the primary components of sludge flocs. Therefore, they are closely related to the settleability of sludge flocs7. Numerous studies of sludge bulking have been performed and a series of technical approaches and measures have been suggested to prevent and control sludge bulking8,9,10, one of which is the addition of an anaerobic step11,12. The setup of the anaerobic step for the anaerobic biological selector can radically inhibit the growth of filamentous bacteria. Moreover, because there is no need to add chemical reagents, it is a sustainable method to inhibit sludge bulking and is therefore widely used in biological wastewater treatment plants13,14. Research on setting up the anaerobic step for the anaerobic biological selector to inhibit sludge bulking is primarily focused on theoretical studies15 and the apparent inhibition effect16. Studies have shown that the setup of the anaerobic step relies on the “selection pressure” resulting from the high load gradient to select zoogloea. Based on the storage selection theory, the organic carbon sources in the influent are largely consumed by various biochemical reactions, such as storage and phosphorus release during the anaerobic step. This will cut the nutritional supplies for the subsequent growth of filamentous bacteria during the aerobic step17, inhibiting the growth of filamentous bacteria.
Although research capabilities and technical approaches have improved in recent years, few studies have taken advantage of these advances to explain the inhibition of sludge bulking in the anaerobic step. Therefore, it is particularly important to carry out studies in this area. In the present study, after sludge was bulked in a sequencing batch reactor (SBR), an anaerobic step was added after the influent step to investigate its effect on the settleability of sludge. High-throughput sequencing was used to analyse the changes in the bacterial community composition of the sludge after adding the anaerobic step. In addition, the EPS of the activated sludge was also analysed. This study aims to provide a theoretical basis and support for the prevention and resolution of sludge bulking issues in the design and operation of activated sludge processes in wastewater treatment plants.
Results
Pollutant removal
During the entire running cycle, the Chemical Oxygen Demand (COD) effluent concentration of the reactor was 6~47 mg·L−1. The sludge bulking stage from day 109 to day 130 was caused by the decreased DO concentration. Because there was not enough DO for nitration, the reaction was incomplete, and the effluent ammonium concentration was 14.90~43.76 mg·L−1. In other operation stages, the effluent ammonium concentration was 0.80~4.92 mg·L−1, exhibiting an excellent ammonium removal efficiency.
Variation of sludge settleability
Sludge settleability was evaluated by determining the SVI of activated sludge. When the SVI is greater than 150 mL·g−1, the sludge is considered to be bulked. From day 1 to day 118, the SVI of the sludge was 26~123 mL·g−1, and the sludge had excellent settleability. From day 119 to day 153, the SVI of sludge was 166~212 mL·g−1, and the sludge was in a bulking state. The anaerobic step was added on day 154, and after only 10 operation cycles on day 158 the sludge settleability was restored, with the SVI of the sludge gradually decreasing to 133 mL·g−1. Thus, the addition of anaerobic step rapidly inhibited sludge bulking. The average rate at which the SVI of the activated sludge decreased was 8.9 mL·(g·cycle)−1. The sludge settleability was restored to normal from day 158 to day 208 with an SVI of 56~133 mL·g−1.
Microscopic examination of sludge
The sludge samples were examined by optical microscopy. As shown in Fig. 1, in sludge samples A1 and A2 a large number of evenly distributed zoogloea were observed with a very small amount of filamentous bacteria. In the A3 bulked sludge sample, very few zoogloea were observed, but an abundance of filamentous bacteria with long and straight filaments were present. The number of filamentous bacteria gradually decreased in the A4 and A5 sludge samples. For the A6 and A7 samples, in which bulking was restored, almost no filamentous bacteria were observed, and the number of zoogloea gradually increased. The microscopic examinations showed that filamentous bulking was the sludge bulking process occurring in the experiment.
Analysis of bacterial community of the activated sludge before and after adding an anaerobic step
Table 1 shows that 30,119~43,862 filtered bacterial sequences were obtained that were grouped into 604~673 Operational Taxonomic Units (OTUs), while 30,711~44,718 filtered fungal sequences were obtained that were grouped into 17~44 OTUs. The bacterial coverage index was greater than 0.996, and the fungal coverage index all reached 1.000.
All bacterial OTUs belonged to 30 bacterial phyla. In at least one sludge sample, 12 bacterial phyla were present with relative abundances of greater than 1.0%. The relative abundances of bacterial phyla are shown in Fig. 2. The primary bacterial phyla included Proteobacteria (37.5~69.6%), Bacteroidetes (13.2~32.3%), Chloroflexi (5.0~8.0%), Ignavibacteriae (3.9~5.6%), Nitrospirae (1.0~5.1%), Elusimicrobia (0.3~4.4%), Spirochaetae (0.1~3.0%), Actinobacteria (0.02~1.4%) and others, which play key roles in the removal of pollutants in wastewater.
