Abstract
The enzyme succinate dehydrogenase (SDH) functions in the citric acid cycle and loss of function predisposes to the development of phaeochromocytoma/paraganglioma (PPGL), wild type gastrointestinal stromal tumour (wtGIST) and renal cell carcinoma. SDH-deficient tumours are most commonly associated with a germline SDH subunit gene (SDHA/B/C/D) mutation but can also be associated with epigenetic silencing of the SDHC gene. However, clinical diagnostic testing for an SDHC epimutation is not widely available. The objective of this study was to investigate the indications for and the optimum diagnostic pathways for the detection of SDHC epimutations in clinical practice. SDHC promoter methylation analysis of 32 paraffin embedded tumours (including 15 GIST and 17 PPGL) was performed using a pyrosequencing technique and correlated with SDHC gene expression. SDHC promoter methylation was identified in 6 (18.7%) tumours. All 6 SDHC epimutation cases presented with SDH deficient wtGIST and 3/6 cases had multiple primary tumours. No case of constitutional SDHC promoter hypermethylation was detected. Whole genome sequencing of germline DNA from three wtGIST cases with an SDHC epimutation, did not reveal any causative sequence anomalies. Herein, we recommend a diagnostic workflow for the detection of an SDHC epimutation in a service setting.
Similar content being viewed by others
Introduction
Loss of function of the succinate dehydrogenase (SDH) enzyme complex leads to intracellular accumulation of succinate as oxidative dehydrogenation of succinate to fumarate in the citric acid cycle is interrupted. Succinate can function as an ‘oncometabolite’ and drive tumourigenesis by competitively inhibiting 2-oxyglutarate dependent enzymes including prolyl hydroxylase and DNA and histone demethylase enzymes resulting in a pseudohypoxic transcriptional response1 and DNA and histone hypermethylation2.
Biallelic inactivation of one of the four SDH subunit genes (SDHA, SDHB, SDHC, SDHD) is the most common mechanism causing SDH deficient (dSDH) tumours. Germline genetic testing for germline SDHx mutations is now considered best practice for patients presenting with i) PPGL3, ii) wild type gastrointestinal stromal tumours (wtGIST)4 and iii) specific histopathological subtypes of renal cell carcinoma5. wtGIST are defined as GIST that are negative for KIT and PDGFRA somatic gene mutations and account for 15% of adult and 85% of paediatric GIST. Biallelic inactivation of any of the SDHx genes, most commonly results in destabilisation of the SDH enzyme complex, which can be detected by loss of staining for the SDHB protein on IH6 and therefore wtGIST can be further classified based on the loss or preservation of SDHB protein expression on immunohistochemistry as a surrogate marker for loss of function of the SDH complex. Importantly, SDH deficient wtGIST (dSDH wtGIST) account for approximately 7–10% of all GIST4,7.
Identification of a germline pathogenic variant in SDHB informs a higher risk of a malignant PPGL2 and detection of a germline SDHx mutation facilitates personalised surveillance, family screening and potentially the choice of therapy for metastatic disease1,2. In addition to testing for germline SDHx variants, immunostaining for SDHB and SDHA is a valuable approach for identifying dSDH tumours6.
It is now recognized that in a subset of dSDH tumours, SDH inactivation results from promoter hypermethylation and epigenetic silencing of the SDHC gene2,6,7,8,9,10. SDHC promoter hypermethylation has been most frequently found in dSDH-wtGIST8,9,10,11,12,13 with up to a third of all of cases having SDHC promoter methylation2. Distinguishing dSDH tumours with germline SDHx mutations from those with SDHC hypermethylation only is beneficial because i) the relatives of patients with a germline SDHx mutation are at increased tumour risk and ii) an SDHC epimutation is potentially reversible (clinical trials have been initiated to investigate demethylating agents in such cases (ClinicalTrials.gov Identifier: NCT03165721)).
SDHC epimutations appear to be unique to specific tumour types (e.g wtGIST and PPGL)8 but further study is required to determine whether SDHC epimutations might occur in tumours with an associated hypermethylation phenotype other than SDH deficient wt GIST and PPGL. IDH1 mutant gliomas have previously been associated with a global hypermethylation phenotype due to inhibition of alpha ketoglutarate dependent de-methylation enzymes14 and therefore IDH1mutant gliomas are a useful tumour type to test the hypothesis that SDHC promoter hypermethylation is unique to specific tumour types.
