Abstract
While the importance of programmed death-ligand 1 (PD-L1), mutation burden caused by microsatellite instability (MSI), and CD8+ tumor infiltrating lymphocytes (TILs) has become evident, the significance of PD-L1 expression on prognosis still remains controversial. We evaluated the usefulness of combined markers of PD-L1 and MSI or CD8+ TILs as a prognostic biomarker in gastric cancer. A total of 283 patients with gastric cancer were reviewed retrospectively. PD-L1 expression on >5% tumor cells was defined as PD-L1-positive. PD-L1-positive rate was 15.5% (44/283). PD-L1 positivity was significantly correlated with invasive and advanced cancer and also significantly correlated with MSI, whereas no significance was observed with CD8+ TILs. Kaplan–Meier analysis showed that PD-L1 positivity significantly correlated with a poor prognosis (p = 0.0025). Multivariate analysis revealed that PD-L1 positivity was an independent poor prognostic factor (hazard ratio [HR]: 1.97, p = 0.0106) along with diffuse histological type and lymph node metastases. Combinations of PD-L1 and MSI (HR: 2.18) or CD8+ TILs (HR: 2.57) were stronger predictive factors for prognosis than PD-L1 alone. In conclusion, combined markers of PD-L1 and MSI or CD8+ TILs may be more useful prognostic biomarkers in gastric cancer, and better clarify the immune status of gastric cancer patients.
Similar content being viewed by others
Introduction
Immunotherapy has become popular in the field of cancer therapy worldwide, especially since cancer immunotherapy was named “Breakthrough of the Year” by Science magazine in 20131, mainly due to success in immune-checkpoint blockade therapy. One of the most common mechanisms underlying immunotherapy is programmed death-1 (PD-1) and programmed death-ligand 1 (PD-L1). The PD-1/PD-L1 interaction, which works as an inhibitory factor in the last step of the cancer immunity cycle, induces functional impairment of antigen-specific T cells, leading to immune evasion by tumors2,3. PD-1/PD-L1 blockade therapy with antagonistic antibodies has achieved great success in clinical trials for patients with various types of cancer, and has been approved for use in clinical practice for patients with several types of cancer including gastric cancer4,5,6,7,8,9,10.
The importance of PD-L1 expression on solid tumors as a prognostic biomarker has been reported in multiple studies, many of which have noted the association between PD-L1 expression in tumor tissues and a worse prognosis, although this association varied according to tumor type11. In gastric cancer, which is the third leading cause of cancer-related death and fifth most common malignancy worldwide12, PD-L1 overexpression in tumor tissues tends to be associated with a worse prognosis13,14,15, although some reports have noted that overexpression correlates with a better prognosis16,17. Thus, the association between PD-L1 expression and prognosis in gastric cancer still remains a subject of debate.
The Cancer Genome Atlas (TCGA) Research Network recently proposed a novel classification in which gastric cancer is divided into four subtypes based on the underlying molecular biology; this is expected to provide a new roadmap for patient stratification and treatment strategy in gastric cancer18. In this classification, status of microsatellite instability (MSI) and Epstein-Barr virus (EBV), both of which are well recognized as factors associated with the tumor immune environment, was each focused as one of key factors to divide into four subtypes. MSI refers to the hypermutatable state of cells caused by impaired DNA mismatch repair, and MSI-high cancer has an increased number of mutation-associated neoantigens, leading to stimulation of anti-tumor immunity19. EBV-positive gastric cancer, which reportedly accounts for about 8.8% of all gastric carcinoma, has also received attention as a tumor population with elevated PD-L1 expression20.
In this study, we evaluated PD-L1 expression and status of MSI, EBV, and tumor-infiltrating lymphocytes (TILs) on gastric cancer tissues which were surgically removed, and assessed the correlation of PD-L1 expression with the status of these factors and the prognosis of patients. We further investigated the usefulness of PD-L1 and MSI or TIL as a combined marker to better predict prognosis than a single marker of PD-L1. While the importance of each factor as a prognostic or predictive biomarker for anti-PD-1 therapy has been extensively reported, to the best of our knowledge, there have been few reports on these combined markers. The results of this study may help with the evaluation of tumor status from the aspect of tumor immunity in gastric cancer, which could lead to the development of more reasonable and effective treatment strategies including immune checkpoint inhibitors for gastric cancer.
