Abstract
Euphyllia paradivisa is a strictly mesophotic coral in the reefs of Eilat that displays a striking color polymorphism, attributed to fluorescent proteins (FPs). FPs, which are used as visual markers in biomedical research, have been suggested to serve as photoprotectors or as facilitators of photosynthesis in corals due to their ability to transform light. Solar radiation that penetrates the sea includes, among others, both vital photosynthetic active radiation (PAR) and ultra-violet radiation (UVR). Both types, at high intensities, are known to have negative effects on corals, ranging from cellular damage to changes in community structure. In the present study, fluorescence morphs of E. paradivisa were used to investigate UVR response in a mesophotic organism and to examine the phenomenon of fluorescence polymorphism. E. paradivisa, although able to survive in high-light environments, displayed several physiological and behavioral responses that indicated severe light and UVR stress. We suggest that high PAR and UVR are potential drivers behind the absence of this coral from shallow reefs. Moreover, we found no significant differences between the different fluorescence morphs’ responses and no evidence of either photoprotection or photosynthesis enhancement. We therefore suggest that FPs in mesophotic corals might have a different biological role than that previously hypothesized for shallow corals.
Similar content being viewed by others
Introduction
The solar radiation that reaches the earth’s surface includes, among others, ultra-violet radiation (UVR; 280–400 nm) and photosynthetically active radiation (PAR; 400–700 nm). As light travels through the atmosphere and penetrates the sea, it is scattered and reflected. At the end of this process, UVA, which ranges between 315 and 400 nm, is the portion of the UVR spectrum that demonstrates maximum penetration in seawater1. Owing to its short, high-energy wavelength, UVR is known to have mostly negative effects on living organisms. It is already established that UVR is one of the major stressors to coral reefs and, in combination with other stressors (i.e. elevated temperatures, bacterial infections, and pollution), it is responsible for coral bleaching and the degradation of coral reefs around the globe2. The effects of UVR or high intensities of PAR on corals and their symbionts are diverse: changes in community composition3, reduction in primary production4,5, breakdown of metabolic pathways, especially those of photosynthesis6, reduced growth7,8, and organismal mortality9. The indirect UVR-induced damage at the cellular or molecular level is caused by the formation of reactive oxygen radicals (ROS) that attack cellular compartments10. The direct UVR-induced DNA damage is caused by absorption of the high-energy wavelength by the DNA molecules that, in turn, can alter the structure of the DNA helix, leading to cell cycle arrest and cell death11,12. Two of the most abundant direct UVR-induced DNA lesions are those of cyclobutane pyrimidine dimers (e.g. CPDs) and 6–4 pyrimidine pyrimidone photoproduct (e.g. 6-4PPs)13. Such damage is highly significant, since it can occur in all organisms, from simple to complex. Being sessile organisms, corals have evolved the ability to accumulate UV-absorbing compounds, known as mycosporine-like amino acids (MAAs), which protect them from light-induced damages14,15. MAAs are known to efficiently absorb light in the UVR range16 and also to display moderate antioxidant activity17.
Euphyllia paradivisa is a large-polyp species usually found in shallow (<20 m), turbid lagoons18,19. In the Gulf of Eilat/Aqaba (GoE/A), however, its area of distribution is strictly mesophotic. Despite being found only at depths greater than 36 m in Eilat, this species has demonstrated both a low mortality rate and an ability also to prosper in shallow depths and shallow light environments for prolonged periods of time following transplantation20. When the photoacclimatization potential of this species to a shallow light environment was tested21, the corals lost their original mesophotic ability to efficiently utilize low-light intensities, but were still able to withstand higher light intensities before suffering from photoinhibition (Ben-Zvi et al.; in preparation). The high survivorship and successful photoacclimatization of E. paradivisa to high-light conditions tend to support the idea of mesophotic coral ecosystems (MCEs) serving as refugia or as a source of replenishment for the degraded shallow-coral ecosystems, a concept that was first presented by Glynn22 and postulated later as the “Deep Reef Refugia Hypothesis” (DRRH) by Bongaerts et al.23.
E. paradivisa also displays a dramatic color polymorphism as a result of its intense fluorescence. The fluorescence can be observed with the naked eye due to the mesophotic light environment, which naturally excites the fluorescent proteins (FPs). Corals can express one or multiple fluorescent and non-fluorescent chromoproteins24,25,26,27, and therefore alter their color morph during the course of different life stages28, under changing stressors or environmental conditions29,30,31, or along a depth gradient32,33. The plasticity of coral color phenotypes, which is partially a consequence of different types of FPs and the rapid regulation of the FPs24, may play a potential role in the biology of corals under changing environments. Two of the widely accepted and studied hypotheses regarding FP function in corals are: (1) a role in the enhancement of photosynthesis where light is scarce34,35,36; and (2) photoprotection under high-light conditions by means of altering the light inside the coral tissues37,38. Early studies on the photosynthesis enhancement hypothesis posited that FPs are able to absorb less suitable wavelengths for photosynthesis (<400 nm) and convert them to wavelengths that are more efficiently utilized by the zooxanthellae (>400 nm)39,40, or that they may provide light to shaded zooxanthellae by reflecting and scattering PAR41. A more recent study has presented evidence that a photoconvertible red FP (pcRFP) may provide longer wavelengths to the zooxanthellae present deeper in the coral host tissue42. Regarding their role in photoprotection, FPs have been shown to be upregulated by light25, specifically by blue light24, and to accumulate around the reproductive organs43 or light-exposed parts of the colony44. Furthermore, higher expression of red fluorescent protein (RFP) in Acropora millepora showed a correlation with reduced photo-damage in the algal symbionts38. We therefore sought to investigate here the possibility of UVR being the potential cause of E. paradivisa’s absence from the shallower reefs of Eilat, by monitoring the response of different fluorescence morphs under UVR stress. Additionally, we sought to test the two major hypotheses regarding FPs, in a mesophotic coral that displays fluorescence polymorphism.