Despite the implications for patient management and family testing and screening, diagnostic testing for SDHC epimutations has not been adopted as routine clinical practice because the indications for testing and a suitable methodology for a clinical service laboratory have not been well defined8. The aims of this study were; i) to investigate a pyrosequencing-based assay for the diagnosis of SDHC promoter methylation and ii) to determine the role for SDHC epimutation testing in a clinical diagnostic pathway using pooled data from this study and available literature.
Methods
Clinical sample collection
Cases were ascertained from the Neuroendocrine Tumour, the National Pediatric and Adult wild type GIST (PAWS GIST UK) and clinical genetics clinics at Cambridge University Hospital NHS Foundation Trust. Details of clinical phenotype, family history and germline molecular testing results were collated from patient records.
Study design
All cases of identified PPGL wtGIST, for whom formalin fixed paraffin embedded (FFPE) tumour blocks were available, were considered for inclusion in the study. All participants (and or legal guardians) gave written informed consent. 32 cases (15 wtGIST and 17 PPGL) were included in the analysis. For each case studied, DNA was extracted from FFPE tumour tissue and adjacent normal tissue (31/32 cases) and blood when available (21/32 cases). mRNA was extracted from FFPE tumour tissue and adjacent normal FFPE tissue. SDHB immunohistochemistry (IH) was performed on all 32 samples. Tumour samples with evidence of SDHB preservation on SDHB IH were included in SDHC promoter methylation analysis in order to confirm if SDHB IH was a sensitive triaging test for the diagnosis of an SDHC epimutation.
Methylation analysis was performed on DNA extracted from FFPE tumour and matched normal tissue/blood. SDHC expression analysis was performed on RNA extracted from FFPE tumour and matched normal tissue and finally sequencing of tumour DNA was performed to identify somatic SDHx mutations.
A further 17 IDH1 mutant glioma samples (anonymised tumour DNA from consented patients provided by Professor Colin Watts) were included in the study.
Germline and tumour genetic sequencing
Clinical germline DNA sequencing
DNA was extracted from peripheral blood samples according to standard protocols. Next generation sequencing of a clinical gene panel including; SDHA, SDHB, SDHC, SDHD, KIT, PDGFRA and NF1 (for GIST) and SDHA, SDHB, SDHC, SDHD, SDHAF2, MAX, TMEM127, VHL, RET, FH for (PPGL) was performed by the laboratory staff at Cambridge University Hospital NHS Foundation Trust or Birmingham Women’s and Children’s Hospital NHS Trust using the TrusightOne or Trusight Cancer sequencing panels (Illumina Inc., UK).
An average coverage depth of >20 fold was achieved for 98% of the regions sequenced. All detected variants were confirmed by Sanger sequencing. Whole exon deletions and duplications and large rearrangements are not detected using this method and multiple ligation probe analysis (MLPA) was performed for VHL, SDHB, SDHC and SDHD.
Tumour DNA sequencing using a custom gene panel
Tumour sequencing was performed on those cases with sufficient DNA quantity following methylation analysis (27/32 cases of PPGL/GIST and 17 gliomas) by the staff at the Stratified Medicine Core Laboratory within the Department of Medical Genetics, Cambridge University. Sequencing was performed using a custom panel based on the Ion AmpliSeq™ 142 Cancer Hotspot Panel v2 (catalogue number 4475346).
Variant filtering was performed on variant calling files (VCF). Variants were removed if the variant allele frequency was <10% or the minor allele frequency (MAF) greater than 0.1% in EVS6500 and/or 1000 genome project (www.internationalgenome.org). Synonymous variants were removed as presumed not to be pathogenic. Those variants that had coverage of less than two standard deviations below the mean coverage were also removed.
Data extracted from whole genome sequencing
Whole genome sequencing (WGS) was performed on germline DNA from three cases as part of the NIHR Rare Disease Bioresource project and sequencing data from two of the three patients was included in a recent publication15. Data was filtered to include data in the regions of interest: the SDHC promoter region and five genes involved in DNA methylation maintenance and regulation: TET1, TET2, TET3, DNMT3A and DNMT3B.
The variants were annotated with variant effect predictor and filtered on i) minor allele frequency of <0.1 or absent in 1000 genome project (www.internationalgenome.org) and UK10K (https://www.uk10k.org), ii) consequence including; truncating, missense, splice site and in frame deletion and insertion variants and iii) quality including; a read depth of >10 and variant allele frequency of >0.3. All filtered variants were then individually interrogated and assigned pathogenicity based on American College of Medical Genetics and Genomics (ACMG) criteria.