Results
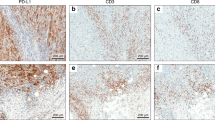
The correlation of PD-L1 expression with clinicopathological characteristics, including status of MSI, EBV, and TILs expressing CD8, CD4, Foxp3, and PD-1, was determined. Based on our evaluation criteria of PD-L1 expression, the PD-L1-positive (score 2+ and 3+) rate was 15.5% (44/283) while the PD-L1-negative rate (score 0 and 1+) was 84.5% (239/283). MSI was observed in 7.8% (22/283) and EBV was detected in 8.1% (23/283). Table 1 shows the correlation of PD-L1 expression with clinicopathological findings. PD-L1 positivity was observed in more male patients (p = 0.0187). In histological findings, PD-L1 positivity was observed more in intestinal type than in diffuse type (p = 0.0201), and in invasive and advanced cases based on status of ly (p = 0.0014), v (p = 0.0154), T (p = 0.0063), and stage (p = 0.0258). PD-L1 positivity tended to be observed more in tumors located in the upper third of the stomach (p = 0.0660). PD-L1 positivity was significantly correlated with MSI (p = 0.0006) whereas no correlation was observed with EBV (p = 0.3926). With respect to TILs, while CD8 and CD4 expression was not correlated with PD-L1 expression (Fig. 1a,b), Foxp3 positivity was more observed in PD-L1-negative tumors (p = 0.0261) (Fig. 1c) and PD-1 positivity was more observed in PD-L1-positive tumors (p < 0.0001) (Fig. 1d).
Correlation of PD-L1 expression with TIL surface markers. Median value of TILs with expression of CD8 (a), CD4 (b), Foxp3 (c), and PD-1 (d) was calculated from counting on three different fields of immunohistochemical staining, and classified into two groups (positive and negative) based on each cutoff value. The cutoff value was set at 20 for CD8, 1 for CD4, 1 for Foxp3, and 1 for PD-1. IQR, interquartile range
Impact of PD-L1 expression on overall survival
In this study, 104 of 283 patients died, including 65 patients who died of gastric cancer and 39 patients who died of other diseases. PD-L1 positivity was correlated with a significantly poor prognosis compared to PD-L1 negativity (p = 0.0025) (Fig. 2a), whereas MSI and EBV did not have much influence on overall survival (OS) (Fig. 2b,c). On analysis of 106 patients who had lymph node metastasis, the PD-L1-positive rate was 15% (16/106) on primary tumors and 13% (14/106) on metastatic lymph nodes (Supplementary Table S1). On these 106 patients, PD-L1 expression on metastatic lymph nodes did not make much difference on OS, whereas PD-L1-positive expression on primary tumors caused a significantly poor prognosis (Supplementary Fig. S1), which suggested that PD-L1 expression on primary tumors likely influenced OS more strongly than PD-L1 expression on metastatic sites. When OS was analyzed based on the combination of PD-L1 expression on primary tumors and metastatic lymph nodes, PD-L1 negativity seemed to correlate with a better prognosis (p = 0.0459) than the other populations (Supplementary Fig. S2). Among the four surface markers of TILs examined in this study, only CD8 had a significant effect on OS. CD8 low correlated with a poorer prognosis (p = 0.0059) (Fig. 2d), whereas CD4, Foxp3, and PD-1 did not have a significant difference on OS (Fig. 2e–g). Univariate and multivariate analyses of factors related to OS showed that PD-L1 positivity was an independent poor prognostic factor (hazard ratio [HR]: 1.97, 95% confidence interval [CI]: 1.18–3.20, p = 0.0106) along with diffuse histological type and the presence of lymph node metastasis (Table 2).
