Abstract
Conspicuous carotenoid ornamentation is considered a signal of individual “quality” and one of the most intensely studied traits found to co-vary with parasitism. Since it has been suggested that only “high quality” individuals have enough resources to express excessive sexual ornaments and resist parasites, current theory struggles to explain cases where the brightest individuals carry the most parasites. Surprisingly little emphasis has been put on the contrasting routes to fitness utilized by different parasite species inhabiting the same host. Using Arctic charr (Salvelinus alpinus) as model species, we hypothesized that skin redness and allocation of carotenoids between skin and muscle (redness ratio) will be positively and negatively associated with parasites using the fish as an intermediate and final host, respectively. Both pigment parameters were indeed positively associated with abundances of parasites awaiting trophic transmission (Diplostomum sp. and Diphyllobothrium spp.) and negatively associated with the abundance of adult Eubothrium salvelini tapeworms. These empirical data demonstrate that contrasting associations between carotenoid coloration and parasite intensities relates to the specific premises of different parasite species and life cycle stages.
Similar content being viewed by others
Introduction
Animal coloration has spurred some of the most controversial research in evolutionary biology. Even before Darwin, color signals such as mimicry, warning colors and sexual ornamentation provided pioneering evidence for sexual selection and evolution. Among the most widespread and spectacular ornamental traits in animals are red, orange and yellow carotenoid-based color displays1,2,3,4,5,6, believed to have arisen through strong mating preference for bright colors. Indeed, conspicuous sexual ornamentation is considered a text book example of an “honest” signal of individual “quality” in the sense that carotenoids are in limited supply and incurs a trade-off between use in external signaling versus use in other critical functions in the body (e.g. immune protection against parasites and pathogen infection).
Indeed, animal pigmentation is one of the most intensely studied phenotypic traits found to co-vary with parasitism1,4,7,8,9. In several species of bird for example, the brightest individuals tend to carry fewer parasites5,10. One example is provided by the blackbird (Turdus merula), where the individuals with the brightest beaks carry fewer intestinal parasites (Isospora Protozoa, Apicomplexa)5. Thus, considering the dual use of carotenoids for host physiological responses against parasites and for sexual ornamentation (i.e. physiological trade-off theory), bright ornamentation can be taken to signal good health and parasite resistance. Under this physiological trade-off framework, however, cases where the brightest individuals are found to carry more parasites than their paler conspecifics are more difficult to reconcile.
In teleost fish with pronounced carotenoid displays, several cases of positive associations between carotenoid-based ornamentation and parasite abundance have been reported2,6,11. For example, the Arctic charr (Salvelinus alpinus L.) with the brightest red breeding coloration carry more eye flukes (trematode Diplostomum spp. metcercariae)1. Theories explaining such positive associations are less straightforward, but include the “immunocompetence handicap-principle” suggesting that bright individuals signal that they are of high quality, despite carrying many parasites1,7,12. The processes described by this theory have, however, proven difficult to demonstrate empirically13. Skarstein and Folstad suggested that higher parasite intensities in more ornamented Arctic charr could be related to immunosuppressive effects of sex hormones necessary for ornament development although sex hormones were not quantified in their study1. They also suggested that increased susceptibility to parasites may represent a cost that ensures honesty of carotenoid ornamentation. This theory is, however, complicated by cases where both positive and negative associations between carotenoid coloration and parasite intensities are observed. In three-spined stickleback (Gasterosteus aculeatus), for example, the individuals with the brightest red breeding coloration suffer from more muscle-dwelling (Diphyllobothrium spp.), but fewer (Schistocephalus solidus) plerocercoids11.
Thus, whether bright carotenoid ornamentation signals parasite resistance (physiological trade-off theory) or high quality despite increased parasite susceptibility (immunocompetence handicap-principle) is still an open question that begs for further exploration. Moreover, since the two existing theories seem somewhat contradictory, other alternative explanations should be explored. For instance, color polymorphisms may be associated with other phenotypic traits affecting encounter probability or resistance14. In this context, we note that little emphasis has been put on the large diversity of parasite species found to co-vary with carotenoid coloration. Indeed, studies investigating carotenoid-based pigmentation in parasitized animals largely focus on host quality and fitness and appear to neglect that parasites also need to heed their own life cycle and fitness.