The bacterial diversity of the sludge from A1 to A7 was principally composed of OTUs associated to the phylum Proteobacteria and Bacteroidetes. The phylum Proteobacteria exhibited the greatest relative abundance among all sludge bacterial phyla, with relative abundances in A1 and A2 samples of 38.4 and 37.5%, respectively. The relative abundance of Proteobacteria increased to 49.3% in the A3 bulked sludge sample, and during the restoration of settleability by adding an anaerobic step, the relative abundance of this phylum gradually increased to 69.6% in A7 sample, which exhibited fully restored settleability. The relative abundance of Bacteroidetes, Chloroflexi, Ignavibacteriae and Acidobacteria were not obvious. The relative abundance of Actinobacteria was 0.05 and 0.02% in A1 and A2 samples before bulking, respectively, increasing to 0.4% in A3 the bulked sludge sample and to 1.4% in the A4 sludge sample at the beginning of the settleability restoration. However, for the A7 sample, which had fully restored settleability, the relative abundance decreased to 0.2%. The relative abundances of the phyla Nitrospirae, Elusimicrobia and Spirochaetae were 5.1, 4.4 and 3.0% in the A1 sludge sample, decreasing to 2.1, 0.5 and 0.1% in the A3 bulked sludge sample, respectively. When the anaerobic step was added, the relative abundances of these bacterial phyla slightly increased.
In total, 342 bacterial genera were observed from sequencing sludge samples. In at least one sludge sample, 14 bacterial genera were present with relative abundances of greater than 1.0%. The relative abundance of bacterial genera is shown in Fig. 3. The primary bacterial genera observed included Candidatus_Competibacter (9.2~31.4%), norank_f_Saprospiraceae (3.0~10.5%), Zoogloea (1.8~6.4%), norank_f_env.OPS_17 (0.2~8.4%), norank_f_PHOS-HE36 (3.1~6.9%), Defluviicoccus (0.9~7.7%), norank_f_Anaerolineaceae (1.9~4.6%) and others. Candidatus_Competibacter had the highest relative abundance among all samples and was the dominant bacterial genus. The relative abundance of Candidatus_Competibacter in the A1 and A2 sludge samples was 9.2 and 10.1%, and that of Defluviicoccus was 0.9 and 1.0%, respectively. The relative abundances of these two genera increased to 13.2 and 4.9% in sample A3 to 22.6 and 7.7% in sample A6 and to 31.4 and 4.1% in sample A7, respectively. Candidatus_Accumulibacter was present at a relative abundance of 1.4% in the A2 sludge samples, decreased to 6.3% in the A3 bulked sludge sample, then gradually increased after adding the anaerobic step, reaching 1.5% in the A7 sludge sample with fully restored settleability. Thiothrix and norank_o_Sphingobacteriales exhibited notable changes in relative abundance throughout the experiment. Thiothrix was present at a relative abundance of 0.2 and 0.3% in the A1 and A2 sludge samples, respectively, which exhibited excellent settleability at the beginning of the experiment. The relative abundance of Thiothrix increased to 6.3% in the A3 bulked sludge sample, then gradually decreased after adding the anaerobic step, reaching 3.0% in the A6 and reaching 0.7% in the A7 sludge sample with fully restored settleability. The relative abundance of norank_o_Sphingobacteriales was 0.2% in the A1 sludge sample, increasing to 6.6% in the A2 sludge sample and was 4.3% in the A3 bulked sludge sample. The relative abundance of norank_o_Sphingobacteriales gradually decreased after adding the anaerobic step to 0.6 and 0.8% in the A6 and A7 sludge samples, respectively. The relative abundance of norank_f_Saprospiraceae was 8.6 and 10.5% in the A1 and A2 sludge samples, respectively, which had excellent settleability at the beginning of the experiment. Because of the impact of sludge bulking, the relative abundance of norank_f_Saprospiraceae decreased to 5.0% in the A3 bulked sludge sample and to 3.0% in the A7 sludge sample with fully restored settleability. Due to sludge bulking, the relative abundance of Nitrospira decreased from 5.1% in the A1 sludge sample to 2.1% in the A3 bulked sludge sample. This value further decreased to 1.0% in the A7 sludge sample with fully restored settleability. Therefore, the relative abundance was greatly affected by the filamentous sludge bulking.