Results
Fluorescence morphs
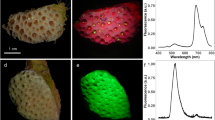
E. paradivisa constituted up to 73% of the total coral cover at our sampling site (Dekel beach), Eilat20. Our field survey revealed that the most dominant fluorescence morph is the green morph. Out of 463 E. paradivisa polyps examined in the field survey, the green morph comprised 51.6% of the total number of polyps, the red morph 41.4%, and the yellow morph 5.4% (all other morphs together comprised 1.6% of the surveyed population). The spectral analysis of the excitation and emission peaks of the collected corals provided a better definition of the different morphs (Fig. 1). Emission peaks (for excitation at 450 nm) had been previously recorded for the species at 515 and 545 nm26. Here we report the green morph as presenting two fluorescence emission peaks, at 480 and 505 nm; the yellow morph also presented two peaks, at 505 and 545 nm; and the red morph presented one emission peak, at 505 nm. The red appearance of the latter morph originates from the fluorescence of chlorophyll (at 680 nm) and not from a RFP. The excitation peaks for the 480 nm, 505 nm, and 545 nm emitting FPs were 405 nm, 450 nm, and 520 nm, respectively.
Response to UVR
Both in nature and in aquaria, E. paradivisa has been observed to expand its tentacles during the day (Fig. 2a). Within a 24-hr period in the controlled light experiment, corals that were exposed only to PAR remained expanded during the light hours (Fig. 2b), while corals that were exposed to PAR + UV contracted fully into their skeleton (Fig. 2c). The contracted corals decreased in size by 73.1% ± 8.35 (mean ± SD, n = 6) compared to those exposed to PAR only, which even expanded slightly (7.94% ± 8.19, n = 6).
Corals from all three morphs under both PAR and PAR + UV treatments presented a decrease in host protein concentration versus the measurement taken prior to the experiment, as indicated in Fig. 3a. There was no significant difference between treatments or morphs (LMM, F = 0.23, p = 0.632 and F = 1.22, p = 0.315, respectively; see material and methods for details). When examining the absorbance at 320 nm as a proxy for MAA concentration, we found that excluding the green morph under PAR + UV and a few outliers, the majority of the corals under both light treatments displayed a decrease in MAA concentration compared with measurements taken prior to the treatments. At the end of the experiment, corals under PAR + UV presented significantly higher mean ± SD absorbance at 320 nm (Fig. 3b; LMM, F = 4.164, p = 0.02) compared to corals under PAR only. When comparing the different morphs, the green and red morphs under PAR + UV treatment, presented higher MAA concentrations than under the PAR treatment while the yellow morph exhibited the opposite response (LMM, F = 9.641, p = 0.001).
Changes in the physiology of Euphyllia paradivisa under two light treatments. The relative change (%) in (a) host protein and (b) MAA concentration of E. paradivisa under two light treatments: full sunlight (“PAR + UV”; light gray boxes) and PAR only (“PAR”; dark gray boxes), in three fluorescence morphs. Boxes represent the upper and lower quartile, center lines represent medians, and whiskers extend to data measurements that are less than 1.5*IQR away from first/third quartile. Outliers are represented by dots. Red lines represent a reference to the measurements taken prior to the light treatments.
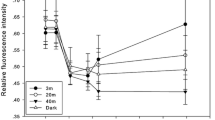
The mean (±SD) effective photosynthetic yield (\({\rm{\Delta }}F/{{\rm{F}}^{\prime} }_{{\rm{m}}}\)) for E. paradivisa as measured in the field survey under ambient light at 45–50 m was 0.579 ± 0.073. We found no significant difference (one-way ANOVA, p = 0.094, n = 12 for each morph) between the photosynthetic yields of the different morphs in their natural habitat (Fig. 4a). In our controlled experiment, the maximal photosynthetic yields (Fv/Fm) of the corals dropped in both PAR and PAR + UV compared to the control measurement, from 0.696 ± 0.032 to 0.263 ± 0.09. Fv/Fm values of polyps under the PAR + UV treatment were found to be ~1.5 fold higher than the PAR-treated polyps in all fluorescence color morphs (LMM, F = 18.306, p = 0.001), with a mean ± SD of 0.318 ± 0.069 under PAR + UV and 0.209 ± 0.076 under the PAR treatment (Fig. 4b). Zooxanthellae density presented with an opposite response in which the corals that were exposed to UVR had fewer zooxanthellae cells than corals who were deprived of UVR (Fig. 4c; LMM, F = 5.1, p = 0.027). Chlorophyll a concentration decreased under both light treatments but differed between treatments and morphs and showed no consistent response (Fig. 4d). In the latter two parameters (i.e. zooxanthellae density and chlorophyll a concentration) we observed a difference between morphs (LMM, F = 3.108, p = 0.071 and F = 2.899, p = 0.088, respectively). We noticed that the green morph showed the greatest decrease in zooxanthellae density (−53.14% ± 30.53) but also the smallest decrease in chlorophyll a concentration (−20.6% ± 26.15) and the yellow morph showed the smallest decrease in zooxanthellae density (−43.7 ± 99.93) and the highest decrease in chlorophyll a concentration (−61.41% ± 27.63).