For example, many helminth parasites (e.g. nematodes, trematodes, cestodes and acantacephalans) depend on a host being eaten by another host (i.e. trophic transmission) to complete their life cycle15,16,17. Hence, whereas adult parasites aiming to maximize reproductive output in the gut of their current and final host should benefit from a long-lived host that stays away from danger, trophically transmitted parasites should actually be under selection pressure to make their host more conspicuous or available to the next host in their life cycle16. Indeed, many trophically transmitted parasites modify the behavior or appearance of their intermediate host to increase the risk of being preyed upon15,16,17. Lafferty16 noted that some parasites may benefit from residing in brighter intermediate hosts. For example, Diphyllobothrium (spp.), uses copepods (a crustacean) as first intermediate host, teleosts as second intermediate host, and birds as definitive hosts. Lafferty hypothesized that this parasite may occur more frequently in brighter colored sticklebacks since copepods represent a rich and important source of carotenoids for these fish. Thus, in addition to making male sticklebacks more attractive to females, carotenoids may also make them easier to spot for predatory birds16. With this assumption, we would expect a positive relationship for all parasites transmitted to sticklebacks via copepods. That is not always the case. On the contrary, the tapeworm, S. solidus, that is also transmitted via carotenoid-rich copepods, is actually more abundant in paler sticklebacks11.
Thus, proximate mechanisms behind positive and negative associations between parasites and carotenoid displays are probably more complex and could instead involve parasites actively modifying carotenoid allocation to or from the skin. However, whether contrasting reports on associations between parasitism and coloration (i.e. high parasite load and bright coloration versus high parasite load and pale coloration) can be explained by the circumstance that individual hosts can be infected with multiple parasite species possessing divergent life strategies (i.e. aiming to maximize reproductive output in final host or ascending the trophic chain to reach its next host) is unknown. In fact, whether parasites benefitting from a more conspicuous host are more abundant in brighter individuals and vice versa has to our knowledge not been tested specifically.
In the present study, we take the parasites’ perspective on conspicuous carotenoid-based ornamentation. To investigate whether parasites benefitting from a more conspicuous host are more abundant in brighter individuals, and vice versa, we used a species that is ideal for studying phenotypic variation in the context of parasites and pigmentation, the Arctic charr. The Arctic charr has been referred to as “the most variable vertebrate on earth”18, and one of its striking characteristics that makes it particularly suitable for studying intraspecific variation is that individual skin color varies from light pink to dark red within a given population (see Fig. 1). As mature Arctic charr enter their reproductive season in the autumn, carotenoid pigments are moved from the muscle to the skin, so that the muscle becomes paler in color while the skin becomes more red and conspicuous. This phenomenon allows for observing the coarse distribution between use of pigment for ornamentation in skin versus use in other somatic functions internally. Moreover, Arctic charr inhabiting lakes in northern Norway harbor a diverse community of parasites (Table 1). Some of these parasites (e.g. Diplostomum sp. and Diphyllobothrium spp.) are trophically transmitted from charr to bird final hosts19,20 and would benefit from a host that is more susceptible to predation. Others (e.g. Eubothrium salvelini, Cyathocephalus truncatus and Protocephalus sp.) are transmitted from various intermediate hosts to definitive charr hosts where they can live for months to years21. Predation or death from other causes associated with high reproductive effort (e.g. immune deficiency) would not constitute a fitness advantage for such species.
Specific objectives of the study were to test the assumption that different parasite species with diverging life history strategies may show contrasting associations with carotenoid pigmentation in male Arctic charr. Also introducing the ratio of carotenoid-based coloration in skin to muscle as an indicator of pigment distribution represents a novel approach that better indicates allocation of pigments from internal stores to external signaling than skin redness per se. Specifically, we hypothesize that by quantifying infection intensities of multiple species of parasites in each individual host, it will be demonstrated that (1) multiple and even contrasting associations between parasite load and carotenoid allocation will be discovered and specifically (2) that skin redness and/or skin to muscle redness ratio will be positively associated with intensity of parasites using charr as an intermediate host and negatively associated with intensity of parasites using charr as final host.
Methods
Capture and sampling
We sampled Arctic charr from lakes Fjellfrösvatn (69°05′N, 19°20′E), Takvatn (69°07′N, 19°05′E) and Sagelvvatn (69°11′N, 19°06′E) in Troms county, northern Norway. All three lakes are dimictic and oligotrophic, and usually ice-covered from late November to May (see Supporting Table S1 for abiotic characteristics of the three study lakes). Charr in these lakes normally spawn from mid-September to early October1,22,23, so we collected fish on their shallow-water spawning grounds between 7–9 September 2016 using gill nets deployed for a maximum of 2 hours. Live fish were removed from the gill nets, placed in buckets containing 0.25 mg l−1 MS-222 (Sigma-Aldrich, St. Louis, Missouri, USA) and transported back to land. For each fish we measured fork length (+/− 1.0 mm), determined skin coloration (see Quantification of muscle coloration and parasite intensities section below), and collected a blood sample from the caudal vein using a 1 ml syringe containing ethylendiamin etetraacetic acid (EDTA), after which the fish were humanely killed by decapitation. Blood samples were centrifuged for 5 min at 700 g in a portable mini centrifuge. Plasma was frozen on dry ice and stored at −20 °C for later analysis of Testosterone (T) and 11-keto Testosterone (11kT) levels. Both eyes were dissected from each fish, each eye bagged separately in a small amount of saline solution, and stored on ice for quantification of Diplostomum sp metacercariae. The rest of the fish was stored on ice for quantification of parasites (Table 2). We caught only 10 sexually mature female Arctic charr. The strength of pigmentation and their association to parasite infection differ between Arctic charr sexes1,23, and we therefore excluded females from our analyses. This study was performed in strict accordance with the Norwegian legislation. For the fish sampling, a fishing permission is required from the fishing right owner. Accordingly, we obtained permissions for the gill net fishing in the lakes Takvatn, Fjellfrösvatn and Sagelvvatn from the County Authority of Troms County (permission reference number: 16/1356-9) with legal authority through LOV 1992-05-15 nr 47, §13. No ethical permission is required from the Norwegian Animal Research Authority for the sampling and described activities (FOR 1996-01-15 no 23, the Norwegian Ministry of Agriculture and Food).