Analysis of fungal community of the activated sludge before and after adding an anaerobic step
Sixteen fungal phyla were observed from in the sludge samples. In at least one sludge sample, 6 fungal phyla were present with relative abundances of greater than 1.0%. The relative abundances of fungal phyla are shown in Fig. 4. The primary fungal phyla included Basidiomycota (14.7~81.2%), norank_k—Fungi (17.1~69.3%), Ascomycota (0.3~26.9%) and others. Their relative abundances of these phyla in the A1 sludge sample were 39.1, 29.5 and 26.9%, respectively, thus exhibiting a relatively even distribution. However, the relative abundances of these phyla exhibited significant changes after sludge bulking. In the A3 bulked sludge sample, the relative abundance of Basidiomycota increased to 81.2% but was only 14.7% in the A7 sludge sample with excellent settleability. The relative abundance of Ascomycota was 26.3% in the A1 sludge sample, which exhibited excellent settleability at the beginning of the experiment, decreasing to below 1.0% after sludge bulking, indicating that the relative abundance was greatly affected by sludge bulking. The relative abundance of norank_k—Fungi was 29.5% in the A1 sludge sample, decreasing to 17.1% in the bulked sludge and then gradually increased after adding the anaerobic step. For example, the relative abundance of this phylum was 28.0 and 69.3% in the A6 and A7 sludge samples, respectively.
Thirty-five fungal genera were observed in the sludge samples. In at least one sludge sample, 10 fungal genera were present with relative abundances of greater than 1.0%. The relative abundances of fungal genera are shown in Fig. 5. The relative abundance of Trichosporon was 38.9% in the A1 sludge sample with excellent settleability at the beginning of the experiment, increasing to 81.2% in the A3 bulked sludge sample and decreasing to 14.7% in the A7 sample after adding the anaerobic step. The relative abundance of norank_k_Fungi decreased from 29.5% in the A1 sludge sample to 17.1% in the A3 sample, gradually increasing to 69.3% in the sample A7. However, the relative abundances of norank_o_Salpingoecidae, norank_p_Blastocladiomycota and Spumella were 1.5, 0.9 and 0.1% in the A1 sludge sample, respectively. These three groups were lower than 1.0% in the A3 bulked sludge and the A6 sludge samples, increasing to 7.6, 3.9, and 2.8% in the A7 sludge sample with fully restored settleability, respectively. Therefore, norank_o_Salpingoecidae, norank_p_Blastocladiomycota and Spumella are favourable for the improvement of sludge settleability.
Relationship between the EPS concentration and settleability of the activated sludge before and after adding an anaerobic step
The sludge EPS concentration in the reactor was measured when the operation from day 109 to 171 of the experiment. Because the measured humic acid and DNA concentrations were low during the experiment, the total EPS was represented by the sum of polysaccharides and protein concentrations. The protein concentration was 26.8~74.8 mg·(gMLSS)−1, accounting for 59.2~92.6% of the total EPS, while the polysaccharide concentration was 3.1~32.9 mg·(gMLSS)−1, accounting for 7.4~40.8% of the total EPS. Thus, proteins were the primary component of EPS in this study.
Discussion
Filamentous sludge bulking occurred to the activated sludge. By adding a 30 min anaerobic step before the aerobic step, the amount of sludge zoogloea was increased while that of filamentous bacteria decreased. The average rate of the SVI decrease of activated sludge was 8.9 mL·(g·cycle)−1. Only 10 running cycles were required to rapidly and effectively inhibit sludge bulking.
Microbial community analysis provided crucial information to understand adding an anaerobic step can rapidly inhibit sludge bulking. High-throughput sequencing was used to analyse the bacterial community composition of the activated sludge in the SBR reactor. The coverage index was greater than 0.996, and the fungal coverage index all reached 1.000, indicating that the depth of the sequence was sufficient. The higher the Chao index value, the richer the species richness, while the higher the Shannon index, the higher the diversity of the communities18. There was little change in the bacterial Chao and Shannon indices for each sample. During the sludge bulking process, the richness of the bacterial community gradually increased, but the diversity gradually decreased. After adding the anaerobic step, the bacterial community richness first decreased and then gradually increased during the sludge settleability restoration process, while the bacterial diversity initially increased and then gradually decreased. The fungal richness and diversity in sample A1 was higher than those of the A3, A6 and A7 samples. Sludge bulking influenced the fungal community in that the fungal richness and diversity decreased due to sludge bulking. After adding the anaerobic step, the fungal richness decreased, while the diversity gradually increased.
The phylum Proteobacteria exhibited the greatest relative abundance among all sludge bacterial phyla, which is consistent with the results of the bacterial communities in soil19,20,21 and activated sludge22,23. Proteobacteria have wide distribution and high diversity24, and their primary function during wastewater treatment is to remove organic pollutants, nitrogen and phosphorus25. Because of the eurytopic nature of the phylum Proteobacteria, its relative abundance was increased after adding the anaerobic step. Bacteroidetes, Chloroflexi, and Acidobacteria were also often observed in activated sludge24,26, Bacteroidetes have strong metabolic capacity for complex organics, proteins and lipids, and can decompose complex macromolecules into simple compounds, which play an important role in the ecosystem27. Chloroflexi is mostly filamentous bacteria, and exists in the form of flocs skeleton in flocculent sludge clump inside body, play a role of sludge flocculation, at the same time with macromolecular organic matter degradation ability and has good biological phosphorus removal effect28. Firmicutes may be related to the degradation of COD and refractory macromolecules22. Research has shown that the excessive growth of Actinobacteria can induce filamentous sludge bulking29. The relative abundances of the phyla Nitrospirae, Elusimicrobia and Spirochaetae decreased in the bulked sludge sample, indicating that the excessive proliferation of filamentous bacteria inhibited the growth of these microorganisms.
