Abstract
Congenital heart diseases (CHDs) are the most common types of birth defects, affecting approximately 1% of live births and remaining the leading cause of mortality. CHD patients often show a higher incidence of heterotaxy syndrome. However, the exact aetiology of CHD and heterotaxy syndrome remains unclear. In this study, targeted sequencing and Sanger sequencing were performed to analyze the exonic regions of 37 primary ciliary dysfunction (PCD)- related candidate genes in 42 CHD patients with heterotaxy syndrome. Variants affecting protein-coding regions were filtered according to databases of known variants and predicted in silico using functional prediction program. Thirty-four potential disease-causing heterozygous variants in 11 genes were identified in the 19 CHD patients with heterotaxy syndrome (45.2%, 19/42). The DNAH11 gene showed the highest mutation rate (16.7%; 14 of 84 alleles) among the CHD patients with heterotaxy. Fisher’s exact test revealed a significant association of DNAH11 variants with CHD and heterotaxy (P = 0.0001). In families, six different compound heterozygous variants of DNAH11 were validated in family 1-5031 (p.W802X/p.M282I), family 2-5045 (p.T3460K/p.G4425S), family 3-5065 (p.G447R/p.L1157R), family 4-5130 (p.I2262T/p.D3800H), family 5-5707 (p.S1823fs/p.F2759L/p.R4395X) and family 6-5062 (p.D3610V/p.I243V). These findings suggest that the DNAH11 variants are significantly associated with CHD and heterotaxy syndrome and that compound heterozygous DNAH11 variants may be the common genetic cause of the development of familial CHD and heterotaxy syndrome.
Similar content being viewed by others
Introduction
Congenital heart diseases (CHDs) are the most common types of birth defects, affecting approximately 1% of live births and remaining the leading cause of mortality1. Interestingly, CHD patients often show a higher incidence of heterotaxy syndrome. Studies have found that approximately 5–10% of CHD patients present with heterotaxy syndrome2. Heterotaxy(HTX) is a rare birth defect involving left-right (LR) asymmetry with an incidence of 1 in 10,000 newborns, and approximately 90% of HTX patients have complex CHDs3. CHD and heterotaxy syndrome have been shown to be associated with primary cilia dysfunction (PCD) or cilia dysfunction (CD). The mortality and respiratory complications of CHD and heterotaxy in patients after cardiac surgery are significantly higher than those in the same type of patients without heterotaxy4. However, PCD is considered to be a monogenic heterogeneous recessive disorder, while CHD and heterotaxy syndrome are multiple-gene complex inherited diseases5. The linkage between PCD and CHD /HTX needs to be investigated.
Studies have shown that mutations in genes causing PCD may be associated with the development of heterotaxy and/or CHD syndrome. Approximately 50% of PCD patients exhibit heterotaxy associated with complex CHDs6. PCD-related genes in heterotaxy are thought to be responsible for the function of motile cilia in LR patterning, and CHD/heterotaxy patients also show increased airway CD similar to that seen in PCD patients7.
Researchers have shown that CHD and heterotaxy syndromes are multiple, complex, inherited diseases caused by numerous genes that are responsible for inherited and sporadic cases8. Mutations in over 15 genes related to LR patterning have been observed in heterotaxy patients, but these findings account for fewer than 20% of heterotaxy cases9. You Li et al. performed whole-exome sequencing in 218 CHD mouse models and identified 91 recessive CHD mutations in 61 genes, including 34 cilia-related genes, 16 genes involved in cilia-transduced cell signalling, and 10 genes regulating vesicular trafficking (a pathway important for ciliogenesis and cell signaling), including a loss-of-function DNAH11 mutation known to cause PCD10.
DNAH11, located on chromosome 7p15.3, encodes a ciliary outer dynein arm protein (520 kDa) and is a member of the dynein heavy chain family, localizing exclusively to the proximal region of respiratory cilia11. DNAH11 mutations have been found to result in abnormal ciliary ultrastructure and hyperkinetic ciliary beating12. Gene editing of DNAH11 mutations can restore normal cilia motility in primary ciliary dyskinesia13. An overwhelming majority of previous studies related to DNAH11 have focused on PCD and situs inversus totalis, and few studies have concentrated on heterotaxy and CHD.
To date, over 40 PCD-related genes have been found in heterotaxic patients with PCD. However, whether PCD-related gene mutations are associated with CHD and heterotaxy syndrome remains unclear. In this study, we performed targeted sequencing and Sanger sequencing analysis of PCD-related genes in Chinese CHD patients with heterotaxy syndrome to explore the genetic aetiology of CHD and heterotaxy syndrome.