Photobiology of Euphyllia paradivisa. (a) Effective photosynthetic yield (\({\rm{\Delta }}F/{{\rm{F}}^{\prime} }_{{\rm{m}}}\)) of three fluorescence morphs of E. paradivisa in their natural habitat (45 m depth). Change (%) in the (b) maximal photosynthetic yield (Fv/Fm), (c) zooxanthellae density, and (d) chlorophyll a concentration under two light treatments: full sunlight (“PAR + UV”; light gray boxes) and PAR only (“PAR”; dark gray boxes), in three fluorescence morphs. Boxes represent the upper and lower quartile, center lines represent medians, and whiskers extend to data measurements that are less than 1.5*IQR away from first/third quartile. Outliers are represented by dots. Red lines represent a reference to the measurements taken prior to the light treatments.
DNA damage
Corals exposed to PAR + UV revealed a significantly higher accumulation of 6-4PP sites (Fig. 5a; LMM, F = 6.57, p = 0.016), and more, but not significantly, CPD sites than their UVR-deprived counterparts, specifically in the red and yellow morphs (Fig. 5b; LMM, F = 0.017, p = 0.898). There was no significant effect of the morph within each treatment on the amount of 6-4PP or CDP sites (LMM, F = 1.102, p = 0.346 and F = 0.086, p = 0.917, respectively).
Quantification of UVR-induced DNA damage in Euphyllia paradivisa. (a) 6–4 photoproducts (6-4PPs) and (b) Cyclobutane pyrimidine dimers (CPDs) under two light treatments: full sunlight (“PAR + UV”; light gray boxes) and PAR only (“PAR”; dark gray boxes), in three fluorescence morphs. Boxes represent the upper and lower quartile, center lines represent medians, and whiskers extend to data measurements that are less than 1.5*IQR away from first/third quartile. Outliers are represented by dots.
Discussion
In recent years there has been increasing interest in the DRRH23 and the potential of MCEs to serve as refugia for the detrimentally affected shallow corals or as a source for replenishment in case of shallow reef loss45,46. In addition, several recent studies have also focused on the uniqueness of the MCEs, rather than considering them as an extension of the shallow reefs47,48. In Eilat, E. paradivisa is a strictly mesophotic species and does not occur in the shallow reef, unlike other reefs in the world18,19. The absence of this species from the shallow reefs of Eilat might be explained by two possible scenarios: (1) its adaptation to the mesophotic, light-limited environment; and (2) local forces that drive it to the deeper part of the reef. Predation pressure is one suggested and tested explanation for the occurrence of this coral exclusively in the mesophotic reef of Eilat20. Here, we suggest that high-light intensities and UVR are other factors that exclude E. paradivisa from the shallow habitats in Eilat. In the present study, most of the measured physiological parameters (protein concentrations, zooxanthellae densities, and chlorophyll a concentration) dropped when the corals were exposed to higher light intensities than those found in their natural habitat in the GoE/A (both PAR and UVR intensities; Figs 3a and 4c,d). This implies that the mesophotic corals and their algal symbionts were suffering from acute light stress, presenting similar responses to those previously described for corals10. This result was not surprising in view of the known negative effects of high-light and UVR on corals. The photosynthetic apparatus is especially sensitive to reactive oxygen species (ROS) production following UVR exposure. Common recorded reactions to the production of ROS are damage to the photosynthetic membranes49, induction of photoinhibition5,7, and a decrease in photosynthetic efficiency50. Such typical outcomes following UVR exposure had been demonstrated in several shallow coral species that were either a-priori acclimated or not to high-light conditions50. High-light intensities or UVR exposure, usually along with elevated temperature, will also increase the tendency of corals to bleach and lose their zooxanthellae51. In this study, E. paradivisa, despite losing symbionts and chlorophyll a, displayed a higher maximal photosynthetic yield under the PAR + UV treatment compared to those that had been exposed to PAR only (Fig. 4b). However, corals from both treatments revealed a significantly lower photosynthetic yield compared to the values at the beginning of the experiment, and to that measured in their natural habitat (Fig. 4a,b). One potential explanation for the less impaired photosynthetic efficiency under UVR is that of the coral’s distinct behavioral reaction observed during the experiment (Fig. 2). E. paradivisa is a fleshy tissue coral, somewhat sea anemone-like, and able to contract almost completely into the skeleton. Corals under UVR exposure almost fully contracted throughout the duration of the experiment (Fig. 2c). This behavior was also observed by Siebeck52 in several massive shallow-coral species but has not been previously described in mesophotic corals. We suggest that this reaction might be triggered by a UVR receptor that has either been found or suggested in other organisms53,54. Such a receptor might have enabled the protection of the remaining zooxanthellae from the intense radiation, and consequently mitigated their impairment. Although this might be helpful as a short-term protection mechanism against UVR damage, it nonetheless did not prevent the loss of zooxanthellae and chlorophyll and the occurrence of DNA damage, nor did it prevent the corals from containing more MAAs under UVR exposure. Hence, we assume that the corals would have not survived for a prolonged light stress experiment while contracted.