Thiothrix and norank_o_Sphingobacteriales exhibited notable changes in relative abundance throughout the experiment, both Thiothrix and norank_o_Sphingobacteriales showed a noticeable increase. The excessive proliferation of filamentous Thiothrix can cause sludge bulking30.These observations indicated that the excessive proliferation of these two bacteria is the primary reason for the observed filamentous sludge bulking in the experiments.
The excessive proliferation of Thiothrix and norank_o_Sphingobacteriales in the bacterial genera resulted in sludge bulking. After adding the anaerobic step as an anaerobic selector, the growth of Thiothrix and norank_o_Sphingobacteriales were inhibited. The sludge bulking was rapidly and effectively inhibited.
Similar results were found in studies by Donkin and Nielsen. The presence of an anaerobic tank ahead of a completely mixed aerobic reactor as an anaerobic selector was to limit/suppress Thiothrix-caused bulking in dairy wastewater treatment plants31. Nielsen’s32 research shows that Thiothrix remained physiologically active under prolonged anaerobic conditions, but it seemed that the growth of Thiothrix was limited in such environments with an anaerobic selector ahead of a completely mixed aerobic conditions. In addition, other research shows that12 adding the anaerobic step as an anaerobic selector generally showed good performances in controlling filamentous bacteria when treating both municipal and industrial wastewaters, namely when bulking occurred following Type 0041 overgrowth. Constantinos et al.33 proved that under the conditions prevailing in anaerobic selector tank, the growth of M. parvicella was limited.
In activated sludge, both glycogen accumulating organi (GAO) and phosphorusaccumulatingorganism (PAO) were reported to help structuring the biomass into dense and stable aggregates34,35 and help increasing the settleability of sludge. Most common GAO reported were the Gammaproteobacterium Candidatus_Competibacter and the Alphaproteobacterium Defluviicoccus36,37, most common PAO were the Betaproteobacterium Candidatus_Accumulibacter. In the anaerobic step, these two glycogen-accumulating bacteria Candidatus_Competibacter, Defluviicoccus and Candidatus_Accumulibacter adsorb large amounts of organic substrates, such as volatile fatty acids, converting them to intracellular polymers such as polyhydroxyalkanoates for storage and inhibiting the growth of filamentous bacteria. This is also beneficial for the formation of high-density sludge particles, further increasing the settleability of sludge38. The filamentous bulking was solved after adding the anaerobic step and a significant population of Candidatus_Competibacter, Defluviicoccus and Candidatus_Accumulibacter was established.
Although sludge bulking caused a decrease in the relative abundance of Nitrospira, members of which played an important role in the biodenitrification process, the effluent ammonia nitrogen concentration did not increase. This result occurred because the DO concentration in the mixed solution was greater than 2.0 mg·L−1, which improved the activity of Nitrospira39,40 and its ammonia nitrogen oxidation capability.
High-throughput sequencing was used to analyse the fungi community composition of the activated sludge in the SBR reactor also. The primary fungal phyla included Basidiomycota, norank_k—Fungi and Ascomycota, which are typically present in the activated sludge of urban wastewater treatment plants41. In the bulked sludge sample, the relative abundance of Basidiomycota increased and decreased in sludge sample with excellent settleability after adding an anaerobic step before the aerobic step. These results indicate that the excessive proliferation of Basidiomycota is unfavourable to the settlement of sludge, while the addition of the anaerobic step can effectively inhibit the proliferation of this fungal phylum.
The relative abundance of norank_k—Fungi was increased after adding an anaerobic step before the aerobic step, indicating that the improvement in the relative abundance of norank_k—Fungi in sludge is favourable to the settleability of sludge, and the addition of an anaerobic step is favourable for the growth of norank_k—Fungi and can promote the growth and proliferation of norank_k—Fungi, thus improving the settleability of sludge.
The relative abundance of Trichosporon was 38.9% in the sludge sample with excellent settleability at the beginning of the experiment, increasing to 81.2% in the bulked sludge sample and decreasing to 14.7% in the sample after adding the anaerobic step. Trichosporon can induce sludge bulking6, the excessive proliferation of Trichosporon resulted in sludge bulking in the SBR reactor in this study. After adding the anaerobic step, the growth of these bacteria and fungi were inhibited.