A comparison of variant allele frequencies in our samples compared to a control group with low neoplastic risk within the bio resource project (NIHR rare disease controls, n = 4053), was also performed and calculated using a Fishers exact test and corrected for a false discovery rate using the Benjamini-Hochberg procedure. Finally, cases were evaluated for structural variants (SV) including copy number variation, using the SV calling tools; Canvas and/or Manta16,17.
Tissue dissection for DNA and RNA isolation
Pre-selected paraffin blocks containing tumour and adjacent normal tissue were used for nucleic acid extraction. Tumour tissue and normal tissue suitable for DNA isolation was identified by an experienced molecular histopathologist (OG). Tumour cell content in the tumour enriched areas ranged between 50–80%. Normal tissue used as control was histologically confirmed to be tumour free. 6–10 µm thick FFPE sections were mounted on glass slides. Tumour and normal tissue were scraped of the slides barring a security margin between tumour and normal of 2 mm.
Bisulfite modification
The assay was proven to work reliably with 10 ng input DNA, however 500 ng of DNA was used as a standard for bisulfite modification with the Zymo Research EZ DNA Methylation kit (D5001) according to the manufacturer’s instructions. Bisulfite converted DNA was eluted from the spin colums with 50ul of elution buffer and directly processed for PCR or frozen at −20 °C. Complete bisulfite modification was monitored by an internal bisulfite control position after 5 consecutive cytosines in the genomic sequence in the pyrosequencing assay.
Polymerase chain reaction and pyrosequencing
CpG27 was chosen over CpG17 as the CpG27 island was located proximal to the transcription start site for the SDHC gene. A 198 bp sized PCR amplicon in the CpG27 island located in the SDHC promoter region of the SDHC gene was amplified from 50 ng of CT bisulfite converted DNA with 375 nM of forward primer (GAAAATAATTAGTAAATTAGTTAGGTAG) and 187.5 nM of biotinylated reverse primer (ACTAAAATCACCTCAACAACAAC) with the Qiagen PyroMark kit (Qiagen 978703). The PCR conditions were 7 min at 95 °C, followed by 20 sec at 95 °C, 30 sec at 53 °C, and 20 sec at 72 °C for 42 cycles, and an end incubation at 72 °C for 5 min. The resulting PCR amplicon was quality assessed for purity and yield on a 2% agarose gel. A nested sequencing primer (GTTATATGATATTTTTAATTT) at a concentration of 500 nM was used to analyse 12 CpGs in 10ul of the sample on the Qiagen Q24 pyrosequencer. Fully methylated and unmethylated human control DNA that had been treated with bisulfite were used as controls on each pyrosequencing run.
Ten percent of the bisulfite conversion eluate (approximately 50 ng) was used as a PCR template. The lower detection limit of the assay was 10% eluate of 10 ng input DNA for bisulfite conversion (approximately 1 ng) for fresh frozen and DNA isolated from FFPE. Methylation percentage differences of 25% were reliably detectable for 10 ng and 50 ng of template bisulfite converted DNA.
Development of a clinical diagnostic assay for SDHC methylation
In order to facilitate the translation of SDHC promoter methylation analysis into clinical practice we set out to develop an assay using technology that is available in a service setting and that would provide robust results on DNA extracted from FFPE. Tumours from 32 patients with wtGIST15 and PPGL17 and a further 17 glioma tumour samples were studied.
Additional methods in supplementary data: (i) Tumour DNA extraction, (ii) Analysis of TCGA tumour set, (iii) RNA extraction, (iv) cDNA synthesis, (v) Expression Analysis with quantitative RT PCR, (vi) Statistical analysis.
All methods were performed in accordance with the relevant guidelines and recommendations.
Ethical approval and consent to participate
All participants gave written informed consent for study participation and publication and the study was approved by Cambridge South Research Ethics Committee (REC Reference Number: CA/5175).
Results
Genotype and clinical phenotype of patient cohort
wtGIST and PPGL cases
The mean age of tumour diagnosis was 36.6 years (range 15–71, SD 18.8). The fifteen cases of wtGIST included 10 cases of dSDH-wtGIST and 5 cases of SDH preserved wtGIST, as defined by loss or preservation respectively of SDHB protein expression on immunohistochemistry (Table 1). The 17 PPGL cases included 13 SDH preserved PPGL, 3 dSDH-PPGL and 1 PPGL with an equivocal SDHB result (diffusely weak SDHB expression) (case # 026) (Table 2). Thirteen participants were male, 19 female and nine cases had metastatic disease (Tables 1 and 2). Five patients had a clinical history of multiple primary tumours (Tables 1 and 2).
