Abstract
Accumulating evidence suggests neuroinflammation to be an integrated feature of neurodegeneration. Profiling inflammatory mediators across diseases may reveal common and disease-specific signatures. Here, we focused on progressive supranuclear palsy (PSP), a tauopathy presenting motor and cognitive dysfunction. We screened for 21 cytokines and growth factors in the dorsomedial prefrontal cortex of 16 PSP and 16 control brains using different quantitative techniques. We found and validated increased interleukin (IL)-2 protein levels in the PSP group expressed locally by neurons and glia cells. We further investigated central players in neuroinflammatory pathways and found increased mRNA expression of glycogen synthase kinase 3 beta (GSK3B). IL-2 and GSK3B proteins are T and natural killer (NK) cell regulators and have previously been associated with other neurodegenerative diseases such as Alzheimer’s disease, Parkinson’s disease and multiple system atrophy. In addition, we identified a shift in peripheral CD4+ and CD8+ T cell populations toward increased numbers of memory and reduced numbers of naive T cells. We also observed increased numbers of CD56+ NK cells, but not of CD56+CD57+ or CD57+ NK cells. Our findings suggest a role for IL-2 in PSP disease processes and point toward active and possibly dysfunctional peripheral immune responses in these patients.
Similar content being viewed by others
Introduction
Progressive supranuclear palsy (PSP) is a progressive, neurodegenerative disease that shares clinical features with other parkinsonian disorders and Alzheimer’s disease (AD)1. Neuropathologically, PSP is characterized by accumulation of tau protein in neurofibrillary tangles (NFT) in tufted astrocytes. The highest load of NFTs are localized in different areas of the basal ganglia and the brainstem, but cortical areas including the frontal lobe are also affected2,3. Brain imaging studies have shown that brain atrophy in PSP patients is accompanied by microglial activation in the frontal cortex4,5. This suggests that neuroinflammatory processes are part of the course of the disease. Still, there is a knowledge gap regarding how neuroimmunomodulating factors are affected in PSP brains. The few existing studies approaching this topic have focused solely on gene expression without reporting protein levels6,7. This information is important, not only for understanding the pathophysiology behind this disease, but also for illustrating to what extend neuroinflammatory processes are a common manifestation in neurodegenerative diseases. To our knowledge, no study has investigated the immune cell profile in PSP patients to shed light on the possible involvement of the peripheral immune system in PSP.
The primary aim of the present study was to investigate the neuroinflammatory signaling profile in the dorsomedial prefrontal cortex (dmPFC) of PSP patients. Using multiplex assays, we screened for protein levels of 21 cytokines and growth factors in post-mortem brain samples from 16 PSP patients and 16 normal controls (NCs). Significant differences were validated using sensitive electrochemiluminescence technology. To depict the underlying processes associated with cytokine aberrancies, we performed quantitative gene expression analyses of up- and down-stream targets as well as of signaling effector molecules. Lastly, using flow cytometry we screened for peripheral changes in CD4+ and CD8+ T cell, and NK cell numbers in PSP patients.
Results
IL-2 protein levels are increased in PSP patients
Of the 21 protein targets, IL-2 (F(4,15) = 7.931, p = 0.001, R2 = 0.68) and G-CSF (F(4,11) = 16.141, p < 0.001, R2 = 0.85) passed the correction for multiple testing, and both cytokines were predicted by group (t = 3.888, p = 0.002 and t = −7.819, p < 0.001, respectively). We then validated these findings using a more sensitive singleplex assay. The observed differences in G-CSF protein levels did not pass validation (F(4,11) = 0.501, p > 0.050, R2 = 0.25) but we confirmed differences in IL-2 protein levels (F(4,23) = 3.698, p = 0.018, R2 = 0.39; Fig. 1A). PSP patients showed increased IL-2 protein levels (t = 3.425, p = 0.002), while in general, males showed lower levels than females (t = −2.804, p = 0.010). Scores for the presence of dementia and resting tremor (Table 1) within the first year of diagnosis correlated with Luminex measurements of IL-2 protein levels (Spearman’s rho(ρ) = −0.881, p < 0.001, and ρ = 0.798, p = 0.006, respectively; Supplementary Fig. S3), with dementia scores showing a negative correlation whereas tremor scores showed a positive correlation. Conversely, no correlations were found with MSD measurements of IL-2 protein levels (p > 0.050; Supplementary Fig. S3). No other clinical scores correlated significantly to IL-2 protein levels (p > 0.050; data not shown).