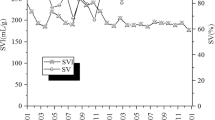
Akkache et al.42 showed that the presence of EPS improved the settleability of activated sludge. However, other studies43,44 concluded that the EPS concentration is positively correlated to the sludge SVI values and is thus unfavourable for the settleability of sludge. The variation in the EPS concentration and SVI values in the sludge in the experiment are shown in Fig. 6. From day 117 to day 131, the polysaccharide concentration in the EPS of the sludge rapidly increased from 3.61 to 25.86 mg·(gMLSS)−1. The rapid increase in the polysaccharide concentration causes an increase of electrophoretic mobility and an increase of the repulsive force between the sludge flocs. As a result, the settleability of sludge was worsened45, and the SVI value increased from 123 to 201 mL·g−1. The change in the bacterial community composition in the activated sludge from day 154 to day 161 after adding the anaerobic step resulted in a change in the EPS concentration46, which increased from 84.4 to 104.0 mg·(gMLSS)−1, and the concentration of proteins secreted by microorganisms rapidly increased from 60.2 to 74.3 mg·(gMLSS)−1. The increase in the protein concentration decreases the effective critical potential on the surface of microorganisms and promotes bioflocculation47. The sludge Settling Velocity (SV) rapidly decreased from 92 to 39%, and the sludge SVI value decreased from 222 to 74 mL·g−1. The increase in the polysaccharide concentration in the EPS of sludge is unfavourable to the settlement of activated sludge. The change in the bacterial community composition after adding the anaerobic step resulted in an increase of protein concentration in the EPS in the sludge, promoting the settlement of activated sludge. The growth and proliferation of filamentous bacteria was inhibited after adding the anaerobic step. The bacterial and fungal community compositions in the sludge exhibited significant changes. The change of dominant bacteria and fungi resulted in the increase of EPS concentration in the sludge and promoted the restoration of the settleability of sludge.
Materials and Methods
Experimental equipment and operation mode
SBR reactor with an effective volume of 11 L were used as the experimental equipment, running for 2 cycles every day with a 10 min water influent step, a 360 min aerobic step, a 60 min precipitation step, a 10 min drainage step with a drainage ratio of 38.5%, and a 290 min standing step. After adding the anaerobic step, the equipment was run with a water 10 min water influent step, a 30 min anaerobic step, a 360 min aerobic step, a 60 min precipitation step, a 10 min drainage step, and a 260 min standing step.
Seed sludge and experimental water
The seed sludge was taken at the end of the aerobic step of the oxidation ditch process in a wastewater treatment plant in the Changji city of Xinjiang.The MLSS of the seed sludge was 4,512 mg·L−1 and the SVI was 202 mL·g−1. The experimental water used was synthetic water that simulated domestic wastewater. The primary components of the synthetic water included CH3COONa·3H2O, NH4Cl, KH2PO4, MgSO4·7H2O and CaCl2, while the trace components included FeSO4·7H2O, CuSO4·5H2O, H2BO4, MnSO4·H2O, NaMo4·2H2O, ZnCl·7H2O and CoCl·6H2O. The COD concentration was 520 mg·L−1, the Total Nitrogen (TN) concentration was 52 mg·L−1 and the Total Phosphorus (TP) concentration was 5.2 mg·L−1.
Experimental process and sampling
Bulked sludge was used as the seed sludge used in this study. After excellent settleability was maintained, the experiment was initiated and lasted for 208 days. The experimental process was as follows. From day 1 to day 108, the operation status remained unchanged, with a (DO) concentration of 1.09~4.98 mg·L−1. From day 109 to day 130, while other parameters remained constant, the DO concentration decreased to 0.20~0.65 mg·L−1, resulting in the gradual bulking of sludge. From day 131 to day 153, the aeration rate was restored such that the DO concentration was 2.19~3.50 mg·L−1, although the settleability of sludge was not restored, even with sufficient DO. From day 154 to day 208, after water was added to the reactor, a 30 min anaerobic step was added prior to the aerobic step.
The sampling and numbering scheme is shown in Table 2. Sludge samples A1 and A2 had excellent settleability at the beginning of the experiment; sludge sample A3 was taken when the sludge bulking occurred; sludge samples A4, A5 and A6 were taken from the stage when the settleability of sludge was restored after adding the anaerobic step; and sludge sample A7 had fully restored settleability after the addition of an anaerobic step.
Analytical methods for water quality and sludge monitoring
Ammonium and COD were analyzed according to standard procedures. DO was measured with a portable DO analyser; pH was measured with a pH test pen; and the water temperature was measured with a mercury thermometer. Sludge volume index (SVI) were determined by reading the volume of the settled bed in a column after 30 min settling and calculated from the dry weight in (MLSS). Microscopic observations were performed with a photonic microscope. The morphology of filaments and flocs was evaluated on a day-to-day basis.