Results
Assessment of the ciliary movement status in CHD patients with heterotaxy syndrome
A cohort of 42 CHD patients with heterotaxy syndrome were recruited from unrelated families, including 14 females (33.3%) and 28 males (66.7%) with ages ranging from 0.1 to 20.1 years (mean ± SD: 5.6 ± 4.7 years). To assess the ciliary movement status, we examined the ciliary beat pattern using a slow-motion playback of the video sequence to generate tracings of the ciliary beat and found that 29 cases (69%, 29/42) showed CD (18 males and 11 females), and 13 cases (31%, 13/42) presented normal ciliary function (10 males and 3 females).
The clinical features of the study subjects are summarized in Table 1.
Targeted sequencing analysis of gene variants in CHD patients with heterotaxy syndrome
To investigate the role of gene variants in CHD/heterotaxy diseases, we carried out targeted sequencing on the exonic regions of the following 37 PCD-related candidate genes in 42 CHD patients with heterotaxy: ABCC4, ARMC4, C21orf59, CCDC39, CCDC40, CCDC65, CCDC114, CCDC151, CCNO, DNAAF1, DNAAF2, DNAFF3, DNAAF5, DNAH5, DNAH8, DNAH11, DNAI1, DNAI2, DNAL1, DRC1, DYX1C1, HEATR2, HYDIN, LRRC6, NAT10, NME8, PTGES, PTGES2, PTGES3, PTGER4, PTGS1, PTGS2, RSPH1, RSPH4A, RSPH9, SPAG1 and ZMYND10.
For these genes, 758 regions of gene coding exons and their intron/exon junctions were sequenced. The coverage of the sequencing results was 90%-99%, with an average coverage of approximately 97%, and the average depth of sequencing was 100X. By filtering from 1000 Genomes Project and ExAC databases and using SIFT, PolyPhen2 and MutationTaster prediction programs, we focused on novel or rare coding variants (MAF < 0.01%) present in CHD patients with heterotaxy, excluding the common variants, synonymous variants and non-synonymous variants that are predicted to have no deleterious effect on protein function.
Thirty-four potential disease-causing heterozygous variants were identified in 11 of 37 candidate genes, including ARMC4, CCDC40, CCDC65, DHAH8, DNAAF2, DNAH11, DNAH5, DNAH8, DRC1, HYDIN, and SPAG1 (Table 2). These mutated genes were distributed in 19 CHD/HTX patients (45.2%, 19/42). Eighteen CHD patients with heterotaxy and CD had 31 variants in 8 genes, and 1 CHD patient (patient #5030) with heterotaxy and normal ciliary function possessed 3 variants in 3 genes. These findings indicate that the CHD patients with heterotaxy and CD has a significantly higher gene mutation rate than the CHD patients with heterotaxy and normal ciliary function among the CHD patients with heterotaxy (62.1%, 18/29 vs. 7.7%, 1/13; P = 0.0018) (Table 3).
Association of DNAH11 variants with the risk of CHD and heterotaxy syndrome
As shown in Table 4, the DNAH11 gene showed the highest mutation rate (16.7%; 14 of 84 alleles) among the CHD patients with heterotaxy. The HYDIN and DNAH8 genes both showed 6% mutation rate (5 of 84 alleles). The mutation rate of DNAH5 genes was 4.8% (4 of 84 alleles), and the other genes (ARMC4, CCDC40, CCDC65, DHAH8, DNAAF2, DRC1, and SPAG1) all showed a 1.2% mutation rate (1 of 84 alleles).
Concerning the highest mutation rate in the DNAH11 gene, we further analyzed the association of DNAH11 mutations with CHD and heterotaxy syndrome. In this study, there were 14 mutations in the DNAH11 genes in 7 of 42 patients, including 11 missense, 1 frameshift and 2 stop-gain mutations. Eight of these variants were novel and not present in the ExAC or 1000 Genomes Project databases, and 6 were low-frequency variants (MAF < 0.01%). Conservation analysis was processed via UCSC Genome Browser hg19.
In our previous study, whole genome sequencing was performed in 98 CHD patients without heterotaxy, and the methodology used in the previous study is strictly the same as that used in the present investigation. The CHD subtypes of these 98 CHD patients are listed in Table 5.
By consulting the previous exome database in our laboratory, we found no disease-causing mutations in the DNAH11 gene among 98 CHD cases. Moreover, one DNAH11 mutation (c.A9584G:p.N3195S) was found in 1 case (Patient 2073) among 3 CHD patients with heterotaxy. Combined with the results showing 7 of 42 patients with DNAH11 mutations in this study, the prevalence of DNAH11 mutations was higher in CHD patients with heterotaxy (8 of 45 cases) than in CHD patients (0 of 98 cases). The 98 CHD cases were considered controls because these cases exhibited only CHD, and the association of DNAH11 mutations with CHD and heterotaxy was significant (8 of 45 CHD patients with heterotaxy vs. 0 of 98 controls, P = 0.0001 by Fisher’s exact test). These findings suggest a significant association of DNAH11 mutations with the risk of CHD and heterotaxy syndrome (Table 6).