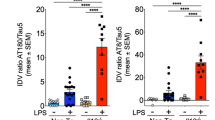
Interleukin-2 (IL-2) and glycogen synthase kinase 3 beta (GSK3B) levels. (A) IL-2 protein levels in progressive supranuclear palsy (PSP) patients normalized to normal control (NC) levels. Protein levels were measured using two commercial assays based on Luminex or MSD technology. Filled symbols mark male subjects, open symbols mark female subjects. (B) mRNA levels of GSK3B. Expression levels normalized to the reference genes ubiquitin-conjugating enzyme 2D2 (UBE2D2), ribosomal protein 13a (RPL13A) and DNA topoisomerase 1 (TOP1). Data presented as mean ± SEM. *p < 0.050; **p < 0.010. (C) Immunofluorescent stainings of IL-2 (red), and NeuN (green) or (D) GFAP (green). Nuclei are stained with DAPI (blue). Scale bars: 20 µm, 100X magnification.
GSK3B mRNA levels are increased in PSP patients
We then measured mRNA levels of central protein players in inflammatory pathways (Table 2). We identified increased mRNA levels of GSK3B in PSP patients (F(5,17) = 4.845, p = 0.006, R2 = 0.59; Fig. 1B) which was predicted by group (t = 3.914, p = 0.001) and RIN (t = −2.200, p = 0.042). Further, investigating downstream targets to GSK3B, only the PD-related NR4A2 was significantly described by our model (F(5,13) = 4.413, p = 0.014), but only by RIN (t = −2.973, p = 0.011) and not group (p > 0.050). Neither NFKBIA nor RELA, both immune regulatory factors, were significantly described by our model (p > 0.050).
IL-2 protein is expressed by NeuN+ and GFAP+ brain cells
To investigate if IL-2 is produced locally by brain cells, we performed double immunofluorescence labelling using specific antibodies against IL-2, neuronal nuclei (NeuN), and glial fibrillary acidic protein (GFAP) on brain sections from both PSP patients and NCs. We observed co-localization of IL-2 with both NeuN and GFAP in both groups (Fig. 1C,D) confirming that IL-2 is produced locally in the brain.
The number of peripheral T and NK cells are altered in blood of PSP patients
Lastly, using flow cytometry on a new cohort (18 NCs, nine PSP patients) we investigated whether the elevated levels of IL-2 in PSP brains could be reflected in the composition of peripheral T lymphocytes. We observed a significant increase in numbers of CD4+ T cells in PSP patients (t = 3.812, p < 0.001; Fig. 2B) accompanied by a shift in the ratio between memory and naive CD4+ T cells (t = 3.915, p < 0.001, and t = 7.940, p < 0.001, respectively; Fig. 2C,D). CD8+ T cells numbers did not differ between the groups (t = 1.602, p = 0.122; Fig. 2E), however, we also observed a shift in the ratio between memory and naive CD8+ T cells (t = 7.667, p < 0.001, and t = 8.595, p < 0.001, respectively; Fig. 2F,G) in PSP patients. We did not see any differences in the levels of total T cells (t = 0.318, p = 0.754; Fig. 2A). Since IL-2 protein not only affects classical T cells but also interacts with different types of natural killer (NK) cells, we included NK cell markers in our setup. We saw no differences in the numbers of total NK cells (t = 1.071, p = 0.295; Fig. 2H) between the groups, however, CD56+ NK cell numbers were increased in PSP patients (t = 7.007, p < 0.001; Fig. 2I). We found a tendency towards increased CD57+ NK cells (t = 1.956, p = 0.062; Fig. 2K) whereas there was no difference in CD56+CD57+ NK cells (t = 0.699, p = 0.491; Fig. 2J).
T and NK cell populations in blood of PSP patients. We investigated peripheral blood mononuclear cells in a new cohort consisting of 18 normal controls and nine PSP patients. We investigated fractions of (A–G) T cells and (H–K) natural killer (NK) cells. For gating strategies, see Supplementary Figs. 1 and 2. Data are presented as mean ± SEM; n.s. non-significant; ***p < 0.001.