EPS extraction methods and component analysis methods
EPS was extracted through an ethylene diamine tetraacetic acid (EDTA) method, the activated sludge was harvested by centrifugation at 5600 rpm for 10 min at 4°C, the collected activated sludge was re-suspended in fresh sterile and harvested by centrifugation at 5500 rpm for 20 min at 4°C, and the collected activated sludge was re-suspended in fresh sterile and 2%EDTA (1:1) at 4 °C for 3 h, then harvested by centrifugation at 12300 rpm for 20 min at 4°C.Extractant residues in the solution were removed by the dialysis membrane filtration in the subsequent treatment. The supernatants were collected as EPS solution and stored at 4 °C or freeze-dried for further analysis.The carbohydrate content in EPS was measured by the anthrone method48 using glucose as the standard. The contents of protein in EPS were measured by the modified Lowry method49 using bovine serum albumin as the respective standards.
DNA extraction, PCR amplification and Illumina sequencing
Microbial DNA was extracted from sludge samples collected from the SBR reactor using the E.Z.N.A.® soil DNA Kit (Omega Bio-tek, Norcross, GA, U.S.). The final DNA concentration and purification were determined by NanoDrop 2000 UV-vis spectrophotometer (Thermo Scientific, Wilmington, USA), whereas DNA quality was checked by 1% agarose gel electrophoresis. The V4-V5 hypervariable regions of the bacteria 16 S r RNA gene was amplified with primers 515 F (5ʹ-GTGCCAGCMGCCGCGG-3ʹ) and 907 R (5ʹ-CCGTCAATTCMTTTRAGTTT-3ʹ), the fungal 16 S r RNA gene was amplified with primers 0817 F (5′-TTAGCATGGAATAATRRAATAGGA-3′) and 1196 R (5′-TCTGGACCTGGTGAGTTTCC-3′) by thermocycler PCR system (GeneAmp 9700, ABI, USA). The PCR reactions were conducted using the following program: 3 min of denaturation at 95 °C, 27cycles of 30 s at 95 °C, 30 s for annealing at 55 °C, and 45 s for elongation at 72 °C, and a final extension at 72 °C for 10 min. PCR reactions were performed in triplicates of 20 μL mixture containing 4 μL of 5 × FastPfu Buffer, 2 μL of 2.5 mM dNTPs, 0.8 μL of each primer (5 μM), 0.4 μL of FastPfu Polymerase and 10 ng of template DNA. The PCR products were extracted from a 2% agarose gel and further purified using the AxyPrep DNA Gel Extraction Kit (Axygen Biosciences, Union City, CA, USA). Then, the products were quantified using QuantiFluor™-ST (Promega, USA).
Purified amplicons were pooled in equimolar and paired-end sequenced (2 × 300) on an Illumina MiSeq platform (Illumina, San Diego, USA) according to the standard protocols of Majorbio Bio-Pharm Technology Co. Ltd. (Shanghai, China). The raw reads were deposited into the NCBI Sequence Read Archive (SRA) database (Accession Number: SRP127708).
Data analysis
Data analysis was conducted using the i-sanger platform (http://www.i-sanger.com/) provided by Majorbio Bio-Pharm Technology Co. Ltd. (Shanghai, China). The microbial phylotype richness levels were calculated using the Chao estimator, the Shannon diversity index. The Chao estimator, the Shannon diversity index and the coverage percentage were also calculated by the Mothur program version v.1.30.1 (http://www.mothur.org/wiki/Schloss_SOP#Alpha_diversity). These analyses were performed using the R Programming Language software.
References
Wagner, M. & Loy, A. Bacterial community composition and function in sewage treatment systems. Curr. Opin. Biotechnol. 13, 218–227 (2002).
Novák, L., Larrea, L., Wanner, J. & García-Heras, J. L. Non-filamentous activated sludge bulking caused by zoogloea. Water Sci. Technol. 29, 301–304 (1994).
Watanabe, K., Teramoto, M. & Harayama, S. An outbreak of nonflocculating catabolic populations caused the breakdown of a phenol-digesting activated-sludge process. Appl. Environ. Microbiol. 65, 2813–2819 (1999).
Burger, W. et al. The influence of protruding filamentous bacteria on floc stability and solid-liquid separation in the activated sludge process. Water Res. 123, 578–585 (2017).
Yang, Q., Zhao, H. & Du, B. Bacteria and bacteriophage communities in bulking and non-bulking activated sludge in full-scale municipal wastewater treatment systems. Biochem. Eng. J. 119, 101–111 (2017).
Zheng, S., Sun, J. & Han, H. Effect of dissolved oxygen changes on activated sludge fungal bulking during lab-scale treatment of acidic industrial wastewater. Environ. Sci. Technol. 45, 8928–8934 (2011).
Sheng, G. P., Yu, H. Q. & Li, X. Y. Extracellular polymeric substances (EPS) of microbial aggregates in biological wastewater treatment systems: a review. Biotechnol. Adv. 28, 882–894 (2010).