Another studied mechanism of UVR damage avoidance is that of MAAs accumulation. Corals and other marine organisms have been shown to accumulate UV-absorbing MAAs when exposed to UVR14,55, thereby improving their ability to mediate excess light. Although presenting a decrease in MAA concentration (Fig. 3b) compared to the measurements taken prior to the experiment (excluding the response of the green morph), the corals indeed presented higher MAA levels when exposed to UVR, despite the short-term exposure of eight days and the fact that these corals are exposed to only minor levels of UVR at the mesophotic depths20. The differences in MAA concentration found among the fluorescent morphs might also be accompanied by a change in the composition of MAAs among these morphs, as demonstrated in Porites astreoides8. Corals have a rapid and efficient DNA repair mechanism compared to other organisms56, but since they were sampled during midday, we infer that the results represent the maximal damage of an average day. Both CPDs and 6-4PPs result in the deformation of the DNA helix, which causes a downstream effect. Our results demonstrate that the corals that were exposed to UVR suffered from approximately two-fold more 6-4PP sites and that there was no difference in the DNA damage between the morphs (Fig. 5). While known to be able both to photoacclimatize to shallow habitats20 and to be found in those habitats (5–20 m)18,19, here we demonstrated that E. paradivisa suffered from light stress, especially in the presence of UVR. The previous successful transplantation of this coral to shallow environments was performed on adult corals that were kept covered by a mesh or by lighting filters, assumedly reducing the light received by them20. Furthermore, the UVR or high-light intensities found in the shallow reefs of Eilat may not be fatal for adult corals but might be fatal for E. paradivisa recruits or juveniles. Therefore, UVR or high-light should be considered as a factor that drives E. paradivisa and other species to the deeper mesophotic reefs of Eilat.
Previous studies have shown that different non-fluorescent color morphs are correlated to differences in stress response, such as different compositions of MAAs8, changes in photodamage38, and differences in photosynthetic yield57. Considering the FP ability to manipulate light, we therefore sought to explore the physiological response of different fluorescent morphs in corals. In most of our measurements, we found no difference between the different fluorescence morphs under either light treatment. Moreover, we assume that E. paradivisa is not subjected to any high-light or UVR stress in its native habitat, and none of the excitation peaks found here is in the range of UVR. Consequently, the reason for the intense fluorescence and fluorescence polymorphism displayed by E. paradivisa may not be related to photoprotection, unlike that suggested for shallow corals37,38. The relationship between FPs and photosynthesis at the mesophotic depth has also been previously investigated, but measurements were not performed under ambient mesophotic light environment32. This study, in contrast, presents in-situ measurements of photosynthetic yield under the unique and well-defined light environment of the mesophotic reef in Eilat20. As no differences were found in the photosynthetic performances among the morphs in the field survey or in the controlled experiment, there is also no evidence of photosynthesis enhancement by the coral FPs, as also suggested for other shallow and mesophotic corals32,58. The lack of evidence supporting this role in mesophotic environments was previously demonstrated in Leptoseris spp. by Roth et al.32. In the case of E. paradivisa, the role of fluorescence remains unknown and other hypotheses, such as prey attraction59, inflammation-like response60,61, and antioxidant activity62, should be further tested.
Materials and Methods
Field surveys and measurements
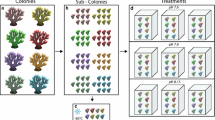
Belt transects (50 m × 0.5 m) were surveyed using closed-circuit rebreather dives at the collection site at Dekel Beach, GoE/A at 45 m (29°32′20.02″N 34°56′44.80″E), where E. paradivisa is most abundant20. Transects were photographed and analyzed as follows: each polyp within each transect was categorized as one of five morphs (green, yellow, red, red-green, and orange). The abundance (%) of each morph was calculated by dividing the total number of polyps of each morph by the total number of polyps (463) in the transect. Effective photosynthetic yield (\({\rm{\Delta }}F/{{\rm{F}}^{\prime} }_{{\rm{m}}}\)) of 12 colonies from three different fluorescence morphs (n = 12 for each morph; green, red, and yellow) was measured at the survey site using a Diving-PAM (Walz, Germany). Measured colonies were at least 1.5 m away from each other, therefor the colonies were genetically distinct, with high certainty, from one another. Corals were measured during the day under ambient light, and “measuring light intensity” and “gain” values were adjusted to reach optimal signals followed by an auto-zero calibration in site.
Coral sampling and experimental design
Fourteen colonies (ca. six polyps in each colony) from three fluorescent morphs of the coral E. paradivisa (i.e. “green” n = 8, “yellow” n = 3, and “red” n = 3 colonies) were sampled under permit 015/41127 of the Israel Nature and Parks Authority, from Dekel Beach and transferred to running seawater aquaria under a filter that mimics light conditions of ca. 40 m (“Lagoon blue”, Lee Filters) at the Interuniversity Institute in Eilat (IUI). Each colony was fragmented into individual polyps, resulting in 49 green polyps, 13 yellow polyps, and 17 red polyps. Tentacles from each polyp were sampled prior to exposure to the light treatments, weighted, and preserved at −80 °C for physiology analyses, following a 5 sec blotting on an absorbing tissue, and flash-freezing in liquid nitrogen. The polyps were then assigned, with equal representation from each colony, to one of two light treatments: (1) full ambient sunlight (i.e. PAR + UV treatment), receiving 1,624 ± 69 µmol m−2 s−1 of PAR and 117 ± 16 µmol m−2 s−1 of UVA and UVB (mean ± SD at 1200 hours during the experiment); or (2) full ambient sunlight but covered with a UV absorption filter (Ultra-Violet Absorption, Lee Filters) that cuts the light spectrum below 400 nm (see Supplementary Fig. SI1) therefore receiving only the PAR portion of the spectrum (i.e. PAR treatment). Each treatment contained three independent tanks with individual water inlet. Mean (±SD) seawater temperature during the experiment was 21.6 ± 0.22 °C as measured by a data logger (HOBO, Onset computer corporation). Corals were exposed to the different light treatments for eight consecutive days and tentacles were sampled again at midday (1200 hours) on Day 8 and were preserved for DNA damage analysis in RNA Save stabilization solution (Biological industries, Israel) and for physiology.