A likely pathogenic or pathogenic germline variant was identified in 12/32 patients (37.5%; 6/15 GIST and 6/17 PPGL). No CNV was identified by MLPA testing in the cohort.
Methylation analysis by pyrosequencing of tumour DNA from wtGIST and PPGL cohort
The % methylation at each of the 12 CpG’s in CpG island 27(CpG27) in the promoter region of SDHC was tested. The percentage methylation ranged between 1% and 73% but was highly correlated within an individual tumour sample with no significant variability detected across individual CpGs (p = 0.08) (see Fig. 1). A mean % methylation index (MI = % of methylated CpGs) of 2.2% (±SD 1.98) across 12 CpG’s, was detected in all but 6 tumour samples (Table S1). The mean MI in these six tumours was 50.8% (±SD 16.4) (Fig. 1B) (cases: #001, #002, #003, #004, #021, #022).
Figure (A) illustrates the distribution of methylation across the 12 individual CpG’s for the six cases demonstrated to have SDHC promoter methylation (epimutant cases), and the wt GIST, glioma and PPGL cases with no SDHC epimutation. Figure (B) demonstrates the methylation levels across the 12 individual CpG’s for the six epimutated cases (#001, #002, #003, #004, ##021, #022).
All cases identified as having an SDHC epimutation in this study had a dSDH wtGIST as the presenting phenotype. Comparing 6 tumours with evidence of SDHC hypermethylation to those with low methylation revealed statistically significant associations with wtGIST (6/15 versus 0/17 PPGL; P = 0.005), female sex (6/19 versus 0/13 males; P = 0.02); metastatic disease (5/6 versus 5/26 (P = 0.035), younger age at diagnosis (mean age 24 years versus mean age 39.2 years) (P = 0.0002) and multiple primary tumours (3/6 versus 2/26, P = 0.03). No significant association was found for the presence of a germline pathogenic SDHx variant (P = 0.2).
Methylation analysis by pyrosequencing of blood and adjacent normal tissue DNA from wtGIST and PPGL cohort
The purpose of this analysis was to further investigate whether SDHC promoter hypermethylation is a constitutional, mosaic or somatic event.
Pyrosequencing of blood DNA was performed on 22/32 (69%) wtGIST and PGL cases and matched normal tissue for 31/32 cases (97%). No evidence of SDHC promoter hypermethylation was detected in blood or normal tissue (MI <10% in all samples) including the 6 samples with tumour SDHC hypermethylation. No statistically significant difference was identified between the mean MI in blood DNA or adjacent normal tissue for those cases identified as having tumour hypermethylation compared with those cases without tumour methylation (p = 0.6) (Fig. 2A).
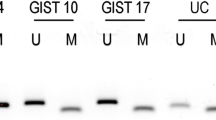
Figure (A) shows the difference in the mean % methylation of the SDHC promoter locus across 12 CpG’s in the tumour of the six hypermethylated cases and tumours of the non-epimutant cases and blood DNA and normal tissue of cases with and without an identified SDHC epimutation. Figure (B) shows reduced SDHC expression in the tumour versus normal tissue of 5/6 cases with an identified SDHC epimutation.
As expected, a significant difference was noted for the MI in the tumour compared to the adjacent normal tissue for the 5 hypermethylated tumour cases for which adjacent normal tissue was available for testing (p = 0 .003) (Fig. 2A). ROC curve analysis (see Supplementary Data and statistical methods) demonstrated that a methylation of >8.5% separated the cases with an identified epimutation and silencing of SDHC from those without (AUC 1.0, p = < 0.0001).
Analysis of SDHC gene expression in tumour tissue from wtGIST and PPGL cohort
To determine whether SDHC promoter methylation was associated with transcriptional silencing, analysis of SDHC mRNA in both tumour tissue and adjacent normal tissue was performed in 31/32 cases. In 5/5 tumour samples with SDHC hypermethylation the mean fold difference was −6.41(SD 5.4) (Fig. 2B) compared to 1.41 (SD 4.41) in 26 tumours without SDHC hypermethylation (P = 0.0002) (Figure S1).
Tumour sequencing and additional functional analysis for SDH deficiency in the hypermethylated cases
Tumour sequencing was performed on 4/6 (#001, #002, #003, #004) cases with evidence of SDHC hypermethylation and no somatic SDHx variants were detected. SDHB immunohistochemistry was performed on all tumours and loss of SDHB expression was confirmed in all 6 cases with SDHC hypermethylation (Table 1, examples for #001 and #003 displayed in Fig. 3A,B).