Combined marker of PD-L1 expression with MSI or CD8+ TILs
When Kaplan–Meier survival analysis was performed on four groups divided based on each status of PD-L1 expression and MSI, patients with PD-L1 positivity and non-MSI had a significantly poor prognosis (p = 0.0050), whereas the other three groups had a similar prognosis (Fig. 3a). The same results were observed with the combination of PD-L1 and CD8+ TILs, as patients with PD-L1 positivity and CD8 low had a significantly poor prognosis compared to the other populations (p = 0.0002) (Fig. 3b). To compare the impact of these combined markers to a single marker of PD-L1 on prognosis, the same univariate and multivariate analyses performed in Table 2 were performed, in which PD-L1 was replaced with either PD-L1/MSI or PD-L1/CD8. This multivariate analysis revealed that PD-L1-positive/non-MSI and PD-L1-positive/CD8 low were both independent poor prognostic factors, and interestingly, the HRs of PD-L1-positive/non-MSI (2.18) and PD-L1-positive/CD8 low (2.57) were higher than that of PD-L1-positive alone (1.97) (Table 3), which suggested that the combined markers of PD-L1 with MSI or CD8 were more important and useful as a prognostic biomarker than a single marker of PD-L1.
Kaplan–Meier survival curve of gastric cancer patients based on the combined markers of PD-L1 and MSI (a), and PD-L1 and CD8 (b). In the right graph, three groups other than “PD-L1 positive/non-MSI” in (a) or “PD-L1 positive/CD8 low” in (b) were combined as “others”. Censored cases are shown as tick marks in each graph.
Discussion
It has become widely recognized that the interaction of PD-1 and PD-L1 plays an important role in immune evasion by tumors, and PD-L1 expression on tumor tissues may be a good biomarker to predict the efficiency of anti-PD-1/PD-L1 antibodies21. The importance of PD-L1 expression on tumor tissues as a prognostic factor has also been reported in many studies11. However, the issue remains that the evaluation criteria of PD-L1 expression is not standardized, for example, it remains unknown which antibody to use. Staining pattern and intensity vary according to the difference of antibody and the method of staining22. We employed a PD-L1 antibody of E1L3N in this study because it was considered the most appropriate staining to use to evaluate PD-L1 expression based on the preliminary comparison of three different antibodies to human PD-L1 (E1L3N, 28–8 and SP142) prior to initiation of this study. It is also unknown which area of tumor tissues should be evaluated, and which type of cells (i.e., tumor cells, immune cells, stromal cells). In this study, we simply focused on PD-L1 expression on tumor cells, but the importance of PD-L1 expression on stromal cells or immune cells such as dendritic cells and macrophages was recently reported23. The appropriate cutoff value of PD-L1 expression is also unknown. Percentages of 1%, 5%, 10%, and even 50% were reportedly used as a cutoff value of PD-L1 expression24. Although the most appropriate cutoff value is not determined yet, a higher cutoff value seems to better predict the prognosis of patients and the efficacy of anti-PD-1/PD-L1 antibodies. Actually in a clinical trial, PD-L1 expression in at least 50% of tumor cells in non-small cell lung cancer tissue was correlated with better efficacy of pembrolizumab than one in less than 50%25. In this study, we classified PD-L1 expression into four groups, and PD-L1 positivity was an independent poor prognostic factor when 5% was selected as a cutoff value. However, when 1% was set as a cutoff value, no significant difference was observed between PD-L1 positive and negative.