Behavioral experiment
Following Day 8 of the experiment, and in order to quantify an observed contraction of the tentacles, a second experiment was set up with six new polyps (two from each fluorescence morph). The polyps were subjected first to PAR treatment for 24 hr, followed by 24 hr under the PAR + UV. After 48 hr of recovery under the Lagoon blue filter, the polyps were first exposed to PAR + UV for 24 hr followed by 24 hr under PAR only. The polyps were photographed at the beginning and end of each exposure, the top-projection area of tentacles was measured using Photoshop software, and contraction/extension percentage was calculated by dividing the area at the end of the exposure by the area at the beginning of the exposure.
Spectral analysis of fluorescence color morphs
Fluorescence excitation and emission peaks of the three fluorescence color morphs used for the experiment were determined from the collected colonies using the Varian Cary Eclipse Fluorescence Spectrometer (Agilent, USA) from host protein extracts.
Coral physiology
Coral tissue was thawed on ice and homogenized mechanically. Host tissue and zooxanthellae were separated by a second homogenization and centrifuging the tissue at low speed (5,000 rpm for 5 min). The host supernatant was centrifuged again at high speed (14,000 rpm for 5 min) to cleanse the supernatant of any additional zooxanthellae prior to the host protein concentration analysis. The symbiont pellet was used for zooxanthellae density calculations and chlorophyll concentration analysis. Since E. paradivisa is a fleshy coral with high tissue volume, and only coral tentacles were sampled, the common methods for determining coral surface area (e.g., the paraffin or aluminum foil methods) are irrelevant in this case. Protein and MAA concentrations were therefore normalized to tissue net wet weight of the sampled tentacles following removal of the excess water in the sample. Chlorophyll a concentrations were normalized to zooxanthellae cells.
Host protein concentration
Host protein concentrations were measured in triplicate using the Coommassie blue assay (Thermo Fisher Scientific, USA) according to the manufacturer’s protocol. The standard microplate protocol (100–1500 µg/ml) was used based on previous experience with E. paradivisa, and absorbance was read with a Multiskan microplate spectrophotometer (Thermo Fisher Scientific, USA).
Mycosporine-like Amino Acid (MAA) concentration
Absorbance at 320 nm of the host protein extractions was measured as approximation for MAA relative concentration, using a Multiskan microplate spectrophotometer (Thermo Fisher Scientific, USA). Since the maximum absorbance of MAAs in E. paradivisa had not been examined yet, absorbance at 320 nm was used as described by Shibata63.
Photobiology
Zooxanthellae cells were counted in triplicate under a light microscope using a hemocytometer in a 1:10 dilution. Chlorophyll was extracted using 90% acetone for 15 hr incubation at 4 °C and concentrations were determined using a Multiskan microplate spectrophotometer (Thermo Fisher Scientific, USA) and the equations previously described by Jeffery and Humphrey64. Maximal photosynthetic yield (Fv/Fm) was measured using a Diving-PAM (Walz, Germany) for each polyp. Polyps were measured approximately 90 min after sunset to ensure a proper dark acclimation state prior to the measurement, both prior to their exposure to the light treatments and at the end of the experiment. The “measuring light intensity” and “gain” values were adjusted to reach optimal signals followed by an auto-zero calibration.
UV-induced DNA damage
Total DNA was extracted using DNeasy Blood & Tissue kit (Qiagen, Germany) according to the manufacturer’s protocol. DNA concentrations were assessed spectrometrically and pools of DNA were created from polyps of the same colony under the same treatment (three polyps in each pool). UV-induced DNA damage was quantified using OxiSelect™ UV-Induced DNA Damage ELISA Kits (Cell Biolabs, Inc., USA) according to the manufacturer’s protocol. This method of DNA damage quantification is based on specific primary antibodies that target either 6-4PPs or CPD sites, and secondary antibodies that create a color reaction that can be quantified spectrometrically.
Statistical analyses
All statistical analyses were conducted with the statistical computing language R, version 3.5.165. In-situ effective photosynthetic yield data were tested with one-way ANOVA, examining the effect of morph on the ∆F/Fm′. In all other analyses (except DNA damage, where we did not have a measurement prior to the light treatments), the change between the measurements taken both prior to and after exposure to the different light treatments was calculated and considered as the dependent variable. In order to account for the effect of both treatment and morph, while also considering the possible effect of the polyp genotype (colony) and the multiple tanks in each of the treatments, data were tested with linear mixed effects model (LMM) using lme4 package version 1.166 and lmerTest67. Treatment (PAR vs. PAR + UV) and morph (green, red, and yellow) were treated as crossed fixed effects while colony (nested within morph) and tank (nested within treatment) were considered as random effects. LMM analyses were followed by ANOVA tests between each full model and a model lacking either treatment or morph. Differences were considered significant for a p-value < 0.05. Normality and homogeneity of variance of the models’ residuals were inspected visually.
Data Availability
The datasets generated during and/or analyzed during the current study are available in the GitHub repository, https://github.com/orbzvi/Response-of-fluorescence-morphs-of-the-mesophotic-coral-Euphyllia-paradivisa-to-ultra-violet-radiati.
References
Kirk, J. T. O. Light and photosynthesis in aquatic ecosystems. Third edn, (Cambridge university press, 1994).
Ban, S. S., Graham, N. A. J. & Connolly, S. R. Evidence for multiple stressor interactions and effects on coral reefs. Global Change Biology 20, 681–697, https://doi.org/10.1111/gcb.12453 (2014).
Shick, J. M., Lesser, M. P. & Jokiel, P. L. Effects of ultraviolet radiation on corals and other coral reef organisms. Global Change Biology 2, 527–545 (1996).