Guo, J. et al. Control filamentous bulking caused by chlorine-resistant Type 021N bacteria through adding a biocide CTAB. Water Res. 46, 6531–6542 (2012).
Meunier., C. et al. Influence of feeding pattern and hydraulic selection pressure to control filamentous bulking in biological treatment of dairy wastewaters. Bioresour. Technol. 221, 300–309 (2016).
Nittami, T. et al. Quantification of Chloroflexi Eikelboom morphotype 1851 for prediction and control of bulking events in municipal activated sludge plants in Japan. Appl. Microbiol. Biotechnol. 101, 3861–3869 (2017).
Lee, Y. & Oleszkiewicz, J. A. Bench-scale assessment of the effectiveness of an anaerobic selector in controlling filamentous bulking. Environ. Technol. 25, 751–755 (2004).
Guo, J. H. et al. Energy saving achieved by limited filamentous bulking sludge under low dissolved oxygen. Bioresour. Technol. 101, 1120–1126 (2010).
Kruit, J., Hulsbeek, J. & Visser, A. Bulking sludge solved?! Water Sci. Technol. 46, 457–464 (2002).
Beccari, M., Mappelli, P. & Tandoi, V. Relationship between bulking and physicochemical–biological properties of activated sludges. Biotechnol. Bioeng. 22, 969–979 (1980).
Martins, A. M., Pagilla, K., Heijnen, J. J. & van Loosdrecht, M. C. Filamentous bulking sludge–a critical review. Water Res. 38, 793–817 (2004).
Mueller, J. A. et al. Full-scale demonstration of improvement in aeration efficiency. J. Environ. Eng. 126, 549–555 (2000).
Beun, J. J., Verhoef, E. V., Van Loosdrecht, M. & Heijnen, J. J. Stoichiometry and kinetics of poly-B-hydroxybutyrate metabolism under denitrifying condition in activated sludge cultures. Biotechnol. Bioeng. 68, 496–507 (2000).
Shu, D., He, Y., Yue, H., Zhu, L. & Wang, Q. Metagenomic insights into the effects of volatile fatty acids on microbial community structures and functional genes in organotrophic anammox process. Bioresour. Technol. 196, 621–633 (2015).
Roesch, L. et al. Pyrosequencing enumerates and contrasts soil microbial diversity. Isme J. 4, 283–290 (2007).
Spain, A. M., Krumholz, L. R. & Elshahed, M. S. Abundance, composition, diversity and novelty of soil Proteobacteria. Isme J. 3, 992–1000 (2009).
Sun, M., Xiao, T., Ning, Z., Xiaom, E. & Sun, W. Microbial community analysis in rice paddy soils irrigated by acid mine drainage contaminated water. Appl. Microbiol. Biotechnol. 99, 2911–2922 (2015).
Hu, M., Wang, X., Wen, X. & Xia, Y. Pyrosequencing analysis of bacterial diversity in 14 wastewater treatment systems in China. Appl. Environ .Microbiol. 78, 7042–7047 (2012).
Gao, P. et al. Correlating microbial community compositions with environmental factors in activated sludge from four full-scale municipal wastewater treatment plants in Shanghai, China. Appl. Microbiol. Biotechnol. 100, 4663–4673 (2016).
Zhang, T., Shao, M. F. & Ye, L. 454 Pyrosequencing reveals bacterial diversity of activated sludge from 14 sewage treatment plants. Isme J. 6, 1137–1147 (2012).
Bucci, V., Majed, N., Hellweger, F. L. & Gu, A. Z. Heterogeneity of intracellular polymer storage states in enhanced biological phosphorus removal (EBPR) - observation and modeling. Environ. Sci. Technol. 46, 3244–3252 (2012).
Zhao, D. et al. Pyrosequencing analysis of bacterial community and assembly in activated sludge samples from different geographic regions in China. Appl. Microbiol. Biotechnol. 98, 9119–9128 (2014).
Hill, V. R. et al. Multistate evaluation of an ultrafiltration-based procedure for simultaneous recovery of enteric microbes in 100-liter tap water samples. Appl. Environ. Microbiol. 73, 4218–4225 (2007).
Kragelund, C. et al. Identity, abundance and ecophysiology of filamentous Chloroflexi species present in activated sludge treatment plants. Fems Microbiol. Ecol. 59, 671–682 (2007).
Seviour, R. J. et al. Ecophysiology of the Actinobacteria, in activated sludge systems. Anton. Leeuw. Int. J. G. 94, 21–33 (2008).
Henriet, O., Meunier, C., Henry, P. & Mahillon, J. Filamentous bulking caused by Thiothrix species is efficiently controlled in full-scale wastewater treatment plants by implementing a sludge densification strategy. Sci. Rep. 7 (2017).