DNAH11 compound heterozygous mutations in CHD families with heterotaxy
Disease-causing DNAH11 mutations are inherited by autosomal recessive inheritance. Because there were no homozygous mutations in DNAH11 in this study, we focused on compound heterozygous mutations in the DNAH11 gene, which may be the main cause of the development of CHD/heterotaxy. The 14 disease-causing heterozygous mutations in DNAH11 were distributed among 7 CHD patients with heterotaxy, with 6 patients having two or more DNAH11 mutations. These DNAH11 mutations were further confirmed to be present in the available DNA of parents and other family members of the patients by Sanger sequencing. Interestingly, six different compound heterozygous variants in DNAH11 were validated respectively in six different families, including family 1-5031 (p.W802X/p.M282I), family 2-5045 (p.T3460K/p.G4425S), family 3-5065 (p.G447R/p.L1157R), family 4-5130 (p.I2262T/p.D3800H), family 5-5707 (p.S1823fs/p.F2759L/p.R4395X) and family 6-5062 (p.D3610V/p.I243V) (Table 7).
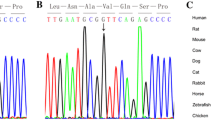
In family 1 (Fig. 1A), there were four members. The proband (#5031), who carried 2 heterozygous mutations (c.G2406A:p.W802X and c.G846C:p.M282I), was male and diagnosed with CHD and heterotaxy, including isolated right heart, complete atrioventricular canal (CAVC), double outlet right ventricle (DORV) and atrial septal defect (ASD), and showed abnormal ciliary function. His parents and younger brother were all without clinical manifestations, but their ciliary movements were abnormal as evidenced by uncoordinated ciliary waves. In this family, the heterozygous variant c.G2406A (p.W802X) was a novo stop-gain mutation located on exon 14 of DNAH11. This variant was present in the proband and his young brother and inherited from their mother. Functional analysis showed the variant p.W802X was predicted to be damaging by SIFT software and conserved among human, rhesus and dog species. The other heterozygous variant c.G846C (p.M282I) located in exon 4 in the proband was transmitted from his father and was not observed in his young brother and mother. This mutation was reported in the Exome Aggregation Consortium (ExAc) (0.00006547) and 1000 Genomes Project (0.000199681) databases. The p.M282I change was also predicted to be damaging by SIFT software and conserved among human, rhesus and mouse species (Fig. 1B).
Identification of mutations in DNAH11 in family 1. (A) The pedigree of family 1 (#5031). The proband from family 1 has two heterozygous mutations in DNAH11. The other members in this family are carriers. Sanger sequencing confirmation is shown below the pedigrees. A square represents male and a circle represents female. A black arrow indicates the proband. (B) Locations and conservation of mutations in DNAH11. The positions of mutations are indicated in the genomic structure of DNAH11. The amino acid changes were compared among eight mammalian species by conservation analysis. MT1: c.G846C (p.M282I); MT2: c.G2406A (p.W802X).
In family 2, the proband (#5045) was male and diagnosed with CHD and heterotaxy, showing abnormal ciliary movement. His parents were both heterozygous carriers and showed normal phenotypes. The heterozygous variants (c.C10379A:p.T3460K/c.G13273A:p.G4425S) of the DNAH11 gene were confirmed in family 2 by Sanger sequencing (Fig. 2A). Of the two heterozygous variants, c.C10379A (p.T3460K) was a missense variant type located in exon 64 and was inherited from the proband’s mother and presented in the ExAc (0.0001879) and 1000 Genomes Project (0.000199681) databases. The amino acid p.T3460K alteration is predicted to be damaging by SIFT, PolyPhen2 and MutationTaster and is highly conserved among mammalian species but not in chickens. The other variant, c.G13273A (p.G4425S), was a novel missense variant type located in exon 81 and which was transmitted from the proband’s carrier father. The p.G4425S change was predicted to be damaging by the SIFT, PolyPhen2 and MutationTaster software programs and is highly conserved among mammalian species (Fig. 2B).
Identification of mutations in DNAH11 in family 2. (A) The pedigree of family 2 (#5045). The proband has two heterozygous mutations in DNAH11. The other members in this family are carriers. Sanger sequencing confirmation is shown below the pedigrees.A square represents a male, and a circle represents a female. A black arrow indicates the proband. (B) Locations and conservation of mutations in DNAH11. The positions of the mutations are indicated in the genomic structure of DNAH11. The amino acid changes were compared among eight mammalian species by conservation analysis. MT3: c.C10379A (p.T3460K), MT4: c.G13273A (p.G4425S).