Figure (A) and (B) shows loss of SDHB protein expression on immunohistochemical analysis of the primary wtGIST tumour in case #001 and #003 respectively. In Figure (B) SDHB expression is preserved in adjacent normal tissue as highlighted by the red arrow. Figure (C) shows a pulmonary chondroma in case #021 as demonstrated by the white arrow and Figure (D) demonstrates the histology of a pulmonary chondroma from case #004, with evidence of normal collapsed lung tissue illustrated by the black arrow and chondrocytes in the tumor marked by the red arrow.
Data extracted from whole genome germline sequencing analysis (WGS) of hypermethylated cases
WGS data was analysed for three cases with tumour SDHC hypermethylation for whom sufficient DNA was available (cases; #002, #021 and #022). No candidate pathogenic structural or single nucleotide variants were identified in these three cases in the SDHC locus (between 161314257-161375340) containing the SDHC promoter, exons and 3′UTR. In the absence of an in cis genetic cause, additional analysis for potential pathogenic variants in genes implicated in genome methylation (TET1, TET2, TET3, DNMT3B, DNMT3A, DNMT1), was performed.
10/965 filtered variants (in test and control samples) were detected in 3 genes (Table S2). A comparison of the identified variant frequencies in the three SDHC hypermethylation samples compared to 4053 control genomes with low neoplastic risk (from the NIHR Rare Diseases BioResource BRIDGE project) did not yield any statistically significant findings (Benjamini Hochberg correction for a false discovery rate of p values was applied and based on 965 tested hypotheses).
None of the variants identified in the SDHC methylation cases were considered to be pathogenic by ACMG criteria. A missense variant of uncertain significance in TET2 (p.Ile1762Val) was identified in all three cases with SDHC promoter hypermethylation, but this variant was absent from 1000 genomes and UK10K databases and was identified in 1876/4053 controls (Table S2).
Investigating SDHC hypermethylation in non PPGL and wtGIST tumour sets
To further investigate the apparent specificity of SDHC epimutations in dSDH wtGIST we explored whether SDHC epimutations might occur in non-wtGIST tumours with (a) DNA hypermethylation or (b) low SDHC expression in order to test the hypothesis that an SDHC epimutation is specific to particular tumour types and/or is not a consequence of generalised tumour DNA hypermethylation.
Firstly we undertook SDHC promoter methylation analysis on 17 IDH1 mutant glioma samples. IDH1 mutant gliomas have previously been associated with a global hypermethylation phenotype due to inhibition of alpha ketoglutarate dependent de-methylating enzymes20. The mean SDHC promoter methylation in the IDH1 mutant glioma samples was 2% (±SD 1.28, range 1–4%) (Fig. 1A and Table S3).
Secondly, from non-wtGIST tumours with SDHC gene expression data and sequencing data from cancer genomic studies (accessed at http://www.cbioportal.org/), we identified 25 tumour samples with very low SDHC transcript levels and no SDHC mutation (Table S4). Methylation array (Illumina 450k) data for these 25 tumours was accessed and beta values for 13 SDHC promoter probes inspected. None of the tumours showed evidence of SDHC promoter hypermethylation (Table S4).
Discussion
A search of PubMed (using the terms SDHC and methylation or epimutation) identified 8 publications containing 34 cases of SDHC promoter region hypermethylation in a variety of tumour types including dSDH wtGIST, sympathetic (PGL) and parasympathetic (HNPGL) paragangliomas1,9,10,12,13,18 (Table S5). The majority of patients (94%, 32/34) identified with SDHC hypermethylation had a dSDH-wtGIST and 53% (18/34) of these cases also had an additional tumour(s) (Table S5).
Phenotype of SDHC epimutation cases detected in the present study
We identified SDHC promoter region methylation in 6/15 wtGIST (all 6 cases were dSDH-wtGIST) but none of the 17 PPGL or SDH-preserved-wtGIST (3/15 wtGIST). All SDHC hypermethylation cases were female and were significantly younger than patients without an SDHC epimutation.
Combining our results with previously published series (see Table S5), the association with dSDH-wtGIST (alone or as the presenting feature of a multi-tumour syndrome), female gender and young age at diagnosis is maintained. Rare reports of isolated sympathetic and parasympathetic PGL with an SDHC epimutation have also been published (Table S5).