Discussion
To our knowledge, this is the first study extensively evaluating cytokine protein levels in brains affected by PSP. We screened for 18 cytokines and three growth factors and identified increased IL-2 protein levels in the prefrontal cortex of the brain in PSP patients. Generally, the effects of IL-2 are maturation and survival of T cells of the peripheral immune system8. However, the expression of IL-2 and IL-2 receptors is found in brain cells, and we have previously shown how NeuN+ cells express IL-2 protein in both healthy brains, and brains from patients diagnosed with Parkinson’s disease or multiple system atrophy9. Concordantly, in this study we observed co-localization of IL-2 inside both NeuN+ and GFAP+ cells. IL-2 protein has been suggested to be a neurokine10. Hence, the increase in IL-2 protein levels could be of neuronal origin and caused by disease processes. In the current study, for IL-2 protein levels, we observed both sex-specific differences and correlations to clinical scores. To our knowledge, no study has identified sex-specific differences in PSP, nor does the incidence rate differ between sexes11. The correlations to the clinical scores were significant for the Luminex measurements, however, this was not replicated for the MSD measurements. This is probably due to the nature of both methods and needs further validation. These observations might be relevant for evaluation of the clinical aspects of changes in brain IL-2 levels. Nevertheless, the current study was not designed to address such issues as the sample size is a limiting factor. Due to our modest sample size included in this experimental setup, additional studies using larger cohorts are needed to validate these findings.
One regulator of IL-2 and T cell proliferation is GSK3B protein12. We identified increased mRNA levels of GSK3B in PSP brains. In the brain, GSK3B protein participates in the production of pro-inflammatory mediators secreted by microglia cells13. GSK3B has previously received considerable attention in relation to PSP as GSK3B protein activity mediates aggregation of tau14. However, interventional strategies targeting GSK3B have been unsuccessful15. Although mRNA and protein levels are not directly correlated, our findings are in addition to the previous observations of a central role of GSK3B in the brains of PSP patients.
For the first time, we report on lymphocyte populations in blood of PSP patients. IL-2 protein is produced by CD4+ and CD8+ T cells as well as CD56+ and CD57+ NK cells either as developing or mature cells8,16,17. Overall, the observed shift in the pool of T cells toward an increase in CD45RA−CD45RO+ memory T cells may be indicative of an active, adaptive immune response. Further, the concurrent decrease in CD45RA+CD45RO− naive T cells, accompanied by increased numbers of CD4+, but not CD8+, T cells, could be indicative of a possible reduced capacity of the adaptive immune system toward novel stimuli18. Lastly, the observed increase in CD56+ NK cells, but not of activated CD56+CD57+ NK cells, points toward an increased capacity to respond to innate immune challenges. These results are supported by the results from Santiago and Potashkin19 reporting on affected gene clusters in PSP that are overrepresented in biological pathways of both leukocyte and lymphocyte activation. Nevertheless, limitations to our study are the low number of individuals as well as the lack of demographic and clinical information for factors affecting peripheral lymphocyte levels. Therefore, we encourage that our observations should be validated in a separate study using a larger and well-defined cohort.
Lately, IL-2 has received much attention as an immune effector in AD since animal studies have shown a beneficial effect of IL-2 on amyloid pathology20,21. We could therefore speculate that the increased levels observed in the PSP brains are reflecting compensatory effects to the disease progression rather than necessarily being the cause of the detrimental effects. Therefore, IL-2 treatment aiming at specifically expanding and activating regulatory T cells recently proposed in AD22,23 could also be an interesting venue to explore in relation to PSP, which at present is a disease without curative treatment.
Other studies have reported on cytokine gene expression in several brain areas of PSP patients with differing results. One study observed increased expression of IL-1beta(β) in the substantia nigra and no change in the expression of transforming growth factor β in the frontal cortex of PSP patients7, whereas another study identified increased expression of the latter in the frontal cortex of PSP patients compared to controls6. In the current study, we did not assess protein levels of transforming growth factor β, nor did we investigate cytokine expression in the substantia nigra. Here, we focused on the prefrontal cortex which is an area that is only mildly affected in PSP4. Our aim was to identify disease pathology distant to the epicenter of the disease that may reflect earlier stages of degenerative processes. This is a limiting factor to our study, and we can therefore not exclude that cytokine expression may be different in other more affected brain areas in PSP patients.
To conclude, in the present study we have identified and validated an increase in IL-2 protein levels in the prefrontal cortex of PSP patients compared with NCs. PSP thereby presents a similar cytokine profile as the one identified for both Parkinson’s disease and multiple system atrophy in the same brain area. Further, we have observed changes in the peripheral immune system. Our results are indicative of an active and dysregulated adaptive and innate immune response. These data warrant future investigations into the possible role of the immune systems in PSP pathophysiology.