The histologic and biologic backgrounds of gastric cancer differ between East and West26. In Asia, gastric cancer is characterized by a diffuse-type histology, tumors primarily located in the distal stomach, and a correlation with Helicobacter pylori status, in contrast to the disease in Western countries. The TCGA project of gastric cancer highlighted MSI and EBV as key molecules to divide gastric cancers into four subtypes18, which has significantly impacted actual clinical practice and basic research in Asia, even though approximately 75% of patients in the TCGA project were from Western countries. EBV was found in 8.8% of 295 gastric cancers and was associated with amplification of PD-L1 in the TCGA report. In our case, EBV was not correlated with PD-L1 expression, while EBV positivity (8.1%) was similar with the TCGA report. MSI seems to have a stronger connection with PD-L1 expression and PD-1/PD-L1 blockade therapy as evidenced by the fact that pembrolizumab was approved for any types of solid tumor with MSI27. While the MSI-high population in this study (7.8%) was a little smaller than that in the TCGA study (21.7%) and other previous studies (11.68–33.82%)28, MSI was significantly correlated with PD-L1 positivity, and more importantly, the combination of PD-L1 and MSI status was a stronger prognostic marker than a single marker of PD-L1. Another main characteristic in cancer immunity was CD8+ TILs, and the clinical significance of TILs has been reported in many studies of gastric cancer29. In our study, tumors with low CD8+ TILs showed a significantly worse prognosis, and combination of PD-L1 and CD8 was also found to be a useful prognostic marker.
With respect to the correlation of PD-L1 with other TIL markers, PD-1 expression on TILs was significantly correlated with PD-L1 expression on tumor cells in this study. This is expected because PD-L1 is a ligand of PD-1, and this interaction leads to immune evasion of tumors. In contrast, Foxp3, another TIL marker, was negatively correlated with PD-L1 in this study. Although high Foxp3 is theoretically associated with poor prognosis based on the fact that Foxp3+ TILs work to prevent the immune system from attacking tumor cells, some studies have actually reported high Foxp3 as a better prognostic factor, which suggests that the correlations of Foxp3 expression on TILs with PD-L1 expression on tumor cells are still controversial in terms of prognosis14,30.
Patil et al. recently reported a correlation between the expression of PD-L1, TILs, and mismatch repair (MMR) proteins and clinicopathologic findings in gastric adenocarcinomas in which PD-L1 was expressed in a large proportion (70%) of patients. They found that PD-L1 expression and MMR deficiency were associated with increases in TIL numbers and larger tumor size31. Although their study included more advanced cases than our study, Patil et al. found higher PD-L1 positivity (70%) compared with our study (15.5%). This could have been caused by differences in evaluation criteria, including the cutoff value of >1% in their study versus >5% in our study, and the type of PD-L1 antibody used. Patil et al. reported no significant difference between PD-L1 positivity and survival, which could have been related to the large population of PD-L1-positive tumors, whereas we found no significant difference when the PD-L1 cutoff value was set at 1%.
While this study provides some important information with potential impact in clinical practice, it has several limitations. First, it was a retrospective, single-center study. Second, many patients with early stage gastric cancer, who are unlikely to die of gastric cancer, were included in the OS analysis. When patients with only advanced (Stage II or higher) gastric cancer were analyzed, the influence of PD-L1 expression on prognosis was actually estimated smaller. Third, four mononucleotide repeat microsatellite targets of BAT26, NR27, NR21, and CAT25 were used for MSI analysis in this study, according to previously described protocols32,33. However, MLH1, MSH2, MSH6, and PMS2 are the most commonly used target markers in MSI analyses.
In conclusion, we demonstrated that PD-L1 overexpression on tumor cells was an independent prognostic factor in gastric cancer, and moreover, combination of PD-L1 with MSI or CD8 was a stronger prognostic marker than a single marker of PD-L1. Evaluation of MSI status and CD8+ TILs in addition to PD-L1 expression on tumor cells is considered important from the perspective of assessing the condition of the tumor microenvironment based on the immunity cycle, which is expected to lead to the development of more appropriate treatment strategies for gastric cancer.
Materials and Methods
All experiments were conducted in accordance with the relevant guidelines and regulations of Okayama University.