Quantification of parasite intensities
An overview of the parasite species found and their life cycles are given in Table 1. The number of Diphyllobothrium sp. cysts on the stomach wall was counted. There are two species of Diphyllobothrium present in charr from these systems, D. dendriticum and D. ditremum, and cyst counts provide a reliable estimate of their combined total number24. Intestines were frozen and later screened for parasites as described by Kuhn et al., (2017). Four metazoan parasites were identified from the intestines: Crepidostomum sp., Cyathocephalus truncatus, Eubothrium salvelini and Proteocephalus sp. We dissected the eyes and counted the number of Diplostomum sp. metacercariae within 24 hours of removal from the fish (with the exception of one individual counted after 48 hours). Finally we counted the total number of Cystidicola farionis nematodes from swimbladders preserved in 96% ethanol.
Determination of carotenoid coloration
To rate skin and muscle coloration, we created a redness scale based on the picture in Fig. 1, a gradient of 11 colors from bright pink to dark red, which yielded a redness score ranging from 0–100 (Fig. S1). If the coloration of skin or muscle was considered to be intermediate between two consecutive colors in the scale, we used the average score between the two colors. Skin coloration was determined for the abdominal area. If skin coloration varied considerably across the abdominal area, an average score was given (for example see Supplementary Fig. S2). Muscle coloration was determined in an area of the muscle located dorsolaterally. The ratio between skin and muscle coloration (redness ratio) was determined by dividing the redness score for the skin by the redness score for the muscle. To estimate reproducibility of color determination using the color scale, the average correlation between 8 different observers using the scale to score skin color of 8 fish was assessed. Average correlation was 94% (linear regression, average ± s.e.m. r2 = 0.94 ± 0.05).
Steroid measurement
Plasma concentrations of T and 11kT (ng/ml) were measured by means of radioimmunoassay (RIA) according to the method described by Frantzen et al.25. Briefly, steroids were first extracted from plasma samples using diethyl ether and then resolubilized in 300 µl cold RIA buffer. Samples were assayed in duplicate, together with 200 µl antiserum (gift from Dr. Helge Tveiten, University of Tromsø) and 50 µl 3H-labelled T or 11kT (Perkin-Elmer, USA). The tubes were vortexed and incubated overnight at 4 °C, after which 300 µl cold dextran-coated charcoal solution was added to all tubes, incubated for 5 minutes and then centrifuged for 5 minutes at 4000 rpm. The supernatant was decanted into scintillation tubes containing 4 ml scintillation fluid (Ultima Gold, Perkin-Elmer, USA) and counted in a liquid scintillation counter (Packard, TRI-CARB 2100TR). The cross-reactivity of the T and 11kT antisera is given by Frantzen et al.25. Blood sampling failed for two individuals, and these were treated as missing values in the statistical analysis.
Statistical analysis
For statistical analyses R v. 3.5.1. was used26. To test whether pigmentation was predicted by parasite infection we used two separate linear mixed models with skin redness and redness ratio as the respective response variables using the ‘lmer’ function from the ‘lmerTest’ package in R27. Initial fixed effects included the abundance of individual parasite species (listed in Table 1), their two-way interactions as well as fish length, T and 11-kT. Fixed effects were standardized by subtracting the mean and dividing by the standard deviation for each data point. Lake was included as a random effect to account for potential variation between populations. Models were stepwise simplified based on AIC values using the ‘dregde’ function from the ‘MuMIn’ library in R26. We compared models with random slope and intercept to random intercept only models to see if pigmentation and parasitism associated differently between populations. Random intercept only models performed better (lower AIC value) and are thus presented here. We assessed variance homogeneity and normal distribution of the residuals from diagnostic plots to ensure that we did not violate model assumptions. One fish from Fjellfrösvatn with extreme redness ratio was removed following inspection of model diagnostic plots. The predicted association between fixed effects and pigmentation by the model were plotted using the ‘Effect’ function from the ‘effects’ library in R28.