Materials and Methods
Patient material
The tissue for the present study originated from donated human brains. All donors provided written informed consent prior to death. PSP samples were analyzed alongside NC samples9. Brain samples were provided by the Bispebjerg Brain Bank (University Hospital of Copenhagen, Bispebjerg Hospital, DNK), the Netherlands Brain Bank (Netherlands Institute for Neuroscience, NLD), and the Harvard Brain Tissue Resource Center (Harvard Medical School Teaching Hospital, USA). A summary of demographic data for the 16 NC and 16 PSP brain samples are shown in Table 3. All brains were neuropathologically examined to verify the clinical diagnosis1,24. Medical records were available for 13 of the 16 patients. The clinical characteristics are shown in Table 1. Clinical scorings were based on the clinicians’ notes as well as appropriate, clinical tests, e.g., the Mini-Mental State Examination, the Montreal Cognitive Assessment, or the Addenbrooke's Cognitive Examination tests for dementia and cognitive dysfunction. All brain samples have been collected and handled in accordance with Danish ethical standards of the Brain Bank and the Danish Health and Medicine Authorities. Samples were stored at −80 °C prior to handling. Flow cytometry was performed on a new cohort of samples, that included 18 NC and nine PSP samples, a summary of the demographic data are shown in Table 3. These samples were generously donated by Bispebjerg Movement Disorder Biobank. This project was approved by the Regional Ethics Committee of the Capital Region of Denmark (DNK), jr.no. H-16025210. All experiments have been performed in accordance with the Declaration of Helsinki25.
Protein analyses
Protein extraction and quantification of total levels were performed as earlier described9. In short, app. 50 mg brain tissue was disrupted and homogenized using a MagNA Lyzer Instrument (Roche Life Science) in a buffer containing protease inhibitors. Samples were spun to pellet cell debris. Sample concentrations were determined using the Bradford protein quantification assay26. The protein concentrations in the NC sample extracts (mean 3.61 ± SD 1.04 mg/ml) did not differ from the PSP sample extracts (mean 3.17 ± SD 0.56 mg/ml; Welch’s unpaired t-test, t = 1.421, p = 0.171). All samples were aliquoted to avoid thawing prior to analysis. Cytokine and neurotrophin protein levels were investigated on a Bio-Plex Pro Human Cytokine 17-plex Assay (BIO-RAD, USA; #M5000031YV) which included antibodies for the targets interleukin(IL)-1β, IL-2, ILs 4–8, IL-10, IL-12, IL-13, IL-17, granulocyte colony-stimulating factor (G-CSF), granulocyte macrophage colony-stimulating factor, interferon-gamma, monocyte chemoattractant protein 1, macrophage inflammatory protein(MIP)1β, and tumor necrosis factor(TNF)alpha(α). Further, the following singleplex targets were added to the assay: basic fibroblast growth factor (BIO-RAD; #171B5016M); MIP1α (BIO-RAD; #171B5022M); platelet-derived growth factor BB (BIO-RAD; #171B5024M); and vascular endothelial growth factor (VEGF; BIO-RAD; #171B5027M). The assays were analysed on a Bio-Plex 200 System (Luminex xMAP Technology, BIO-RAD) following the manufacturer’s instructions. Samples were added in a concentration of 1 mg protein/ml after 1:2 dilution in sample diluent.
Validation of IL-2 and G-CSF protein levels were investigated on MSD V-PLEX Human IL-2 Assays (Meso Scale Discovery (MSD), USA; #K151QQD; lower limit of detection (LLOD): 0.09 pg/ml) and MSD U-PLEX G-CSF Assays (MSD; #K151VGK; LLOD: 1.6 pg/ml), respectively, following the manufacturer’s instructions. Plates were analyzed on a SECTOR S 600 instrument (MSD).