Patients
The medical records of 283 patients with gastric cancer who underwent gastrectomy at Okayama University Hospital (Okayama Prefecture, Japan) between 2002 and 2009 were reviewed retrospectively. Patient characteristics included age and sex. Histological type was classified into intestinal type and diffuse type, and status of lymphatic invasion (ly), venous invasion (v), tumor location, depth of tumor invasion (T), lymph node metastasis (N), and stage were described according to the 3rd English edition of the Japanese classification of gastric carcinoma34. This study was reviewed and approved by the institutional review board of Okayama University (No. 1505–023). Informed consent was obtained by the opt-out method.
Immunohistochemistry
Formalin-fixed, paraffin-embedded (FFPE) tissue samples cut at 4 μm were deparaffinized in xylene and rehydrated in a graded ethanol series. After blocking endogenous peroxidases by incubation with 3% H2O2 for 10 min, the samples were boiled in citrate buffer (pH 6.0) for 14 min in a microwave oven for antigen retrieval. The samples were incubated with primary antibodies overnight at 4 °C and then with peroxidase-linked secondary antibody for 30 min at room temperature. After 3, 3-diaminobenzidine (DAB) staining for signal generation and counterstaining with Mayer’s hematoxylin, the samples were dehydrated and mounted onto coverslips. Antibodies to human PD-L1 (E1L3N; Cell Signaling Technology, Danvers, MA, USA), CD8, CD4, Foxp3, and PD-1 were used.
Classification of PD-L1
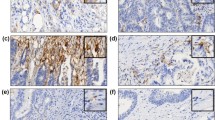
PD-L1 expression was classified into four groups according to PD-L1-positive rate on tumor cells, as determined by immunohistochemical staining (0: <1%, 1+: 1–5%, 2+: 5–10%, 3+: ≥10%) (Fig. 4a), and 2+ and 3+ were defined as PD-L1-positive. The evaluation was blindly performed by a pathologist.
Representative pictures and data of each PD-L1 expression level (0, 1+, 2+ and 3+) (a), immunohistochemical staining for CD8, CD4, Foxp3, and PD-1 (b), four mononucleotide repeat microsatellite targets (BAT26, NR27, NR21 and CAT25) (c) and electrophoresis for EBV (d). The original gel was presented in Supplementary Fig. S3.
Evaluation of TILs
The average number of TILs with expression of CD8, CD4, Foxp3, or PD-1 (Fig. 4b) was calculated from three different randomly selected fields and classified into two groups (positive and negative) based on a cutoff value of 20 for CD8, 1 for CD4, 1 for Foxp3, and 1 for PD-1.
Analysis of MSI
Genomic DNA was extracted from FFPE gastric cancer tissues using TaKaRa DEXPAT (Takara Bio Inc., Shiga, Japan) following separation of tumor and normal tissue by manual microdissection. MSI was evaluated with polymerase chain reaction (PCR) using primers for four mononucleotide repeat microsatellite targets (BAT26, NR27, NR21, and CAT25) according to a previously described protocol (Fig. 4c)32,33. Tumors showing allelic shifts in ≥two of four markers were defined as MSI-high (hereafter referred to as MSI) and the rest were defined as microsatellite stable (MSS, hereafter referred to as non-MSI).
Detection of EBV
Genomic DNA collected from FFPE tissues was subjected to PCR using EBV primers35. The reaction mixture was fractionated electrophoretically on a 3% agarose gel and the DNA bands were visualized under UV light (Fig. 4d).
Statistical analysis
Statistical analysis was conducted using JMP software (SAS Institute, Cary, NC, USA) by a different researcher from the ones who performed the staining and evaluation. Student’s t-test was used to assess the continuous variable of age, and the Wilcoxon signed-rank test was used for continuous variables of the number of lymphocytes expressing CD8, CD4, Foxp3, and PD-1. Pearson’s chi-square test was used for categorical variables of sex, histological type, ly, v, tumor location, T status, N status, stage, MSI, and EBV. The log-rank test was used for Kaplan–Meier survival analysis. Univariate and multivariate Cox proportional hazards regression analyses were performed to assess the effects of the prognostic factors.