Results
Parasite infections
A total of 62 sexually mature males were caught on the spawning grounds across the three study lakes. There were some differences in parasite community composition between lakes. All fish were infected with Diphyllobothrium spp. and Diplostomum sp. (Table S2). Crepidostomum sp., C. farionis and E. salvelini were common in Takvatn and Fjellfrösvatn, whereas charr from Sagelvvatn had high infections of Proteocephalus sp. (Table S2). Cyathocephalus truncatus was common only in Fjellfrösvatn (62% prevalence) at a low abundance. Within lakes, individual charr varied considerably in levels of parasitic infection.
Coloration
There was an inverse relationship between skin and muscle redness (Pearson’s r = −0.30, P = 0.02). There were no significant differences in skin redness between lakes (one-way ANOVA, F60, 2 = 1.11, P = 0.34), and average redness scored between 50 and 60 for all lakes (Supplementary Table S2). Muscle redness was higher in Sagelvvatn than in the two other lakes (Tukey’s HSD test, both P < 0.001), and consequently redness ratios were comparatively lower in Sagelvvatn than in both Fjellfrösvatn (Tukey’s HSD test, P = 0.003) and Takvatn (Tukey’s HSD test, P < 0.001). There was a positive correlation between skin redness and redness ratios across all fish (Pearson’s r = 0.54, P < 0.001).
Final predictors of skin redness following model simplification are listed in Table 2. The total R2 for the model was 0.49, and the random factor lake accounted for zero variation (i.e. the model converged to a linear model). Skin redness increased with 11kT plasma concentrations, the abundance of Diplostomum parasites and fish length (Fig. 2A–C, Table 2). In contrast, skin redness associated negatively with the abundance of E. salvelini (Fig. 2D, Table 2). The model included interaction terms indicating that the effect of some parasite species on skin redness depend on levels of co-infection. For instance, the predicted negative association between E. salvelini and skin redness becomes positive at high intensities (>1000) of C. farionis (see Supplementary Fig. S3).
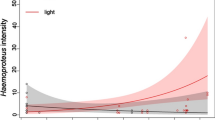
Effects plots illustrating predicted associations between fixed effects and pigmentation in wild-caught male Arctic charr caught in Takvatn, Fjellfrösvatn and Sagelvvatn in Troms County, Northern Norway during spawning season. Predicted associations (significant at P < 0.05) between (A) 11 keto-Testosterone (ng/ml), (B) infection intensity of Diplostomum species, (C) fish fork length (mm) and (D) infection intensity of Eubothrium salvelini and skin redness from a linear mixed model (Table 2). Predicted associations (significant at P < 0.05) between (E) 11 keto-Testosterone (ng/ml), infection intensity of (F) Diplostomum species, (G) Diphyllobothrium species and (H) Eubothrium salvelini and log-transformed ratio between skin and muscle redness (redness ratio) from a linear mixed model (Table 3). Inward ticks on x-axes indicate individual data points and bands indicate 95% confidence.
Predictors of redness ratios (i.e. allocation of pigment between skin and muscle) following model selection are listed in Table 3. The R2 for the model was 0.80 with the random factor lake accounting for 0.49 and fixed factors 0.31. There was a positive association between redness ratios and plasma concentrations of 11kT as well as the abundance of Diphyllobothrium spp. and Diplostomum sp. (Fig. 2E–G, Table 3). In addition, there was a negative association between redness ratio and the abundance of E. salvelini (Fig. 2H, Table 3). Interaction terms indicated that this negative association reversed at higher intensities of Diphyllobothrium spp. and C. farionis (see Supplementary Fig. S4).
Discussion
The objective of the current study was to test whether parasites benefitting from a more conspicuous host are more abundant in brighter individuals, and vice versa. Using wild-caught naturally infected Artic charr, we found that abundances of parasites awaiting trophic transmission to bird hosts associate positively with skin redness (Diplostomum sp.) as well as the ratio of redness in skin versus muscle (Diplostomum sp. and Diphyllobothrium spp.). We also show that abundance of a parasite species that use charr as definitive host (E. salvelini) and will gain fitness from hosts investing less in reproduction and secondary sexual characters, is negatively associated with the same phenotypic traits. To our knowledge, this is the first report of contrasting associations between carotenoid-based pigmentation and parasites species at different life history and transmission stages in a teleost host.
Pigmentation varied considerably among individuals in all three study lakes. Intriguingly, this variation in carotenoid pigmentation was predicted by parasite species and also by the sex steroid 11kT (Fig. 2, Tables 2 and 3). In agreement with our findings, Skarstein and Folstad found that the Arctic charr with the brightest red breeding coloration carry more Diplostomum eye flukes1. A novel aspect of the current study is that redness ratio (ratio of coloration in skin versus flesh) could also be predicted by parasite intensity (e.g. Diplostomum, Diphyllobothrium, and Eubothrium). Scoring the redness ratio is a novel approach that better indicates allocation of pigments from internal stores to external signalling than skin redness per se.