mRNA analyses
Extraction, quality assessment of total RNA, DNA contamination, and analysis of mRNA levels was performed as previously described9. All RNA experiments were performed in accordance with the MIQE guidelines27. Extraction of RNA was performed using the miRNeasy Mini Kit (Qiagen, NLD; #217004). In short, app. 30 mg brain tissue was homogenized by intensive pipetting in a lysis reagent. Samples were then phase separated in chloroform, and subjected to on-column DNase treatment. Samples were eluted in 30 µl nuclease-free water. RNA integrity was measured using the Agilent RNA 6000 Nano Kit (Agilent Technologies, USA; #5067-1511) on an Agilent 2100 Bioanalyzer (Agilent Technologies). All samples had a RIN > 3.95 as suggested earlier28,29. DNA contamination was investigated using primers for glyceraldehyde 3-phosphate dehydrogenase (GAPDH). cDNA synthesis was performed using qScript (Quanta BioSciences, USA; #95048) with 100 ng input RNA. cDNA concentrations were measured on a NanoDrop 2000C (Thermo Fisher Scientific), and diluted to 100 ng/µl in nuclease-free water. RT-qPCR was performed with SYBR Green Master Mix 2X (Thermo Fischer Scientific; #4309155) on a Stratagene Mx3005P qPCR system, or a QuantStudio 3 Real-Time PCR System (Thermo Fischer Scientific). All primers had an efficiency of 93–108% with an R2 > 0.98. Primer concentrations ranged from 300 to 500 nM with an annealing temperature of 56–62 °C. An acquisition step was added when necessary at 77–80 °C. The amplicons were 72 to 146 base pairs long. A melting curve was included in each reaction for product verification. Targets of interest were: glycogen synthase kinase 3β (GSK3B), forward primer: 5-ACAACAGTGGTGGCAACTCC-3, reverse primer: 5-TTCTTGATGGCGACCAGTTCT-329; nuclear factor kappa-light-chain-enhancer of activated B cells inhibitor alpha (NFKBIA), forward primer: 5-AGCCTACAAGAAAGTTTGCCTAT-3, reverse primer: 5-TCTTCTTCCGGTAGTGGATCTTGGC-3; nuclear receptor subfamily 4 group A member 2 (NR4A2; also known as Nurr1), forward primer: 5-CCGGGATCTCTCCACAACTT-3, reverse primer: 5-GGAGACTGGCGTTTTCCTCT-3; and transcription factor p65 (RELA; also known as NFKB-p65), forward primer: 5-CTGCCGGGATGGCTTCTAT-3, reverse primer: 5-CCGCTTCTTCACACACTGGAT-330. A calibrator sample (cDNA from Human Reference Total RNA; Clontech, USA; #636538), was included on each plate31. Sample cycle threshold (Ct) values were normalized to the reference genes29: ubiquitin-conjugating enzyme E2D2 (UBE2D2), forward primer: 5-TGCCTGAGATTGCTCGGATCT-3, reverse primer: 5-TCGCATACTTCTGAGTCCATTCC-332; ribosomal protein 13a (RPL13A), forward primer: 5-AGCCTACAAGAAAGTTTGCCTAT-3, reverse primer: 5-TCTTCTTCCGGTAGTGGATCTTGGC-3; and topoisomerase 1 (TOP1), forward primer: 5-GGCGAGTGAATCTAAGGATAATGAA-3, reverse primer 5-TGGATATCTTAAAGGGTACAGCGAA-333. Normalized values were calculated using the geometric mean34.
Immunohistochemistry
Immunostainings on FFPE tissue samples were performed as previously described9. In short, brain samples from three PSP patients and three NCs containing both white and grey matter were excised and fixed in formalin for min. 48 h in 10% buffered formalin before embedding in paraffin on a Leica ASP300 S tissue processor (Leica, DEU). Samples were cut on a sliding microtome into 5 µm sections. All antibodies were tested alone using HRP and 3,3′-diaminobenzidine tetrahydochloride hydrate as previously described9. For immunofluorescent double labelling, slides were deparaffinized before antigen retrieval at pH 6 (NeuN) and/or 9 (IL-2, GFAP) followed by incubation with primary antibodies: Monoclonal rabbit anti-human IL-2 (1:250; Abcam; #ab92381), and monoclonal mouse anti-human NeuN (1:500; Merck Millipore; #MAB377) or monoclonal mouse anti-human GFAP (1:200; Dako; #M0761). Secondary antibodies were goat anti-mouse IgG Alexa Fluor 488 (1:200; Invitrogen; #A11001) and goat anti-rabbit IgG TRITC (1:1000; Abcam; #ab6718). Cover slides were mounted with medium containing DAPI. Stainings were investigated using a Nikon Eclipse 80i microscope. Specificity of the IL-2 antibody was tested on tonsils as a positive control (data not shown). Additionally, appropriate isotype controls were included to verify specificity.
Flow cytometry
Blood was collected in 9 ml EDTA tubes (Greiner Bio-One; #455036). Briefly, blood was diluted 1:1 in Dulbecco’s PBS (DPBS; Sigma-Aldrich; #D8537) containing 2 mM EDTA (Sigma-Aldrich; #E9884) and 1:5 in a Ficoll-Paque PLUS gradient (GE Healthcare Life Science; #17144003). Cells were washed twice with PBS and frozen in RPMI-1640 (Sigma-Aldrich; #R5885) containing 40% fetal calf serum (FCS; Gibco; #26010-074) and 10% dimethyl sulfoxide (Sigma-Aldrich; #D4540) in cryotubes at −80 °C in a Mr. Frosty Freezing Container (Thermo Scientific), then stored at −130 °C until use.