Kinzie, R. A. Effects of ambient levels of solar ultraviolet radiation on zooxanthellae and photosynthesis of the reef coral Montipora verrucosa. Marine Biology 116, 319–327, https://doi.org/10.1007/bf00350022 (1993).
Lesser, M. P. Elevated temperatures and ultraviolet radiation cause oxidative stress and inhibit photosynthesis in symbiotic dinoflagellates. Limnology and Oceanography 41, 271–283 (1996).
Lesser, M. P. & Shick, J. M. Effects of irradiance and ultraviolet radiation on photoadaptation in the zooxanthellae of Aiptasia pallida: primary production, photoinhibition, and enzymic defenses against oxygen toxicity. Marine Biology 102, 243–255, https://doi.org/10.1007/bf00428286 (1989).
Jokiel, P. L. & York, J. R. H. Solar ultraviolet photobiology of the reef coral Pocillopora Damicornis and symbiotic zooxanthellae. Bulletin of Marine Science 32, 301–315 (1982).
Gleason, D. F. Differential effects of ultraviolet radiation on green and brown morphs of the Caribbean coral Porites astreoides. Limnology and Oceanography 38, 1452–1463 (1993).
Gleason, D. & Wellington, G. M. Variation in UVB sensitivity of planula larvae of the coral Agaricia agaricites along a depth gradient. Marine Biology 123, 693–703 (1995).
Lesser, M. P. & Farrell, J. H. Exposure to solar radiation increases damage to both host tissues and algal symbionts of corals during thermal stress. Coral Reefs 23, 367–377, https://doi.org/10.1007/s00338-004-0392-z (2004).
Roos, W. P. & Kaina, B. DNA damage-induced cell death by apoptosis. Trends in Molecular Medicine 12, 440–450, https://doi.org/10.1016/j.molmed.2006.07.007 (2006).
Buma, A. G., Van Hannen, E. J., Veldhuis, M. J. & Gieskes, W. W. UV-B induces DNA damage and DNA synthesis delay in the marine diatom Cyclotella sp. Scientia Marina 60, 101–106 (1996).
Mitchell, D. L. & Karentz, D. The induction and repair of DNA photodamage in the environment. in Environmental UV Photobiology (eds Antony R. Young, Johan Moan, Lars Olof Björn, & Wilhelm Nultsch) 345–377 (Springer US, 1993).
Shick, J. M. & Dunlap, W. C. Mycosporine-like amino acids and related gadusols: biosynthesis, accumulation, and UV-protective functions in aquatic organisms. Annual review of Physiology 64, 223–262 (2002).
Al-Utaibi, A. A., Niaz, G. R. & Al-Lihaibi, S. S. Mycosporine-like amino acids in six scleractinian coral species. Oceanologia 51, 93–104 (2009).
Dunlap, W. C. & Shick, J. M. Ultraviolet radiation-absorbing mycosporine-like amino acids in coral reef organisms: a biochemical and environmental perspective. Journal of Phycology 34, 418–430 (1998).
Yakovleva, I., Bhagooli, R., Takemura, A. & Hidaka, M. Differential susceptibility to oxidative stress of two scleractinian corals: antioxidant functioning of mycosporine-glycine. Comparative Biochemistry and Physiology Part B: Biochemistry and Molecular Biology 139, 721–730, https://doi.org/10.1016/j.cbpc.2004.08.016 (2004).
Veron, J. E. N. In Corals of the World Vol. 2 (ed Stafford-Smith. M.) 77 (Australian Institue of marine science, 2000).
Turak, E., Sheppard, C. & Wood, E. The IUCN red list of threatened species. at, http://www.iucnredlist,org/. (2018)
Eyal, G. et al. Euphyllia paradivisa, a successful mesophotic coral in the northern Gulf of Eilat/Aqaba, Red Sea. Coral Reefs 35, 91–102, https://doi.org/10.1007/s00338-015-1372-1 (2016).
Eyal, G. et al. Photoacclimation and induction of light-enhanced calcification in the mesophotic coral Euphyllia paradivisa. Royal Society Open. Science 6, 180527, https://doi.org/10.1098/rsos.180527 (2019).
Glynn, P. W. Coral reef bleaching: facts, hypotheses and implications. Global Change Biology 2, 495–509 (1996).
Bongaerts, P., Ridgway, T., Sampayo, E. M. & Hoegh-Guldberg, O. Assessing the ‘deep reef refugia’ hypothesis: focus on Caribbean reefs. Coral Reefs 29, 309–327 (2010).
D’Angelo, C. et al. Blue light regulation of host pigment in reef-building corals. Marine Ecology Progress Series 364, 97–106 (2008).
Ben-Zvi, O., Eyal, G. & Loya, Y. Light-dependent fluorescence in the coral Galaxea fascicularis. Hydrobiologia 759, 15–26, https://doi.org/10.1007/s10750-014-2063-6 (2014).
Eyal, G. et al. Spectral diversity and regulation of coral fluorescence in a mesophotic reef habitat in the Red Sea. Plos One 10, e0128697, https://doi.org/10.1371/journal.pone.0128697 (2015).
Klueter, A., Loh, W., Hoegh-Guldberg, O. & Dove, S. Physiological and genetic properties of two fluorescent colour morphs of the coral Montipora digitata. Symbiosis (Rehovot) 42, 123–134 (2006).
Roth, M. S., Fan, T. Y. & Deheyn, D. D. Life history changes in coral fluorescence and the effects of light intensity on larval physiology and settlement in Seriatopora hystrix. Plos One 8, e59476, https://doi.org/10.1371/journal.pone.0059476 (2013).