In two of the cases reported here, somatic SDHC promoter methylation was detected in the presence of a germline pathogenic SDHC variant. This would be consistent (though not proven) with a two hit model of tumourigenesis in which SDHC hypermethylation resulted in silencing of the wild-type allele in the tumour. Two of the cases with a germline SDHC mutation had multiple tumours including case #004 (Fig. 3C,D). The association of synchronous or metachronous gastric wtGIST, PPGL and pulmonary chondroma (PCHO) is referred to as Carney triad whereas the combination of GIST and PPGL is designated as the Carney-Stratakis syndrome (CSS) or dyad. Although it was previously suggested that PCHO occurred exclusively in CT (a non-inherited disorder), this study and others11,19 have demonstrated that the triad of wtGIST, PPGL and PCHO can occur in association with a germline SDHx mutation and highlights the overlapping features of CT and CSS19,20,21. However, we did not (from interrogation of TCGA, literature and original data) find evidence that SDHC promoter methylation occurs outside of wtGIST and, occasionally, PGL.
We identified 4 cases of tumour SDHC promoter methylation with no detectable germline or somatic SDHC mutations. Furthermore there was no evidence of a germline SDHC epimutation. In such cases the SDHC promoter hypermethylation might be a somatic event as occurs in many types of cancer and multiple tumour suppressor genes22. In the case of the mismatch repair gene MLH1, somatic MLH1 promoter methylation is relatively common in older individuals with colorectal cancer with microsatellite instability but there are rare cases of patients with a constitutional MLH1 epimutation who present at a younger age23. In contrast to MLH1, there has been no evidence to date that SDHC epimutations may result from in cis promoter region genetic variants24, although some studies have described mosaic constitutional SDHC promoter hypermethylation in association with tumour hypermethylation8. In the absence of a detectable in cis or in trans genetic variant in these cases, low level postzygotic tissue mosaicism for SDHC promoter hypermethylation, provides an alternative hypothesis for this multiple tumour phenotype at a young age.
Translating the diagnosis of an SDHC epimutation into clinical practice
A primary aim of this study was to develop a proposed methodology for diagnostic SDHC promoter methylation testing in a clinical setting. We developed a pyrosequencing-based method because it is well established on FFPE material, allows a low level variant detection and is frequently used in diagnostic pathology services for other types of somatic methylation analysis (e.g. MGMT promoter methylation analysis in glioma). Our method worked well on DNA extracted from archived routine diagnostic FFPE material (an important consideration as fresh frozen tumour is rarely available) and pyrosequencing is less expensive compared to alternative methods e.g. methylation arrays.
We found that the methylation status of 12 CpG’s in CpG27 in the promoter region of the SDHC gene could be accurately assessed and that detection of hypermethylation of the SDHC promoter correlated with reduced SDHC mRNA on mRNA extracted from the same FFPE tissue block. Recently described methods for the detection of ex-vivo and in vivo succinate accumulation are useful adjuncts to SDHB IH for the detection of SDH deficiency25,26. However, these methods cannot identify the cause of SDH deficiency. The authors recommend that whenever possible, cases with SDHC promoter hypermethylation should be analysed by RT-PCR of both tumour and adjacent normal tissue to confirm silencing of SDHC in the tumour tissue.
Given that SDHB immunohistochemistry is a relatively accessible and sensitive test, this should be considered as a first-line triaging test for the detection of SDH deficiency in PPGL and wtGIST21. We recommend that germline genetic testing is always considered as the next diagnostic step in dSDH tumours to rule out a potential syndromic cause. If germline genetic testing (including MLPA) is negative and SDHB IH suggests loss of SDHB protein expression, the first step for PPGL should be somatic sequencing27 to investigate for somatic SDHx or VHL mutations, which can account for loss of SDHB protein expression6. However, as SDHC epimutations are more frequent in wtGIST than in PPGL, we recommend SDHC promoter methylation analysis as the next step after germline genetic testing for wtGIST (Fig. 4B). If an SDHC epimutation is diagnosed, somatic tumour sequencing should be performed to identify a co-existing somatic SDHx mutation, which may affect the efficacy of any potential demethylating therapy (Fig. 4).
Illustrates a proposed work flow for the investigation of SDHC promoter methylation in a clinical setting for (A) PPGL and (B) wtGIST (defined as a GIST with no identified somatic mutation in KIT, PDGFRA OR BRAF) *Next generation sequencing panel for PPGL including the genes; SDHA, SDHB, SDHC, SDHD, SDHAF2, FH, TMEM127, RET, VHL, MAX and including multiplex ligation dependent probe amplification for deletions and duplication. **Next generation sequencing panel for wtGIST including the genes; SDHA, SDHB, SDHC, SDHD, KIT, PDGFRA, NF1 and including multiplex ligation dependent probe amplification for deletions and duplication.