On the day of analysis, PBMCs were thawed at 37 °C for 10 min before washing in 10% FCS in RPMI-1640 followed by centrifugation at 200 g for 10 min. 200,000 cells were added to a well on a round bottom plate. The plate was spun down at 2,600 RPM for 2 min before washing in 1X Foxp3 TF Wash Buffer (Invitrogen; A24261) in 1% BSA (Sigma-Aldrich; #05482) in PBS followed by another spin down. Cells were incubated for 30 min in the dark at 4 °C in 45 µl antibody staining solution. The staining solution contained antibodies against TCR-α/β (1:8; BD Biosciences; #555548), CD3ε (1:50; BioLegend; #300431), CD4 (1:50; BD Biosciences; #564976), CD8 (1:50; BD Biosciences; #565310), CD14 (1:25; BioLegend; #325618), CD16 (1:25; BioLegend; #302012), CD45R0 (1:50; BD Biosciences; #562327), CD45RA (1:50; BD Biosciences; #563963), CD56 (1:25; BioLegend; #318334), and CD57 (1:25; BD Biosciences; #561906) in Brilliant Stain Buffer (BD Biosciences; #563794). Cells were washed in 180 µl 1X Foxp3 TF Wash Buffer and centrifuged at 600 × g for 5 min before 150 µl 1X Fixation and Permeabilization Solution (Buffer A: Invitrogen; #A24217; Buffer B: Invitrogen; #A24218) was added for 15 min at room temperature. After centrifugation at 600 × g for 5 min, cells were resuspended in 200 µl 1% BSA in PBS for analysis.
Samples were quantified on a BD LSR II flow cytometer (BD Biosciences). A minimum of 20,000 events were counted per sample. Data were analyzed using FlowLogic v. 7.2.1 (Inivai Technologies). The gating strategies are illustrated in Supplementary Figs. S1 and S2 which are based on compensation and FMO analyses.
Statistics
ILs 4, 5, 8, 10, 12, 17, MIP1α, TNFα and VEGF proteins were omitted from the analyses as detection sensitivity was less than 50%. One sample did not present detectable protein levels in any measurements on the Luminex assays and was excluded from further analyses. For each target, samples that presented measurements below the minimum detection limit were omitted from the analysis. Outliers were identified using the ROUT method with Q = 1% in GraphPad Prism v. 7.02 (GraphPad Software Inc., USA). Demographic data were analyzed using an unpaired t-test (age), a Mann-Whitney test (RNA Integrity Number (RIN) and post-mortem interval (PMI)), or Fischer’s exact test (sex). Spearman’s Rank-Order Correlation test was applied to correlation analyses of clinical characteristics and IL-2 protein levels. Student’s t-test or Welch’s t-test was used for flow cytometry analyses. The remaining statistical analyses were performed in R v. 3.4.135. Normality was assessed using the Shapiro-Wilk Normality test. Scedasticity and collinearity were assessed using ncvTest and vif, respectively, from the car package36. If necessary, data were log10-transformed. Data were analyzed using multiple linear regression with models that included relevant variables that have previously been shown to possibly impact results9. These variables included group (NC vs. PSP), age, sex (female vs. male), and PMI as well as RIN for the mRNA targets. Familywise error rate was adjusted using Bonferroni correction for the multiplex data (adj. p = 4.17*10−3, n = 12). Graphs were made in GraphPad Prism v. 7.02.
Data Availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
References
Höglinger, G. U. et al. Clinical diagnosis of progressive supranuclear palsy: The movement disorder society criteria. Mov Disord 32, 853–864, https://doi.org/10.1002/mds.26987 (2017).
Li, F. et al. Regional quantitative analysis of tau-positive neurons in progressive supranuclear palsy: Comparison with Alzheimer's disease. J Neurol Sci 159, 73–81, https://doi.org/10.1016/S0022-510X(98)00136-1 (1998).
Tawana, K. & Ramsden, D. Progressive supranuclear palsy. Mol Pathol 54, 427 (2001).
Sakurai, K. et al. Beyond the midbrain atrophy: wide spectrum of structural MRI finding in cases of pathologically proven progressive supranuclear palsy. Neuroradiology 59, 431–443, https://doi.org/10.1007/s00234-017-1812-4 (2017).