The family 3 proband (#5065) had severe CHD and heterotaxy, with abnormal ciliary movement. His parents were normal and healthy. This family carried two heterozygous missense mutations (c.G1339A:p.G447R/c.T3470G:p.L1157R) in the proband (Fig. 3A). The c.G1339A (p.G447R) in exon 7 was a novel variant and absent in his parents, indicating that this variant was de novo. The altered amino acid p.G447R was conserved among the human, rhesus, dog and elephant species. Functional analysis of this change was predicted to be benign by prediction software. The other c.T3470G (p.L1157R) in exon 18 was a missense mutation type and presented in the ExAc (0.000149) and 1000 Genomes Project (0.000199) databases. The mutation c.T3470G was inherited from his mother, resulting in the substitution of the 1157 amino acid Leu (L) with Arg (R) (p.L1157R) and was predicted to be a damaging change in the protein. The altered amino acid p.L1157R is highly conserved among many species. Although the p.G447R variant was predicted to be not damaging, considering the proband’s mother normal phenotype, we concluded that the combined effects of the two heterozygous variants (p.G447R/p.L1157R) may be an important factor causing CHD/heterotaxy disease (Fig. 3B).
Identification of mutations in DNAH11 in family 3. (A) The pedigree of family 3 (#5065). The proband carries two heterozygous mutations in DNAH11. The proband’s mother is a carrier, and his father shows no mutation in DNAH11. Sanger sequencing confirmation is shown below the pedigrees. A square represents male and a circle represents female. A black arrow indicates the proband. (B) Locations and conservation of mutations in DNAH11. The positions of mutations are indicated in the genomic structure of DNAH11. The amino acid changes were compared among eight mammalian species by conservation analysis. MT5: c.G1339A (p.G447R); MT6: c.T3470G (p.L1157R).
The family 4 proband (#5130), who was female and diagnosed with CHD, heterotaxy and CD, carried two heterozygous missense variants (c.T6785C:p.I2262T/c.G11398C:p.D3800H). We obtained the proband and her mother’s blood sample for validating the detected variants by Sanger sequencing. The blood sample of the proband’s father was not obtained. However, the parents both had normal phenotypes according to the medical history record (Fig. 4A). The c.T6785C (p.I2262T) variant was a novel heterozygous type and was located in exon 41 of DNAH11, which was inherited from the proband’s mother. Functional analysis indicated that the p.I2262T variant was predicted to be damaging by the SIFT, PolyPhen2 and Mutation Taster software programs and was highly conserved among many species. The variant c.G11398C (p.D3800H) in exon 70 was also a novel heterozygous variant and was absent from the ExAc and 1000 Genomes Project databases. Functional analysis predicted that the variant p.D3800H was damaging (by MutationTaster software) and was conserved among different species (Fig. 4B). We could not collect a blood sample from the proband’s father; therefore the inherited origin of the c.G11398C (p.D3800H) variant could not be determined, and we proposed that the compound heterozygous variants (p.I2262T/p.D3800H) may be associated with the development of the proband’s disease.
Identification of mutations in DNAH11 in family 4. (A) The pedigree of family 4 (#5130). The proband has two heterozygous mutations in DNAH11. The proband’s mother is a carrier, and the blood sample of his father was not obtained. Sanger sequencing confirmation is shown below the pedigrees. A square represents a male and a circle represents a female. A black arrow indicates the proband. (B) Locations and conservation of mutations in DNAH11. The positions of mutations are indicated in the genomic structure of DNAH11. The amino acid changes were compared among eight mammalian species by conservation analysis. MT7: c.T6785C (p.I2262T); MT8: c.G11398C (p.D3800H).
In family 5, patient #5707, diagnosed with CHD, heterotaxy and CD, had three variants in DNAH11, one frameshift variant (c.5470dupC:p.S1823fs), one missense variant (c.T8275C:p.F2759L) and one stop-gain variant (C13183T:p.R4395X). We did not obtain a blood sample from the patient’s parents, so we only confirmed these variants in the DNA sample of patient #5707 by Sanger sequencing. One frameshift variant (c.5470dupC:p.S1823fs) and two variants (c.T8275C:p.F2759L and C13183T:p.R4395X) were validated (Fig. 5A). The variant (c.5470dupC:p.S1823fs) was a novel frameshift insertion located in exon 32 and affected the protein function, which was conserved among different species. The c.T8275C variant was a missense variant located in exon 50 and was presented in the ExAc (0.0003) and 1000 Genomes Project (0.000599042) databases. The p.F2759L change was predicted to be damaging by the PolyPhen2 and MutationTaster software programs and was conserved among different species. The variant (C13183T:p.R4395X) in exon 81 was a novel stop-gain type that influenced protein function. The p.R4395X variant was highly conserved among different species (Fig. 5B).