Rodriguez-Lanetty, M., Harii, S. & Hoegh-Guldberg, O. Early molecular responses of coral larvae to hyperthermal stress. Molecular Ecology 18, 5101–5114, https://doi.org/10.1111/j.1365-294X.2009.04419.x (2009).
Roth, M. S. & Deheyn, D. D. Effects of cold stress and heat stress on coral fluorescence in reef-building corals. Scientific reports 3 (2013).
Grinblat, M., Fine, M., Tikochinski, Y. & Loya, Y. Stylophora pistillata in the Red Sea demonstrate higher GFP fluorescence under ocean acidification conditions. Coral Reefs 37, 309–320 (2018).
Roth, M. S. et al. Fluorescent proteins in dominant mesophotic reef-building corals. Marine Ecology Progress Series 521, 63–79 (2015).
Vermeij, M. J. A., Delvoye, L., Nieuwland, G. & Bak, R. P. M. Patterns in fluorescence over a Caribbean reef slope: the coral genus Madracis. Photosynthetica 40, 423–429, https://doi.org/10.1023/a:1022635327172 (2002).
Dove, S. G. et al. Host pigments: potential facilitators of photosynthesis in coral symbioses. Plant, cell & environment 31, 1523–1533 (2008).
Schlichter, D., Meier, U. & Fricke, H. W. Improvement of photosynthesis in zooxanthellate corals by autofluorescent chromatophores. Oecologia 99, 124–131 (1994).
Wiedenmann, J. & Nienhaus, G. U. Photoactivation in green to red converting EosFP and other fluorescent proteins from the GFP family. In Proc. SPIE 6098, Genetically Engineered Probes for Biomedical Applications at, https://doi.org/10.1117/12.657565 (2006).
Salih, A., Larkum, A., Cox, G., Kühl, M. & Hoegh-Guldberg, O. Fluorescent pigments in corals are photoprotective. Nature 408, 850–853 (2000).
Gittins, J. R., D’Angelo, C., Oswald, F., Edwards, R. J. & Wiedenmann, J. Fluorescent protein-mediated colour polymorphism in reef corals: multicopy genes extend the adaptation/acclimatization potential to variable light environments. Molecular Ecology 24, 453–465, https://doi.org/10.1111/mec.13041 (2015).
Kawaguti, S. Effect of the green fluorescent pigment on the productivity of the reef corals. Micronesica 5, 121 (1969).
Schlichter, D., Fricke, H. W. & Weber, W. Light harvesting by wavelength transformation in a symbiotic coral of the Red Sea twilight zone. Marine Biology 91, 403–407, https://doi.org/10.1007/bf00428634 (1986).
Schlichter, D., Fricke, H. W. & Weber, W. Evidence for PAR-enhancement by reflection, scattering and fluorescence in the symbiotic deep-water coral Leptoseris fragilis. Endocytobiosis and Cell Research 5, 83–94 (1988).
Smith, E. G., D’Angelo, C., Sharon, Y., Tchernov, D. & Wiedenmann, J. Acclimatization of symbiotic corals to mesophotic light environments through wavelength transformation by fluorescent protein pigments. Proceedings of the Royal Society B: Biological Sciences 284, https://doi.org/10.1098/rspb.2017.0320 (2017).
Shikina, S. et al. Oocytes express an endogenous red fluorescent protein in a stony coral, Euphyllia ancora: a potential involvement in coral oogenesis. Scientific Reports 6, 25868, https://doi.org/10.1038/srep25868 (2016).
Kahng, S. E. & Salih, A. Localization of fluorescent pigments in a nonbioluminescent, azooxanthellate octocoral suggests a photoprotective function. Coral Reefs 24, 435–435, https://doi.org/10.1007/s00338-005-0002-8 (2005).
Bongaerts, P. & Smith, B. T. Beyond the ‘deep reef refuge’ hypothesis: a conceptual framework to characterize persistence at depth. in Mesophotic Coral Ecosystems (eds Y. Loya, K. A. Puglise, & T. C. L. Bridge) (Springer, 2019).
Shlesinger, T., Grinblat, M., Rapuano, H., Amit, T. & Loya, Y. Can mesophotic reefs replenish shallow reefs? Reduced coral reproductive performance casts a doubt. Ecology (2017).
Rocha, L. A. et al. Mesophotic coral ecosystems are threatened and ecologically distinct from shallow water reefs. Science 361, 281–284, https://doi.org/10.1126/science.aaq1614 (2018).
Semmler, R. F., Hoot, W. C. & Reaka, M. L. Are mesophotic coral ecosystems distinct communities and can they serve as refugia for shallow reefs? Coral Reefs 36, 433–444, https://doi.org/10.1007/s00338-016-1530-0 (2017).
Lesser, M. P. Oxidative stress causes coral bleaching during exposure to elevated temperatures. Coral Reefs 16, 187–192, https://doi.org/10.1007/s003380050073 (1997).
Shick, J. M. et al. Depth-dependent responses to solar ultraviolet radiation and oxidative stress in the zooxanthellate coral Acropora microphthalma. Marine Biology 122, 41–51, https://doi.org/10.1007/bf00349276 (1995).
Lesser, M. P., Stochaj, W. R., Tapley, D. W. & Shick, J. M. Bleaching in coral reef anthozoans: effects of irradiance, ultraviolet radiation, and temperature on the activities of protective enzymes against active oxygen. Coral Reefs 8, 225–232, https://doi.org/10.1007/bf00265015 (1990).
Siebeck, O. Photoreactivation and depth-dependent UV tolerance in reef coral in the Great Barrier Reef/Australia. Naturwissenschaften 68, 426–428, https://doi.org/10.1007/bf01079713 (1981).