Gerhard, A. et al. In vivo imaging of microglial activation with [11C](R)‐PK11195 PET in progressive supranuclear palsy. Mov Disord 21, 89–93, https://doi.org/10.1002/mds.20668 (2006).
López González, I., Garcia-Esparcia, P., Llorens, F. & Ferrer, I. Genetic and Transcriptomic Profiles of Inflammation in Neurodegenerative Diseases: Alzheimer, Parkinson, Creutzfeldt-Jakob and Tauopathies. Int J Mol Sci 17, 206, https://doi.org/10.3390/ijms17020206 (2016).
Fernandez-Botran, R. et al. Cytokine expression and microglial activation in progressive supranuclear palsy. Parkinsonism Relat Disord 17, 683–688, https://doi.org/10.1016/j.parkreldis.2011.06.007 (2011).
Boyman, O. & Sprent, J. The role of interleukin-2 during homeostasis and activation of the immune system. Nat Rev Immunol 12, 180, https://doi.org/10.1038/nri3156 (2012).
Rydbirk, R. et al. Cytokine profiling in the prefrontal cortex of Parkinson’s Disease and Multiple System Atrophy patients. Neurobiol Dis 106, 269–278, https://doi.org/10.1016/j.nbd.2017.07.014 (2017).
Prinz, M., Van Rossum, D. & Hanisch, U.-K. Interleukin-2 as a neuroregulatory cytokine. NeuroImmune Biol 6, 145–165, https://doi.org/10.1016/S1567-7443(07)10008-9 (2008).
Schrag, A., Ben-Shlomo, Y. & Quinn, N. P. Prevalence of progressive supranuclear palsy and multiple system atrophy: a cross-sectional study. Lancet 354, 1771–1775, https://doi.org/10.1016/S0140-6736(99)04137-9 (1999).
Garcia, C. A. et al. Antigenic Experience Dictates Functional Role of Glycogen Synthase Kinase-3 in Human CD4+ T Cell Responses. J Immunol 181, 8363–8371, https://doi.org/10.4049/jimmunol.181.12.8363 (2008).
Cao, Q., Karthikeyan, A., Dheen, S. T., Kaur, C. & Ling, E.-A. Production of proinflammatory mediators in activated microglia is synergistically regulated by Notch-1, glycogen synthase kinase (GSK-3β) and NF-κB/p65 signalling. Plos One 12, e0186764, https://doi.org/10.1371/journal.pone.0186764 (2017).
Hernández, F., de Barreda, E. G., Fuster-Matanzo, A., Lucas, J. J. & Avila, J. GSK3: a possible link between beta amyloid peptide and tau protein. Exp Neurol 223, 322–325, https://doi.org/10.1016/j.expneurol.2009.09.011 (2010).
Koros, C. & Stamelou, M. Interventions in progressive supranuclear palsy. Parkinsonism Relat Disord 22, S93–S95, https://doi.org/10.1016/j.parkreldis.2015.09.033 (2016).
Renard, V. et al. Normal development and function of natural killer cells in CD3 epsilon delta 5/delta 5 mutant mice. PNAS 92, 7545–7549, https://doi.org/10.1073/pnas.92.16.7545 (1995).
Liao, W., Lin, J.-X. & Leonard, W. J. Interleukin-2 at the crossroads of effector responses, tolerance, and immunotherapy. Immunity 38, 13–25, https://doi.org/10.1016/j.immuni.2013.01.004 (2013).
Obar, J. J. & Lefrançois, L. Memory CD8+ T cell differentiation. Ann. N. Y. Acad. Sci. 1183, 251–266, https://doi.org/10.1111/j.1749-6632.2009.05126.x (2010).
Santiago, J. A. & Potashkin, J. A. A network approach to diagnostic biomarkers in progressive supranuclear palsy. Mov Disord 29, 550–555, https://doi.org/10.1002/mds.25761 (2014).
Alves, S. et al. Interleukin-2 improves amyloid pathology, synaptic failure and memory in Alzheimer's disease mice. Brain 140, 826–842, https://doi.org/10.1093/brain/aww330 (2017).
Dansokho, C. et al. Regulatory T cells delay disease progression in Alzheimer-like pathology. Brain 139, 1237–1251, https://doi.org/10.1093/brain/awv408 (2016).
Dansokho, C., Aucouturier, P. & Dorothee, G. Beneficial effect of interleukin-2-based immunomodulation in Alzheimer-like pathology. Brain 140, e39, https://doi.org/10.1093/brain/awx108 (2017).