Identification of mutations in DNAH11 in family 5. (A) The pedigree of family 5 (#5707). The proband carries one frameshift insertion and two heterozygous mutations in DNAH11. The blood samples of the proband’s parents were not obtained. Sanger sequencing confirmation is shown below the pedigrees.A square represents a male, and a circle represents a female. A black arrow indicates the proband. A red square indicates the “dupC”. (B) Locations and conservation of mutations in DNAH11. The positions of mutations are indicated in the genomic structure of DNAH11. The amino acid changes were compared among eight mammalian species by conservation analysis. MT9: c.5470dupC (p.S1823fs); MT10: c.T8275C (p.F2759L); MT11: c.C13183T (p.R4395X).
In family 6 (Fig. 6A), there were three members. The proband (#5062), who carried 2 heterozygous mutations (c.A10829T:p.D3610V and c.A727G:p.I243V), was female and diagnosed with CHD and heterotaxy, including isolated right heart, PA, levo-transposition of the great arteries (L-TGA) and ASD, and showed abnormal ciliary function. His parents both showed normal phenotypes. The c.A10829T (p.D3610V) variant was a novel heterozygous type located in exon 66 of DNAH11 and was inherited from the proband’s mother. Functional analysis showed the variant p.D3610V was predicted to be damaging by SIFT software and conserved among human, rhesus and dog species. The other heterozygous variant c.A727G (p.I243V) was a novel missense mutation type located in exon 4 of DNAH11 and was transmitted from the proband’s father. The p.I243V change was also predicted to be damaging by SIFT software and conserved among human, rhesus and mouse species (Fig. 6B).
Identification of mutations in DNAH11 in family 6. (A) The pedigree of family 6 (#5062). The proband carries two heterozygous mutations in DNAH11. Sanger sequencing confirmation is shown below the pedigrees. Locations and conservation of mutations in DNAH11. A black arrow indicates the proband. (B) Locations and conservation of mutations in DNAH11. The positions of mutations are indicated in the genomic structure of DNAH11. The amino acid changes were compared among eight mammalian species by conservation analysis. MT12: c.A727G (p.I243V); MT13: c.A10829T (p.D3610V). A square represents a male, and a circle represents a female.
Based on these confirmed variants and the medical history of the families, the findings suggest that these DNAH11 compound heterozygote variants are responsible for the development of CHD/heterotaxy syndrome.
Discussion
Human LR asymmetry plays an important role in normal organogenesis and provides the developmental basis for correct heart looping2. LR asymmetry disorders in early embryonic development may result in a series of congenital birth defects, such as heterotaxy syndrome14. Although many genes have been reported to be associated with LR asymmetry disorders15, the exact aetiological mechanism of CHD and heterotaxy remain unknown. Moreover, studies have found that cilia participate in the formation of the left and right asymmetric mode by regular swinging to form a nodal flow during the embryonic period16. PCD and CD have been shown to be associated with CHD and heterotaxy syndrome. PCD is considered a monogenic heterogeneous recessive disorder, and CHD and heterotaxy syndrome are multiple, complex inherited diseases. There may be a link between PCD and CHD/HTX.
In this study, we found 34 potential disease-causing heterozygous variants in 11 genes present in 19 CHD/HTX patients, accounting for 45.2% of 42 CHD/HTX patients. We compared the gene mutation rate in the CHD/HTX patients with CD and CHD/HTX patients without CD and found that CHD/HTX patients with CD had a significantly higher gene mutation rate than CHD/HTX patients without CD. The results suggest that PCD-related gene mutations are significantly associated with CHD with heterotaxy and CD.
DNAH11 is localized to the proximal region of respiratory cilia and is known as a PCD-related gene. Approximately 6% of PCDs are caused by DNAH11 mutations17. Although cilia are required for LR body-axis determination and second heart field (SHF) Hedgehog (Hh) signalling, Burnicka et al. observed that DNAH11 mutations did not disrupt SHF Hh signalling and caused AVSDs only concurrently with heterotaxy, a LR axis abnormality18.
DNAH11 showed the highest mutation rate in this study, followed by HYDIN and DNAH5. We concluded that DNAH11 mutations may be an important risk factor involved in the development of CHD/HTX syndrome. We reanalyzed the exome database in our previous study, including 98 CHD cases and 3 CHD/HTX cases, and found no mutation in the DNAH11 gene among 98 CHD cases and 1 DNAH11 mutation in 1 of 3 CHD patients with heterotaxy. We considered the 98 CHD cases as controls, fisher’s exact test revealed that DNAH11 mutations can significantly increase the risk of developing CHD/HTX syndrome, indicating a significant association of DNAH11 mutations with CHD and heterotaxy syndrome.