Since animals do not biosynthesize carotenoids de novo, but rely on diet for their supply of carotenoids, positive associations between skin redness and parasite abundance could be explained by dietary intake of carotenoid-rich intermediate host29. Put differently, the charr obtains parasites and carotenoids in the same meal. Indeed, the observed trend for a positive association between C. faronis and red skin coloration observed in the current study (p = 0.06, Table 2) could be ascribed to this phenomenon. This parasite can accumulate in charr over many years, indicating high feeding rates of carotenoid-rich amphipods over the host’s lifespan30,31. For Diphyllobothrium and Diplostomum, on the other hand, it is unlikely that the positive association between the infection intensities of these parasites and carotenoid pigmentation is related to diet. Firstly, Diphyllobothrium, transmitted to fish via carotenoid-rich copepods, was a predictor of redness ratio (reflecting allocation of carotenoids), but not of skin redness. Secondly, contrary to all other parasite species investigated in the current study Diplostomum sp. is not transmitted to charr via crustaceans (Table 1). Finally, the negative association between copepod-transmitted E. salvelini and redness/redness ratio in charr further precludes that diet alone can explain positive relationships between parasite intensities and carotenoid coloration.
We also have to consider the possibility that redder individuals (skin redness) or individuals with more pigment allocated to the skin (redness ratio) are more (1) susceptible or (2) exposed to Diplostomum sp. The first alternative seems unlikely for two main reasons. Firstly, Diplostomum sp. reside in the immune-privileged eye (i.e. hidden from the immune system) suggesting that the association between this parasite and host color does not involve an immune system response to infection. Like most digenetic trematodes, the free-swimming Diplostomum cercariae do however infect the fish by penetrating the skin32. Thus, we cannot rule out that penetration and migration of Diplostomum in blood vessels33 provoke an acute humoral (i.e. in blood) immune response in the host that limits infection success of the parasite and that individual variation in immune responsiveness could be related to carotenoid-based ornamentation1. This would be in line with the immunocompetence handicap-principle suggesting that brightly ornamented individuals signal high quality despite being more susceptible to parasites1,7. However, if redder Arctic charr are more susceptible to parasites, we should see positive associations between redness and the total parasite fauna in the animal, which is not the case. In fact, the intestinal cestode E. salvelini was even negatively associated with skin redness (Fig. 2, Table 2).
Such negative associations between parasite infection intensity and carotenoid ornamentation can reflect a trade-off between use of carotenoids in physiological responses against parasite infection and ornamentation (i.e. physiological trade-off theory)5,34. Such a trade-off could potentially explain the negative association between the copepod transmitted tapeworm E. salvelini and skin redness in our study since E. salvelini is a pathogenic parasite in salmonids35,36. This assumption is however complicated by the fact that we also see positive associations between Diphyllobothrium and redness ratio since this tapeworm is also pathogenic37.
In this context, species specific variation in parasite life cycle seems to be a unifying variable that can explain the contrasting associations observed in this study. Thus, if we instead consider the possibility that parasites influence physiological processes involving a redistribution of pigment between muscle and skin, reallocation of pigments available for immune function to external signaling could incur several advantages to parasites awaiting trophic transmission (such as Diplostomum and Diphyllobothrium). Firstly, the interior milieu could become more hospitable due to reduced host immune reactivity. Secondly, in the context of parasite-induced trophic transmission (PITT), costly ornaments such as nuptial coloration are associated with increased predation38,39,40,41,42. Of note, charr display a striking indifference to human presence during spawning as we and other charr researchers observed (see Supplementary Video kindly donated by Brattli et al.43) indicating increased risk-taking behavior. Thus positive relationships between parasite abundance and secondary sexual ornamentation could be caused by the presence of parasites that stand to benefit from host reproductive maturation (for instance those awaiting trophic transmission) and their putative influence on host behavior and physiology.
Conversely, parasites that have reached their final, sexually reproducing adult stage (e.g. E. salvelini) would instead benefit from a long-lived host, which engages itself in mundane matters such as energy intake rather than energetically costly reproduction associated with potentially risky behavior. In many animals, including Arctic charr, there is often a pronounced cessation or decline in feeding during spawning migrations or other reproductive behaviors (e.g. courtship, spawning, territoriality, guarding)44,45,46. Clearly, host anorexia in association with spawning would seem disadvantageous for a gamete-producing organism such as E. salvelini, which inhabits the intestine and pyloric caecae in the charr and feed on intestinal contents. Thus, a negative relationship between parasite abundance and secondary sexual ornamentation could be related to the presence of parasites that do not benefit from neither a conspicuous or anorectic host, let alone both. Of note, Crepidostomum sp. and Proteocephalus sp., also adult parasites in the intestine, did not show any association to pigmentation.