Alves, S., Churlaud, G., Klatzmann, D. & Cartier, N. Reply: Beneficial effect of interleukin-2-based immunomodulation in Alzheimer-like pathology. Brain 140, e40–e40, https://doi.org/10.1093/brain/awx109 (2017).
Lantos, P. The neuropathology of progressive supranuclear palsy. J Neural Transm-Supp, 137–152 (1994).
World Medical Association Declaration of Helsinki. ethical principles for medical research involving human subjects. Jama 310, 2191–2194, https://doi.org/10.1001/jama.2013.281053 (2013).
Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72, 248–254 (1976).
Bustin, S. A. et al. The MIQE guidelines: minimum information for publication of quantitative real-time PCR experiments. Clin Chem 55, 611–622, https://doi.org/10.1373/clinchem.2008.112797 (2009).
Weis, S. et al. Quality control for microarray analysis of human brain samples: The impact of postmortem factors, RNA characteristics, and histopathology. J Neurosci Methods 165, 198–209, https://doi.org/10.1016/j.jneumeth.2007.06.001 (2007).
Rydbirk, R. et al. Assessment of brain reference genes for RT-qPCR studies in neurodegenerative diseases. Sci Rep 6, 37116, https://doi.org/10.1038/srep37116 (2016).
Yan, W. & Chen, X. Identification of GRO1 as a critical determinant for mutant p53 gain of function. The Journal of biological chemistry 284, 12178–12187, https://doi.org/10.1074/jbc.M900994200 (2009).
Pfaffl, M. W. A new mathematical model for relative quantification in real-time RT–PCR. Nucleic Acids Res 29, e45, https://doi.org/10.1093/nar/29.9.e45 (2001).
Massingham, L. J. et al. Proof of concept study to assess fetal gene expression in amniotic fluid by nanoarray PCR. J Mol Diagn 13, 565–570, https://doi.org/10.1016/j.jmoldx.2011.05.008 (2011).
Lanoix, D. et al. Quantitative PCR pitfalls: the case of the human placenta. Mol Biotechnol 52, 234–243, https://doi.org/10.1007/s12033-012-9539-2 (2012).
Hellemans, J., Mortier, G., De Paepe, A., Speleman, F. & Vandesompele, J. qBase relative quantification framework and software for management and automated analysis of real-time quantitative PCR data. Genome Biol 8, R19, https://doi.org/10.1186/gb-2007-8-2-r19 (2007).
R: A Language and Environment for Statistical Computing v. 3.4.1 (R Foundation for Statistical Computing, 2017).
Fox, J. & Weisberg, S. An R Companion to Applied Regression. Second Edition edn, (SAGE Publications, Inc., 2011).
Acknowledgements
The authors are grateful to Hans-Jørgen Jensen and Susanne Sørensen for help with preparation of the samples and conduction of the experiments. We acknowledge Karina Fog and Lundbeck A/S who provided MSD equipment used for sample analyses. We are grateful to Bispebjerg Movement Disorders Biobank at Bispebjerg-Frederiksberg Hospital for donation of PBMC samples. This work has been supported by the Brdr. Hartmann Foundation, the Hørslev Foundation, the Jascha Foundation, Danmodis, the Parkinson Foundation Denmark, the Lundbeck Foundation, the Research Foundation of Bispebjerg-Frederiksberg Hospital, and the Danish National Association for Multiple System Atrophy. The sponsors were not involved in the study design; the collection, analysis and interpretation of data; writing of the report; or in the decision to submit the article for publication.
Author information
Authors and Affiliations
Contributions
R.R. and S.A. conceived the research. R.R., S.A., B.E., T.B. and J.F. designed and conducted the experiments. R.R. analyzed the data. R.R., S.A. and J.F. interpreted the results. R.R. wrote the main manuscript and prepared the figures and tables. K.W., T.B., B.P. and J.F. supplied brain tissue samples. S.A. elaborated the manuscript. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Rydbirk, R., Elfving, B., Folke, J. et al. Increased prefrontal cortex interleukin-2 protein levels and shift in the peripheral T cell population in progressive supranuclear palsy patients. Sci Rep 9, 7781 (2019). https://doi.org/10.1038/s41598-019-44234-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-44234-y
- Springer Nature Limited
This article is cited by
-
Sexual dimorphic impacts of systemic vincristine on lower urinary tract function
Scientific Reports (2022)
-
Brain proteome profiling implicates the complement and coagulation cascade in multiple system atrophy brain pathology
Cellular and Molecular Life Sciences (2022)
-
Epigenetic modulation of AREL1 and increased HLA expression in brains of multiple system atrophy patients
Acta Neuropathologica Communications (2020)