Importantly, a number of potential limitations in the diagnosis of SDHC methylation using pyrosequencing methods on FFPE tumour tissue, were encountered over the course of this study. Identification of these pitfalls has prompted the following practical recommendations; i) using a minimum input of 50 ng of bisulfite converted DNA for the PCR and ii) a minimum volume of 10 microlitre of the PCR product for pyrosequencing can minimize the risk of false elevations in methylation, iii) fully methylated and unmethylated human control DNA, treated with bisulfite should be used as external controls on each pyrosequencing run and iv) the use of matched normal tissue is useful as an internal control to account for any false elevation in methylation which may have been caused by the long term paraffin storage. Limitations of this study also include the retrospective study design and relatively small sample size and diagnostic laboratories wishing to adopt the methodology described herein will need to undertake a formal clinical validation study before implementing it for clinical diagnostic use.
In conclusion, the results from our literature review, experimental studies and interrogation of the TCGA data, suggest that SDHC epimutations are rare in tumours other than wtGIST and PPGL. Improving the accessibility of clinical diagnostic testing for SDHC promoter methylation will facilitate the management of patients with wtGIST by enabling stratification for personalised therapeutic strategies and defining risks for other family members, according to the presence or absence of a germline SDHx mutation and or a SDHC epimutation.
Data Availability
Data is provided in the manuscript and/or Supplementary Data.
References
Dahia, P. L. M. et al. A HIF1α Regulatory Loop Links Hypoxia and Mitochondrial Signals in Pheochromocytomas. PLoS Genet. 1, e8 (2005).
Letouzé, E. et al. SDH mutations establish a hypermethylator phenotype in paraganglioma. Cancer Cell 23, 739–52 (2013).
Lenders, J. W. M. et al. Pheochromocytoma and Paraganglioma: An Endocrine Society Clinical Practice Guideline. J. Clin. Endocrinol. Metab. 99, 1915–1942 (2014).
Mason, E. F. & Hornick, J. L. Conventional Risk Stratification Fails to Predict Progression of Succinate Dehydrogenase-deficient Gastrointestinal Stromal Tumors: A Clinicopathologic Study of 76 Cases. Am. J. Surg. Pathol. 40, 1616–1621 (2016).
Ricketts, C. J. et al. Succinate dehydrogenase kidney cancer: an aggressive example of the Warburg effect in cancer. J. Urol. 188, 2063–71 (2012).
Papathomas, T. G. et al. SDHB/SDHA immunohistochemistry in pheochromocytomas and paragangliomas: a multicenter interobserver variation analysis using virtual microscopy: a Multinational Study of the European Network for the Study of Adrenal Tumors (ENS@T). Mod. Pathol. 28, 807–821 (2015).
Gill, A. J. et al. Immunohistochemistry for SDHB divides gastrointestinal stromal tumors (GISTs) into 2 distinct types. Am. J. Surg. Pathol. 34, 636–44 (2010).
Killian, J. K. et al. Recurrent epimutation of SDHC in gastrointestinal stromal tumors. Sci. Transl. Med. 6, 268ra177–268ra177 (2014).
Haller, F. et al. Aberrant DNA hypermethylation of SDHC: a novel mechanism of tumor development in Carney triad. Endocr. Relat. Cancer 21, 567–77 (2014).
Urbini, M. et al. SDHC methylation in gastrointestinal stromal tumors (GIST): a case report. BMC Med. Genet. 16, 87 (2015).
Boikos, S. A. et al. Molecular Subtypes of KIT/PDGFRA Wild-Type Gastrointestinal Stromal Tumors. JAMA Oncol. 2, 922 (2016).
Bernardo-Castiñeira, C. et al. SDHC Promoter Methylation, a Novel Pathogenic Mechanism in Parasympathetic Paragangliomas1. Bernardo-Castiñeira, C., Valdés, N., Sierra, M. I., Sáenz-de-Santa-María, I., Bayón, G. F., Perez, R. F. et al. SDHC Promoter Methylation, a Novel Pathogenic Mechanism in Parasympa. J. Clin. Endocrinol. Metab. 103, 295–305 (2018).
Richter, S. et al. Epigenetic Mutation of the Succinate Dehydrogenase C Promoter in a Patient With Two Paragangliomas. J. Clin. Endocrinol. Metab. 101, 359–63 (2016).