It is known that DNAH11 mutations are inherited by autosomal recessive inheritance pattern. Bartoloni L et al. found a homozygous nonsense mutation (R2852X) in DNAH11 in a patient with situs inversus totalis19. DNAH11 compound heterozygotes (p.R2250*/p.Q3604*) were observed in two monochorionic biamniotic male twins with PCD13. Nader Nakhleh et al. found DNAH11 compound heterozygotes (p.Q1507P/p.E3133K) in a patient with heterotaxy; these two mutations were predicted to be damaging and involved in hyperkinetic ciliary beats4,20.
DNAH11 homozygous mutations were not observed in our study. We focused on recessive DNAH11 mutations in the families and found six different compound heterozygous variants in six families and concluded that these compound heterozygous variants may be the main factors causing CHD/heterotaxy syndrome.
Recently, a study found that heterotaxy patients with heterozygous DNAH6 mutations also had heterozygous mutations in DNAH5 and DNAHI1 genes, which experimentally showed that the trans-heterozygous interactions of DNAH6 with DNAI1 or DNAH5 may contribute to heterotaxy syndrome21.
In our study, in addition to these DNAH11 compound heterozygotes, we also found that the patients with the DNAH11 heterozygous mutations possessed other heterozygous mutations in known PCD genes, such as HYDIN and DNAH5. Patients 5033, who possessed two different heterozygous variants in the DNAH11 and HYDIN genes (Table 2), presented primary CD and heterotaxy/CHD, indicating that interactions between trans-heterozygous variants of DNAH11 and HYDIN may be involved in heterotaxy/CHD and PCD.
Although over 40 PCD pathogenic genes were revealed, our study found compound heterozygous variants in only DNAH11 among the CHD patients with heterotaxy, which is subject to the limited number of patients recruited in this study. We speculated that larger studies may uncover comprehensive genetic pathogenic factors related to cilia among these patient populations. However, we can still conclude that pathogenic DNAH11 mutations are an important cause of heterotaxy with CHD. Additionally, we did not conduct deeper functional studies of these DNAH11 heterozygous variants for pathogenic confirmation and further interpretation of the genotypic and phenotypic mechanisms, which should be the key aspects of future works.
Conclusion
In summary, we performed targeted sequencing and Sanger sequencing to analyze the exonic regions of 37 candidate genes in 42 HTX patients with CHD from unrelated families and found 34 potential disease-causing heterozygous variants in 11 genes among the 19 CHD patients with heterotaxy syndrome. The association of DNAH11 variants with CHD and heterotaxy was significant (P = 0.0001). In families, six different compound heterozygous variants of DNAH11 were validated in family 1-5031 (p.W802X/p.M282I), family 2-5045 (p.T3460K/p.G4425S), family 3-5065 (p.G447R/p.L1157R), family 4-5130 (p.I2262T/p.D3800H), family 5-5707 (p.S1823fs/p.F2759L/p.R4395X) and family 6-5062 (p.D3610V/p.I243V).
These findings expand the spectrum of DNAH11 gene mutations causing the development of HTX/CHD and provide an important clue for understanding the genetic mechanism of HTX/CHD syndrome.
Methods
Patient cohorts
Blood samples from patients or their family members were collected in accordance with the Declaration of Helsinki, and the Ethics Committees of Children’s Hospital of Fudan University (CHFU) approved this study. Written informed consent was obtained from the parents and guardians of all the probands. Informed consent has been obtained for the blood samples taken from the available family members.
Forty-two participants with CHD and heterotaxy syndrome were recruited from the CHFU, Shanghai, China, including 28 males and 14 females, with ages ranging from 0.1 to 12.1 years. Family medical history was obtained at the cardiovascular centre of the CHFU, and medical records were reviewed to confirm the disease diagnosis. Blood samples from probands and their available family members were obtained for further genetic analysis.
Nasal tissue sampling and ciliary motion analysis
Nasal tissues were collected from patients with Rhino-Probe (Arlington Scientific, Springville, UT) curettage of the inferior nasal turbinate. Exclusion criteria included severe bleeding diathesis or conditions such as haemophilia or hereditary haemorrhagic telangiectasia syndrome. The nasal tissues were suspended in L-15 medium (Invitrogen, CA) for videomicroscopy using a Leica inverted microscope (DMIRE2) with a 67× oil objective under differential interference contrast optics. Movies were recorded at 200 frames/s at room temperature using a 680 PROSILICA GE camera (Allied Vision, PA), and digital recordings were evaluated by a blinded panel of coinvestigators. Abnormal ciliary motion was described as follows: immotile (I), discordance (D), wave (W), restricted (R) and no cilia (None).