Donkin, M. J. Bulking in aerobic biological systems treating dairy processing wastewaters. Int. J. Technol. 50, 67–72 (1997).
Nielsen, P. H., de Muro, M. A. & Nielsen, J. L. Studies on the in situ physiology of Thiothrix spp. present in activated sludge. Environ. Microbiol. 2, 389–398 (2000).
Noutsopoulos, C., Mamais, D. & Andreadakis, A. Long chain fatty acids removal in selector tanks: Evidence for insufficient Microthrix parvicella control. Desalin Water Treat. 23, 20–25 (2010).
de Kreuk, M. K. & van Loosdrecht, M. C. M. Selection of slow growing organisms as a means for improving aerobic granular sludge stability. Water Sci. Technol. 49, 9–17 (2004).
Bin, Z. et al. Denitrifying capability and community dynamics of glycogen accumulating organisms during sludge granulation in an anaerobic-aerobic sequencing batch reactor. Sci. Rep. 5, 12904 (2015).
Burow, L. C., Kong, Y., Nielsen, J. L., Blackall, L. L. & Nielsen, P. H. Abundance and ecophysiology of Defluviicoccus spp., glycogen-accumulating organisms in full-scale wastewater treatment processes. Microbiology. 153, 178–85 (2007).
Kong, Y., Xia, Y., Nielsen, J. L. & Nielsen, P. H. Ecophysiology of a group of uncultured Gammaproteobacterial glycogenaccumulating organisms in full-scale enhanced biological phosphorus removal wastewater treatment plants. Environ. Microbiol. 8, 479–489 (2006).
Seviour, T., Lambert, L. K., Pijuan, M. & Yuan, Z. Selectively inducing the synthesis of a key structural exopolysaccharide in aerobic granules by enriching for Candidatus, “Competibacter phosphatis”. Appl. Microbiol. Biotechnol. 92, 1297–1305 (2011).
Kita, Y., Nishikawa, H. & Takemoto, T. Effects of cyanide and dissolved oxygen concentration on biological Au recovery. J. Biotechnol. 124, 545–551 (2006).
Qian, F. et al. Differentiation in nitrogen-converting activity and microbial community structure between granular size fractions in a continuous autotrophic nitrogen removal reactor. J. Microbiol. Biotechnol. 27, 1798–1807 (2017).
Mazamárquez, P. et al. Community structure, population dynamics and diversity of fungi in a full-scale membrane bioreactor (MBR) for urban wastewater treatment. Water Res. 105, 507–519 (2016).
Akkache, S., Seyssiecq, I. & Roche, N. Effect of exo-polysaccharide concentration in the rheological properties and settling ability of activated sludge. Environ. Technol. 34, 2995–3003 (2013).
Li, X. Y. & Yang, S. F. Influence of loosely bound extracellular polymeric substances (EPS) on the flocculation, sedimentation and dewaterability of activated sludge. Water Res. 41, 1022–1030 (2007).
Liao, B. Q., Allen, D. G., Droppo, I. G., Leppard, G. & Liss, S. N. Surface properties of sludge and their role in bioflocculation and settleability. Water Res. 35, 339–350 (2001).
Yang, S. F., Tay, J. H. & Liu, Y. Inhibition of free ammonia to the formation of aerobic granules. Biochem. Eng. J. 17, 41–48 (2004).
Miao, L. et al. Characterization of EPS compositions and microbial community in an anammox SBBR system treating landfill leachate. Bioresour. Technol. 249, 108–116 (2017).
Liu, Y. et al. Regulation of aerobic granular sludge reformulation after granular sludge. broken: effect of poly aluminum chloride (PAC). Bioresour. Technol. 158, 201–208 (2014).
Gaudy, A. F. Colorimetric Determination of Protein and Carbohydrate. Ind. Water Wastes 7, 17–22 (1962).
Frølund, B., Griebe, T. & Nielsen, P. H. Enzymatic activity in the activated-sludge floc matrix. Appl. Microbiol. Biotechnol. 43, 755–761 (1995).
Acknowledgements
National Natural Science Foundation of China (51568061).
Author information
Authors and Affiliations
Contributions
J.Y. and Y.C. designed the study. J.Y., J.L., Y.Z., Y.H. and S.X. performed all the experiments and analyses. J.L. edited the manuscript. All authors reviewed the manuscript and approved the manuscript for publication.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yao, J., Liu, J., Zhang, Y. et al. Adding an anaerobic step can rapidly inhibit sludge bulking in SBR reactor. Sci Rep 9, 10843 (2019). https://doi.org/10.1038/s41598-019-47304-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-47304-3
- Springer Nature Limited
This article is cited by
-
Microbial community structure of an anaerobic side-stream coupled anoxic–aerobic membrane bioreactor (AOMBR–ASSR) for an in-situ sludge reduction process
Bioprocess and Biosystems Engineering (2024)