References
Couzin-Frankel, J. Breakthrough of the year 2013. Cancer immunotherapy. Science 342, 1432–1433 (2013).
Pardoll, D. M. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12, 252–264 (2012).
Chen, D. S. & Mellman, I. Oncology meets immunology: the cancer-immunity cycle. Immunity 39, 1–10 (2013).
Topalian, S. L. et al. Safety, activity, and immune correlates of anti-PD-1 antibody in cancer. N Engl J Med 366, 2443–2454 (2012).
Borghaei, H. et al. Nivolumab versus Docetaxel in Advanced Nonsquamous Non-Small-Cell Lung Cancer. N Engl J Med 373, 1627–1639 (2015).
Robert, C. et al. Nivolumab in previously untreated melanoma without BRAF mutation. N Engl J Med 372, 320–330 (2015).
Ansell, S. M. et al. PD-1 blockade with nivolumab in relapsed or refractory Hodgkin’s lymphoma. N Engl J Med 372, 311–319 (2015).
Ferris, R. L. et al. Nivolumab for Recurrent Squamous-Cell Carcinoma of the Head and Neck. N Engl J Med 375, 1856–1867 (2016).
Reck, M. et al. Pembrolizumab versus Chemotherapy for PD-L1-Positive Non-Small-Cell Lung Cancer. N Engl J Med 375, 1823–1833 (2016).
Kang, Y. K. et al. Nivolumab in patients with advanced gastric or gastro-oesophageal junction cancer refractory to, or intolerant of, at least two previous chemotherapy regimens (ONO-4538-12, ATTRACTION-2): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet (2017).
Wu, P., Wu, D., Li, L., Chai, Y. & Huang, J. PD-L1 and Survival in Solid Tumors: A Meta-Analysis. PLoS One 10, e0131403 (2015).
Torre, L. A. et al. Global cancer statistics, 2012. CA Cancer J Clin 65, 87–108 (2015).
Liu, Y. X. et al. Prognostic significance of PD-L1 expression in patients with gastric cancer in East Asia: a meta-analysis. Onco Targets Ther 9, 2649–2654 (2016).
Eto, S. et al. Programmed cell death protein 1 expression is an independent prognostic factor in gastric cancer after curative resection. Gastric Cancer 19, 466–471 (2016).
Tamura, T. et al. Programmed Death-1 Ligand-1 (PDL1) Expression Is Associated with the Prognosis of Patients with Stage II/III Gastric Cancer. Anticancer Res 35, 5369–5376 (2015).
Kim, J. W. et al. Prognostic implications of immunosuppressive protein expression in tumors as well as immune cell infiltration within the tumor microenvironment in gastric cancer. Gastric Cancer 19, 42–52 (2016).
Boger, C. et al. PD-L1 is an independent prognostic predictor in gastric cancer of Western patients. Oncotarget 7, 24269–24283 (2016).
Cancer Genome Atlas Research, N. Comprehensive molecular characterization of gastric adenocarcinoma. Nature 513, 202–209 (2014).
Yarchoan, M., Johnson, B. A. 3rd, Lutz, E. R., Laheru, D. A. & Jaffee, E. M. Targeting neoantigens to augment antitumour immunity. Nat Rev Cancer 17, 569 (2017).
Gulley, M. L. Genomic assays for Epstein-Barr virus-positive gastric adenocarcinoma. Exp Mol Med 47, e134 (2015).
Carbognin, L. et al. Differential Activity of Nivolumab, Pembrolizumab and MPDL3280A according to the Tumor Expression of Programmed Death-Ligand-1 (PD-L1): Sensitivity Analysis of Trials in Melanoma, Lung and Genitourinary Cancers. PLoS One 10, e0130142 (2015).
Sheffield, B. S. et al. Investigation of PD-L1 Biomarker Testing Methods for PD-1 Axis Inhibition in Non-squamous Non-small Cell Lung Cancer. J Histochem Cytochem 64, 587–600 (2016).