Blood DNA extraction
A QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) was used to extract blood genomic DNA from probands and available family members according to the manufacturer’s instructions. The concentration and purity of genomic DNA were measured by absorbance at 260 and 280 nm by using a NanoDropTM 1000 Spectrophotometer (Thermo Scientific, Wilmington, USA). The extracted genomic DNA were stored at −80 °C until the samples were ready for further analysis.
Targeted sequencing analysis of gene variants
Targeted sequencing analysis was performed for the 42 probands from the CHFU. We focused on 37 candidate PCD-related genes, namely, ABCC4, ARMC4, C21orf59, CCDC39, CCDC40, CCDC65, CCDC114, CCDC151, CCNO, DNAAF1, DNAAF2, DNAFF3, DNAAF5, DNAH5, DNAH8, DNAH11, DNAI1, DNAI2, DNAL1, DRC1, DYX1C1, HEATR2, HYDIN, LRRC6, NAT10, NME8, PTGES, PTGES2, PTGES3, PTGER4, PTGS1, PTGS2, RSPH1, RSPH4A, RSPH9, SPAG1 and ZMYND10, intending to find novel or rare coding variants present in patients with CHD and heterotaxy syndrome.
Primers covering all exons and at least 10 bp of all intron/splice sites of these genes were designed online (https://www.ampliseq.com/). The libraries were constructed using the Ion AmpliSeq Library Kit v2.0 (Life Technologies, USA) according to the protocol. The concentration of each library was confirmed by a TaqMan Quantification Kit (Life Technologies). The Ion OneTouch 2 system with an Ion PGM Template OT2 200 Kit (Life Technologies, USA) was used to amplify pooled barcode libraries, and ion sphere particles (ISP) were enriched according to the E/S module protocol. The enriched template-positive ISPs were loaded and sequenced on an Ion 316™ Chip by PGM (Life Technologies, USA).
For each subject, base calls were detected with Torrent Suite software. Raw sequencing data were aligned against the human reference genome GRCh37/hg19 (NCBI) using NextGENe software. Single-nucleotide variations (SNVs) were aligned based on the following criteria: 1) the variant was detected on both strands of the sequence reads; 2) the minimum coverage of reads was no less than 10×; 3) the variant reads represented more than 20% of the sequence reads.
The variants filtered from NextGENe software were confirmed by Sanger sequencing and compared with 1000 Genomes (http://www.1000genomes.org) and ExAc databases (http://exac.broadinstitute.org/) as well as our laboratory’s internal databases. Additionally, the risk of SNVs was predicted using the silico tools SIFT (http://sift.jcvi.org/), PolyPhen2 (http://genetics.bwh.harvard.edu/pph2/) and MutationTaster (http://www.mutationtaster.org/). The amino acid changes were compared among eight mammalian species by conservation analysis (UCSC Genome Browser hg19).
Statistical analysis
Statistical analysis was performed using the Chi-square test with GraphPad Prism 6.0 Software. Fisher’s exact test was used to analyze the association of DNAH11 mutations with CHD and heterotaxy. Values were considered significant at P < 0.05.
Data Availability
The datasets generated during and/or analysed in this study are available from the corresponding author on reasonable request.
References
van der Linde, D. et al. Birth Prevalence of Congenital Heart Disease Worldwide A Systematic Review and Meta-Analysis. Journal of the American College of Cardiology 58, 2241–2247, https://doi.org/10.1016/j.jacc.2011.08.025 (2011).
Deng, H., Xia, H. & Deng, S. Genetic basis of human left-right asymmetry disorders. Expert Rev Mol Med 16, e19, https://doi.org/10.1017/erm.2014.22 (2015).
Lin, A. E., Ticho, B. S., Houde, K., Westgate, M. N. & Holmes, L. B. Heterotaxy: associated conditions and hospital-based prevalence in newborns. Genet Med 2, 157–172, https://doi.org/10.1097/00125817-200005000-00002 (2000).
Nakhleh, N. et al. High prevalence of respiratory ciliary dysfunction in congenital heart disease patients with heterotaxy. Circulation 125, 2232–2242, https://doi.org/10.1161/CIRCULATIONAHA.111.079780 (2012).
Harrison, M. J., Shapiro, A. J. & Kennedy, M. P. Congenital Heart Disease and Primary CiliaryDyskinesia. PaediatrRespirRev 18, 25–32, https://doi.org/10.1016/j.prrv.2015.09.003 (2016).
Klena, N. T., Gibbs, B. C. & Lo, C. W. Cilia and Ciliopathies in Congenital Heart Disease. Cold Spring Harb Perspect Biol 9, https://doi.org/cshperspect.a028266 (2017).