In short, reproductive maturation brings about coordinated changes in brain and endocrine function (increased steroid levels and associated neurobiological control), behavior (increased risk taking), external appearance (increased conspicuousness), and immune function (reduced), all of which provide potential pathways of influence and benefit to a trophically transmitted parasite. Thus, an efficient route to achieve substantial manipulation of host phenotype with limited intervention would be to interfere with the neuroendocrine system controlling reproduction (e.g. hypothalamus pituitary gonadal axis, HPG-axis). Intriguingly, the expression of two different secondary sex traits in male sticklebacks, fin size47 and carotenoid ornamentation11 that are both regulated by sex steroids, positively associate with intensity of parasites awaiting trophic transmission, including Diplostomum and Diphyllobothrium. Thus, these parasites could, in theory, achieve both these modifications by interfering with the production of sex steroids in their stickleback host.
So far, few reports have demonstrated that parasites can directly alter HPG-axis activity and production of sex steroids in its host. However, in the extensively studied Toxoplasma gondii model, where T. gondii infection in intermediate rodent hosts attenuates innate fear of predator urine, parasite-induced behavioral manipulation requires an increase in testosterone levels48. In the current study, accurate analyses of all molecular aspects of endocrine control of the reproductive axis were not feasible because the study animals were stressed during capture. Stress rapidly inhibits the HPG-axis, so any molecular measures could be biased by the capture method if tissue samples are not taken within a few minutes of capture. It is also important to remember that background variation in host physiology cannot be controlled for when using wild-caught fish as study animals. Moreover, steroid levels measured directly following capture does not necessarily reflect historic steroid production and exposure. Nonetheless, in this study the sex steroid 11kT (which is the primary end-point in the male HPG-axis in salmonids - as opposed to testosterone in mammals) positively associated with redness ratio. This observation supports that somatic redistribution of carotenoids is mediated by 11kT in Arctic charr49. In summary, it would appear that the individuals that are most heavily infected with parasites awaiting trophic transmission also demonstrate the strongest investment in reproduction.
In conclusion, our findings may represent the first empirical data demonstrating contrasting associations between carotenoid coloration and parasite infection intensities related to parasite species and life cycle. In short, parasites benefitting from a more conspicuous host are more abundant in brighter individuals, and vice versa. Hence, to fully explain the relationship between carotenoid pigmentation and parasitism, it is necessary to consider the wider range of parasites species and associated life histories occurring within single hosts. Moreover, the significant interaction terms (Supplementary Figs 3 and 4) in our models show that the association between parasite intensity and pigmentation depend on levels of co-infection. This substantiates the complex nature of parasite infections that can be observed in wild populations as opposed to in controlled experimental settings. Nonetheless, proximate mechanisms underlying the observed contrasting associations between parasitism and coloration should be addressed in experimental studies where background variation in host physiology, behavior, timing and magnitude of infections etc. can be controlled. The direction of the association should in this context be analysed with respect to expected fitness outcome for specific parasite species and life stages. We hope that the present study can encourage taking on a parasite’s perspective on host carotenoid allocation also in other model systems where sexual ornamentation is reported to associate with parasite abundance.
Data Availability
All relevant data are within the manuscript and its Supplementary Material.
References
Skarstein, F. & Folstad, I. Sexual dichromatism and the immunocompetence handicap: an observational approach using Arctic charr. Oikos 76, 359–367 (1996).
Milinski, M. & Bakker, T. C. M. Female sticklebacks use male coloration in mate choice and hence avoid parasitized males. Nature 344, 330–333 (1990).
Zuk, M., Johnsen, T. S. & MacLarty, T. Endocrine-immune interactions, ornaments and mate choice in red jungle fowl. P. Roy. Soc. B-Biol. Sci. 260, 205–210 (1995).
Lumpkin, D. C., Murphy, T. G. & Tarvin, K. A. Blood parasite infection differentially relates to carotenoid-based plumage and bill color in the American goldfinch. Ecol. Evol. 4, 3210–3217 (2014).
Baeta, R., Faivre, B., Motreuil, S., Gaillard, M. & Moreau, J. Carotenoid trade-off between parasitic resistance and sexual display: an experimental study in the blackbird (Turdus merula). P. Roy. Soc. B-Biol. Sci. 275, 427–434 (2008).
Houde, A. E. & Torio, A. J. Effect of parasitic infection on male color pattern and female choice in guppies. Behav. Ecol. 3, 346–351 (1992).
Hamilton, W. D. & Zuk, M. Heritable true fitness and bright birds: a role for parasites? Science 218, 384–387 (1982).
Roulin, A., Riols, C., Dijkstra, C. & Ducrest, A.-L. Female plumage spottiness signals parasite resistance in the barn owl (Tyto alba). Behav. Ecol. 12, 103–110 (2001).