Portwich, A. & Garcia‐Pichel, F. A novel prokaryotic UVB photoreceptor in the cyanobacterium Chlorogloeopsis PCC 6912. Photochemistry and Photobiology 71, 493–498, https://doi.org/10.1562/0031-8655(2000)0710493anpupi2.0.co2 (2000).
Kräbs, G., Watanabe, M. & Wlencke, C. A monochromatic action spectrum for the photoinduction of the UV‐absorbing mycosporine‐like amino acid Shinorine in the red alga Chondrus crispus. Photochemistry and Photobiology 79, 515–520 (2004).
Torregiani, J. H. & Lesser, M. P. The effects of short-term exposures to ultraviolet radiation in the Hawaiian Coral Montipora verrucosa. Journal of Experimental Marine Biology and Ecology 340, 194–203, https://doi.org/10.1016/j.jembe.2006.09.004 (2007).
Svanfeldt, K., Lundqvist, L., Rabinowitz, C., Sköld, H. N. & Rinkevich, B. Repair of UV-induced DNA damage in shallow water colonial marine species. Journal of Experimental Marine Biology and Ecology 452, 40–46, https://doi.org/10.1016/j.jembe.2013.12.003 (2014).
Quick, C., D’Angelo, C. & Wiedenmann, J. Trade-offs associated with photoprotective green fluorescent protein expression as potential drivers of balancing selection for color Polymorphism in reef corals. Frontiers in Marine Science 5, https://doi.org/10.3389/fmars.2018.00011 (2018).
Mazel, C. H. et al. Green-fluorescent proteins in Caribbean corals. Limnology and Oceanography 48, 402–411 (2003).
Haddock, S. H. D. & Dunn, C. W. Fluorescent proteins function as a prey attractant: experimental evidence from the hydromedusa Olindias formosus and other marine organisms. Biology Open, bio. 012138, https://doi.org/10.1242/bio.012138 (2015).
Palmer, C. V., Mydlarz, L. D. & Willis, B. L. Evidence of an inflammatory-like response in non-normally pigmented tissues of two scleractinian corals. Proceedings of the Royal Society B Biological Sciences. 275, 2687–2693, https://doi.org/10.1098/rspb.2008.0335 (2008).
D’Angelo, C. et al. Locally accelerated growth is part of the innate immune response and repair mechanisms in reef-building corals as detected by green fluorescent protein (GFP)-like pigments. Coral Reefs 31, 1045–1056 (2012).
Bou-Abdallah, F., Chasteen, N. D. & Lesser, M. P. Quenching of superoxide radicals by green fluorescent protein. Biochimica et Biophysica Acta (BBA)-General Subjects 1760, 1690–1695 (2006).
Shibata, K. Pigments and a UV-absorbing substance in corals and a blue-green alga living in the Great Barrier Reef. Plant and Cell Physiology 10, 325–335 (1969).
Jeffrey, S. W. T. & Humphrey, G. F. New spectrophotometric equations for determining chlorophylls a1, b1, c1 and c2 in higher plants, algae and natural phytoplankton. Biochem. Physiol. Pflanz. 167, 191–194 (1975).
R: A language and environment for statistical computing. (R Foundation for Statistical Computing, Vienna, 2013).
Bates, D., Maechler, M., Bolker, B. & Walker, S. Fitting linear mixed-effects models using lme4. Journal of Statistical Software 67(1), 1–48, https://doi.org/10.18637/jss.v067.i01 (2015).
Kuznetsova, A., Brockhoff, P. B. & Christensen, R. H. B. lmerTest package: Tests in linear mixed effects models. Journal of Statistical Software 82(13), 1–26, https://doi.org/10.18637/jss.v082.i13 (2017).
Acknowledgements
We would like to thank the Interuniversity Institute for Marine Sciences for making their facilities available to us. We thank N. Paz for proofreading, R. Tamir for diving assistance, and all of YL’s lab members for their support. We thank Dr. M. Kiflawi, T. Shlesinger, and R. Pickholtz for their statistical advise. We also wish to thank the editors and anonymous referees for their helpful comments. This research was funded by the Israel Science Foundation (ISF) grant agreement No. 1191/16 to YL, by the Ministry of Science, Technology & Space doctoral fellowship grant agreement No. 3–18487 to OBZ, and by the European Union’s Horizon 2020 research and innovation program under the Marie Skłodowska-Curie post-doctoral grant agreement No. 796025 to GE.
Author information
Authors and Affiliations
Contributions
O.B.Z., G.E. and Y.L. conceived the idea of the research, designed and carried out the field surveys and controlled experiments. O.B.Z. analyzed the data, generated the figures, and performed the different forms of data presentation. O.B.Z. wrote the first draft text and all authors reviewed and edited the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Ben-Zvi, O., Eyal, G. & Loya, Y. Response of fluorescence morphs of the mesophotic coral Euphyllia paradivisa to ultra-violet radiation. Sci Rep 9, 5245 (2019). https://doi.org/10.1038/s41598-019-41710-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-41710-3
- Springer Nature Limited
This article is cited by
-
Exploring marine algae-derived phycocyanin nanoparticles as a safe and effective adjuvant for sunscreen systems
Discover Applied Sciences (2024)
-
Coral fluorescence: a prey-lure in deep habitats
Communications Biology (2022)
-
Limited acclimation of early life stages of the coral Seriatopora hystrix from mesophotic depth to shallow reefs
Scientific Reports (2022)
-
Experimental evidence of temperature-induced bleaching in two fluorescence morphs of a Red Sea mesophotic coral
Coral Reefs (2021)