Kim, H. R. et al. PD-L1 expression on immune cells, but not on tumor cells, is a favorable prognostic factor for head and neck cancer patients. Sci Rep 6, 36956 (2016).
Gu, L. et al. PD-L1 and gastric cancer prognosis: A systematic review and meta-analysis. PLoS One 12, e0182692 (2017).
Garon, E. B. et al. Pembrolizumab for the treatment of non-small-cell lung cancer. N Engl J Med 372, 2018–2028 (2015).
Bickenbach, K. & Strong, V. E. Comparisons of Gastric Cancer Treatments: East vs. West. J Gastric Cancer 12, 55–62 (2012).
Lemery, S., Keegan, P., Pazdur, R. & First, F. D. A. Approval Agnostic of Cancer Site - When a Biomarker Defines the Indication. N Engl J Med 377, 1409–1412 (2017).
Zhu, L. et al. Microsatellite instability and survival in gastric cancer: A systematic review and meta-analysis. Mol Clin Oncol 3, 699–705 (2015).
Kang, B. W., Kim, J. G., Lee, I. H., Bae, H. I. & Seo, A. N. Clinical significance of tumor-infiltrating lymphocytes for gastric cancer in the era of immunology. World J Gastrointest Oncol 9, 293–299 (2017).
Zheng, X. et al. Prognostic role of tumor-infiltrating lymphocytes in gastric cancer: a meta-analysis. Oncotarget 8, 57386–57398 (2017).
Patil, P. A. et al. Expression of PD-L1, indoleamine 2,3-dioxygenase and the immune microenvironment in gastric adenocarcinoma. Histopathology 73, 124–136 (2018).
Goel, A., Nagasaka, T., Hamelin, R. & Boland, C. R. An optimized pentaplex PCR for detecting DNA mismatch repair-deficient colorectal cancers. PLoS One 5, e9393 (2010).
Takehara, Y. et al. Accuracy of four mononucleotide-repeat markers for the identification of DNA mismatch-repair deficiency in solid tumors. J Transl Med 16, 5 (2018).
Japanese Gastric Cancer, A. Japanese classification of gastric carcinoma: 3rd English edition. Gastric Cancer 14, 101–112 (2011).
Iwashita, C. et al. Evaluation of Epstein-Barr virus DNA loads in peripheral blood using a plasmid solution calibrated with the 1st WHO International Standard. Japanese. Journal of Medical Technology 64, 48–53 (2015).
Acknowledgements
The authors would like to thank Tomoko Sueishi, Tae Yamanishi, and Akihiro Nyuya for their excellent technical support.
Author information
Authors and Affiliations
Contributions
T.M., N.K., Y.K., T.K. and K.A. conducted the experiments. T.M. and S. Ku. designed the experiments. T.T. performed the pathological assessment. S. Ki., T.N., M.N., S. Ka., H.T. and T.F. provided expertise and feedback. S. Ku. secured funding. T.M. and S. Ku. wrote the manuscript and all authors reviewed the manuscript.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Morihiro, T., Kuroda, S., Kanaya, N. et al. PD-L1 expression combined with microsatellite instability/CD8+ tumor infiltrating lymphocytes as a useful prognostic biomarker in gastric cancer. Sci Rep 9, 4633 (2019). https://doi.org/10.1038/s41598-019-41177-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-41177-2
- Springer Nature Limited
This article is cited by
-
Cervical lymphoepithelioma-like carcinoma with deficient mismatch repair and loss of SMARCA4/BRG1: a case report and five related cases
Diagnostic Pathology (2024)
-
Genomic characterization and immunotherapy for microsatellite instability-high in cholangiocarcinoma
BMC Medicine (2024)
-
Involvement in the tumor-infiltrating CD8+ T cell expression by the initial disease of remnant gastric cancer
World Journal of Surgical Oncology (2022)
-
Tumor-infiltrating CD8+ T cells combined with tumor-associated CD68+ macrophages predict postoperative prognosis and adjuvant chemotherapy benefit in resected gastric cancer
BMC Cancer (2019)