Kittilsen, S., Johansen, I. B., Braastad, B. O. & Øverli, Ø. Pigments, parasites and personality: towards a unifying role for steroid hormones? PLoS One 7, e34281 (2012).
Zuk, M., Thornhill, R., Ligon, J. D. & Johnson, K. Parasites and mate choice in red jungle fowl. Am. Zool. 30, 235–244 (1990).
Folstad, I., Hope, A. M., Karter, A. & Skorping, A. Sexually selected color in male sticklebacks: a signal of both parasite exposure and parasite resistance? Oikos 69, 511–515 (1994).
Svensson, P. A. & Wong, B. B. M. Carotenoid-based signals in behavioural ecology: a review. Behaviour 148, 131–189 (2011).
Balenger, S. L. & Zuk, M. Testing the Hamilton–Zuk hypothesis: past, present, and future. Integr. Comp. Biol. 54, 601–613 (2014).
Côte, J. et al. Melanin-based coloration and host–parasite interactions under global change. P. Roy. Soc. B-Biol. Sci. 285, 20180285 (2018).
Øverli, Ø. & Johansen, I. B. Kindness to the final host and vice versa: a trend for parasites providing easy prey? Front. Ecol. Evol. 7 (2019).
Lafferty, K. D. The evolution of trophic transmission. Parasitol Today 15, 111–115 (1999).
Poulin, R. In Advances in the Study of Behavior Vol. 41 (eds H. Jane Brockmann et al.) 151–186 (Academic Press, 2010).
Klemetsen, A. The most variable vertebrate on Earth. Journal of Ichthyology 53, 781–791 (2013).
Henriksen, E. H. et al. Ontogenetic dynamics of infection with Diphyllobothrium spp. cestodes in sympatric Arctic charr Salvelinus alpinus (L.) and brown trout Salmo trutta L. Hydrobiologia 783, 37–46 (2016).
Ferguson, M. S. & Hayford, R. A. The Life History and Control of an Eye Fluke. Prog. Fish. Cult. 8, 1–13 (1941).
Kennedy, C. R. Studies on the biology of Eubothrium salvelini and E. crassum in resident and migratory Salvelinus alpinus and Salmo trutta and in S. salar in North Norway and the islands of Spitsbergen and Jan Mayen. J. Fish Biol. 12, 147–162 (1978).
Klementsen, A., Amundsen, P. A., Knudsen, R. & Hermansen, B. A profundal, winter-spawning morph of arctic charr Salvelinus alpinus (L.) in Fjellfrøsvatn, North Norway. Nordic J. Freshw. Res. 73, 13–23 (1997).
Skarstein, F., Folstad, I., Liljedal, S. & Grahn, M. MHC and fertilization success in the Arctic charr (Salvelinus alpinus). Behav.l Ecol. Sociobiol. 57, 374–380 (2005).
Kuhn, J. A., Knudsen, R., Kristoffersen, R. & Amundsen, P.-A. A simplified method to estimate Diphyllobothrium spp. infection in salmonids. J. Fish Dis. 40, 863–871 (2017).
Frantzen, M., Arnesen, A. M., Damsgård, B., Tveiten, H. & Johnsen, H. K. Effects of photoperiod on sex steroids and gonad maturation in Arctic charr. Aquaculture 240, 561–574 (2004).
Barton, K. MuMIn: Multi-model inference. R package version 1.42.1. URL, https://CRAN.R-project.org/package=MuMIn (2018).
Kuznetsova, A., Brockhoff, P. B. & Christensen, R. H. B. lmerTest package: tests in linear mixed effects models. J. Stat. Softw. 82 (2017).
Fox, J. & Weisberg, S. Visualizing fit and lack of fit in complex regression models with predictor effect plots and partial residuals. J. Stat. Softw. 87, 1–27 (2018).
Klemetsen, A. et al. Atlantic salmon Salmo salar L., brown trout Salmo trutta L. and Arctic charr Salvelinus alpinus (L.): a review of aspects of their life histories. Ecol. Freshw. Fish 12, 1–59 (2003).
Black, G. A. & Lankester, M. W. The transmission, life span, and population biology of Cystidicola cristivomeri White, 1941 (Nematoda: Habronematoidea) in char, Salvelinus spp. Can. J. Zool. 59, 498–509 (1981).
Giaever, A. A., Klemetsen, A. & Halvorsen, O. Infection of Cystidicola farionis Fischer(Nematoda: Spiruroidea) in the swimbladder of Arctic charr, Salvelinus alpinus(L.), from Takvatn, North Norway. Nordic J. Freshw. Res. Drottningholm. 66, 63–71 (1991).
Goater, T. M., Goater, C. P. & Esch, G. W. Parasitism: The Diversity and Ecology of Animal Parasites. 2 edn, (University Printing House, 2014).
Blasco-Costa, I. & Locke, S. A. In Adv Parasitol Vol. 98 167–225 (Elsevier, 2017).