Turcan, S. et al. IDH1 mutation is sufficient to establish the glioma hypermethylator phenotype. Nature 483, 479–83 (2012).
Whitworth, J. et al. Comprehensive Cancer-Predisposition Gene Testing in an Adult Multiple Primary Tumor Series Shows a Broad Range of Deleterious Variants and Atypical Tumor Phenotypes. Am. J. Hum. Genet. https://doi.org/10.1016/j.ajhg.2018.04.013 (2018).
Roller, E., Ivakhno, S., Lee, S., Royce, T. & Tanner, S. Canvas: versatile and scalable detection of copy number variants. Bioinformatics 32, 2375–7 (2016).
Chen, X. et al. Manta: rapid detection of structural variants and indels for germline and cancer sequencing applications. Bioinformatics 32, 1220–2 (2016).
Remacha, L. et al. Targeted Exome Sequencing of Krebs Cycle Genes Reveals Candidate Cancer–Predisposing Mutations in Pheochromocytomas and Paragangliomas. Clin. Cancer Res. 23, 6315–6324 (2017).
Boikos, S. A. et al. Carney triad can be (rarely) associated with germline succinate dehydrogenase defects. Eur. J. Hum. Genet. 24, 569–73 (2016).
Carney, J. A. & Stratakis, C. A. Familial paraganglioma and gastric stromal sarcoma: a new syndrome distinct from the Carney triad. Am. J. Med. Genet. 108, 132–9 (2002).
Settas, N., Faucz, F. R. & Stratakis, C. A. Succinate dehydrogenase (SDH) deficiency, Carney triad and the epigenome. Mol. Cell. Endocrinol. https://doi.org/10.1016/j.mce.2017.07.018 (2017).
Baylin, S. B. & Jones, P. A. Epigenetic Determinants of Cancer. Cold Spring Harb. Perspect. Biol. 8 (2016).
Gazzoli, I., Loda, M., Garber, J., Syngal, S. & Kolodner, R. D. A hereditary nonpolyposis colorectal carcinoma case associated with hypermethylation of the MLH1 gene in normal tissue and loss of heterozygosity of the unmethylated allele in the resulting microsatellite instability-high tumor. Cancer Res. 62, 3925–8 (2002).
Hitchins, M. P. et al. Dominantly inherited constitutional epigenetic silencing of MLH1 in a cancer-affected family is linked to a single nucleotide variant within the 5′UTR. Cancer Cell 20, 200–13 (2011).
Richter, S. et al. Krebs Cycle Metabolite Profiling for Identification and Stratification of Pheochromocytomas/Paragangliomas due to Succinate Dehydrogenase Deficiency. J. Clin. Endocrinol. Metab. 99, 3903–3911 (2014).
Casey, R. T. et al. Translating In Vivo Metabolomic Analysis of Succinate Dehydrogenase–Deficient Tumors Into Clinical Utility. JCO Precis. Oncol. 1–12, https://doi.org/10.1200/PO.17.00191 (2018).
Currás-Freixes, M. et al. Recommendations for somatic and germline genetic testing of single pheochromocytoma and paraganglioma based on findings from a series of 329 patients. J. Med. Genet. 52, 647–56 (2015).
Acknowledgements
NIHR BioResource, Cambridge University Hospitals, Cambridge Biomedical Campus, Cambridge CB2 0QQ, UK. Funding sources: Health Research Board Ireland and GIST Support UK (RTC), Cambridge NIHR Biomedical Research Centre (EM), NIHR Senior Investigator Award (ERM), European Research Council Advanced Researcher Award (ERM), the British Heart Foundation (ERM). The University of Cambridge has received salary support in respect of EM from the NHS in the East of England through the Clinical Academic Reserve. The views expressed are those of the authors and not necessarily those of the NHS or Department of Health.
Author information
Authors and Affiliations
Contributions
R.T.C., R.t.H., B.C., E.O., O.G., E.R.M. were involved in patient recruitment, study design, data analysis and manuscript preparation. S.M.P., C.W. and V.R.B. were involved with patient recruitment, data analysis and manuscript preparation and approval. J.W., P.S., F.R., M.M., G.C., L.C., T.R., J.A., K.A., M.B., A.M., J.E.M. were involved in data analysis, sample preparation and manuscript preparation and approval.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Casey, R.T., ten Hoopen, R., Ochoa, E. et al. SDHC epi-mutation testing in gastrointestinal stromal tumours and related tumours in clinical practice. Sci Rep 9, 10244 (2019). https://doi.org/10.1038/s41598-019-46124-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-46124-9
- Springer Nature Limited