Harden, B. et al. Increased postoperative respiratory complications in heterotaxy congenital heart disease patients with respiratory ciliary dysfunction. J Thorac CardiovascSurg 147, 1291–1298 e1292, https://doi.org/10.1016/j.jtcvs.2013.06.018 (2014).
Lin, A. E. et al. Laterality defects in the national birth defects prevention study (1998–2007): birth prevalence and descriptive epidemiology. Am J Med Genet A 164A, 2581–2591, https://doi.org/10.1002/ajmg.a.36695 (2014).
Sutherland, M. J. & Ware, S. M. Disorders of left-right asymmetry: heterotaxy and situs inversus. Am J Med Genet C Semin Med Genet 151C, 307–317, https://doi.org/10.1002/ajmg.c.30228 (2009).
Li, Y. et al. Global genetic analysis in mice unveils central role for cilia in congenital heart disease. Nature 521, 520–524, https://doi.org/10.1038/nature14269 (2015).
Chapelin, C. et al. Isolation of several human axonemal dynein heavy chain genes: genomic structure of the catalytic site, phylogenetic analysis and chromosomal assignment. Febs Letters 412, 325–330, https://doi.org/10.1016/S0014-5793(97)00800-4 (1997).
Dougherty, G. W. et al. DNAH11 Localization in the Proximal Region of Respiratory Cilia Defines Distinct Outer Dynein Arm Complexes. Am J Respir Cell Mol Biol 55, 213–224, https://doi.org/10.1165/rcmb.2015-0353OC (2016).
Lai, M. et al. Gene editing of DNAH11 restores normal cilia motility in primary ciliary dyskinesia. J Med Genet 53, 242–249, https://doi.org/10.1136/jmedgenet-2015-103539 (2016).
Mortari, E. P. et al. Heterotaxy syndrome with and without spleen: Different infection risk and management. Journal of Allergy and Clinical Immunology 139, 1981–1984.e1, https://doi.org/10.1016/j.jaci.2016.10.014 (2017).
Brueckner, M. Heterotaxia, congenital heart disease, and primary ciliary dyskinesia. Circulation 115, 2793–2795, https://doi.org/10.1161/CIRCULATIONAHA.107.699256 (2007).
Wang, G. L., Yost, H. J. & Amack, J. D. Analysis of Gene Function and Visualization of Cilia-Generated Fluid Flow in Kupffer’s Vesicle. Jove-Journal of Visualized Experiments, https://doi.org/10.3791/50038 (2013).
Pifferi, M. et al. New DNAH11 mutations in primary ciliary dyskinesia with normal axonemal ultrastructure. European Respiratory Journal 35, 1413–1416, https://doi.org/10.1165/rcmb.2015-0353OC (2010).
Burnicka-Turek, O. et al. Cilia gene mutations cause atrioventricular septal defects by multiple mechanisms. Human Molecular Genetics 25, 3011–3028, https://doi.org/10.1093/hmg/ddw155 (2016).
Bartoloni, L. et al. Mutations in the DNAH11 (axonemal heavy chain dynein type 11) gene cause one form of situs inversus totalis and most likely primary ciliary dyskinesia. Proceedings of the National Academy of Sciences of the United States of America 99, 10282–10286, https://doi.org/10.1073/pnas.152337699 (2002).
Nakhleh, N. et al. High Prevalence of Respiratory Ciliary Dysfunction in Congenital Heart Disease Patients With Heterotaxy. Circulation 125, 2232–U2164, https://doi.org/10.1161/CIRCULATIONAHA.111.079780 (2012).
Li, Y. et al. DNAH6 and Its Interactions with PCD Genes in Heterotaxy and Primary CiliaryDyskinesia. PloSGenet 12, e1005821, https://doi.org/10.1371/journal.pgen.1005821 (2016).
Acknowledgements
This work was supported by grants from the National Key Research and Development Program of China (2016YFC1000500) and the Natural Science Foundation of China (81570282, 81370198 and 81873482) and Science and Research Foundation of Shanghai Municipal Commission of Health and Family Planning for Young Scientists (20144Y0057).
Author information
Authors and Affiliations
Contributions
Conception and study design: Guoying Huang and Wei Sheng. Acquisition of patient information and communication with the patients’ families: Sida Liu, Weicheng Chen and Shuolin Li. NGS design, Sanger sequencing and data analysis: Sida Liu, Yongkun Zhan, Shuolin Li, Duan Ma and Xiaojing Ma. Drafting: Sida Liu. Manuscript editing and revision: Guoying Huang, Wei Sheng and Sida Liu.
Corresponding authors
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Liu, S., Chen, W., Zhan, Y. et al. DNAH11 variants and its association with congenital heart disease and heterotaxy syndrome. Sci Rep 9, 6683 (2019). https://doi.org/10.1038/s41598-019-43109-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-43109-6
- Springer Nature Limited