Fitze, P. S., Tschirren, B. & Heinz Richner, J. G. Carotenoid‐based plumage colors and immune function: is there a trade‐off for rare carotenoids? Am. Nat. 169, S137–S144 (2007).
Saksvik, M., Nilsen, F., Nylund, A. & Berland, B. Effect of marine Eubothrium sp.(Cestoda: Pseudophyllidea) on the growth of Atlantic salmon. Salmo salar L. J. Fish Dis. 24, 111–119 (2001).
Bristow, G. A. & Berland, B. The effect of long term, low level Eubothrium sp.(Cestoda: Pseudophyllidea) infection on growth in farmed salmon (Salmo salar L.). Aquaculture 98, 325–330 (1991).
Bylund, G. Pathogenic effects of a diphyllobothriid plerocercoid on its host fishes. Soc. Scient. Fenn., Comm. Biol. (1972).
Darwin, C., Bonner, J. T. & May, R. M. The descent of man, and selection in relation to sex. REV - Revised edn, (Princeton University Press, 1981).
Endler, J. A. Natural and sexual selection on color patterns in poeciliid fishes. Environ. Biol. Fishes 9, 173–190 (1983).
Godin, J.-G. J. & McDonough, H. E. Predator preference for brightly colored males in the guppy: a viability cost for a sexually selected trait. Behav. Ecol. 14, 194–200 (2003).
Götmark, F. & Olsson, J. A. N. Artificial colour mutation: do red-painted great tits experience increased or decreased predation? Anim. Behav. 53, 83–91 (1997).
Zuk, M. & Johnsen, T. S. Seasonal changes in the relationship between ornamentation and immune response in red jungle fowl. P. Roy. Soc. B-Biol. Sci. 265, 1631–1635 (1998).
Brattli, M. B., Egeland, T. B., Nordeide, J. T. & Folstad, I. Spawning behavior of Arctic charr (Salvelinus alpinus): Spawning synchrony, vibrational communication, and mate guarding. Ecol. Evol. 8, 8076–8087 (2018).
Volkoff, H., Unniappan, S. & Kelly, S. P. In Fish Physiology Vol. 28 421–465 (Academic Press, 2009).
Miquelle, D. G. Why don’t bull moose eat during the rut? Behav. Ecol. Sociobiol. 27, 145–151 (1990).
Tveiten, H., Johnsen, H. K. & Jobling, M. Influence of maturity status on the annual cycles of feeding and growth in Arctic charr reared at constant temperature. J. Fish Biol. 48, 910–924 (1996).
Brønseth, T. & Folstad, I. The effect of parasites on courtship dance in threespine sticklebacks: more than meets the eye? Can. J. Zool. 75, 589–594 (1997).
Lim, A., Kumar, V., Hari Dass, S. A. & Vyas, A. Toxoplasma gondii infection enhances testicular steroidogenesis in rats. Mol. Ecol. 22, 102–110 (2013).
Bjerkeng, B., Johnsen, K., Mayer, I., Storebakken, T. & Nilssen, K. J. Influence of 11-ketotestosterone, 17β-estradiol, and 3,5,3′-triiodo-L-thyronine on distribution and metabolism of carotenoids in Arctic charr, Salvelinus alpinus L.*. Fish Physiol. Biochem. 21, 353–364 (1999).
Acknowledgements
A large group of people participated on this study and we are immensely grateful for valuable contributions from Wayne J. Korzan, Else Beitnes Johansen, Martin Rognli Johansen, Laina H. Dalsbø, Karin Strand Johannesen, Lars Nilssen and Sofie Coucheron Støre. This work was supported by the Research Council of Norway (Project No.: 250048).
Author information
Authors and Affiliations
Contributions
I.B.J. participated in the design of the study, coordinated the study, collected field data, participated in data analysis and drafted the manuscript, E.H.H. collected field data, performed data analysis, carried out statistical analysis and drafted the manuscript, J.C.S. collected field data and drafted the manuscript, I.M. performed steroid analysis and helped draft the manuscript, P.-A.A. participated in the design of the study, coordinated the study, collected field data, participated in data analysis and helped draft the manuscript, Ø.Ø. conceived, designed and coordinated the study, collected field data, participated in data analysis and drafted the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Johansen, I.B., Henriksen, E.H., Shaw, J.C. et al. Contrasting associations between breeding coloration and parasitism of male Arctic charr relate to parasite species and life cycle stage. Sci Rep 9, 10679 (2019). https://doi.org/10.1038/s41598-019-47083-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-47083-x
- Springer Nature Limited
This article is cited by
-
Parental kinship coefficient but not paternal coloration predicts early offspring growth in lake char
Heredity (2024)
-
Longitudinal effects of habitat quality, body condition, and parasites on colour patches of a multiornamented lizard
Behavioral Ecology and Sociobiology (2022)