Abstract
The aim of this study was to determine the effects of sodium selenate (15, 30, 45, 60, 75, 90, and 105 mg kg−1) on the germination and seedling growth of Changnongjing 1 rice (Oryza sativa L.) at 25 °C and 30 °C. Low selenate concentrations induced shorter and more uniform germination periods than did ultrapure water at both temperatures. Seedlings primed with low selenate concentrations were superior to those primed with ultrapure water in terms of plant height, fresh weight, dry matter accumulation, and soluble carbohydrate and protein contents. Lower selenate concentrations (15–75 mg kg−1) induced higher chlorophyll and phenol contents in seedlings than did ultrapure water. Lower selenate concentrations also increased the superoxide dismutase (SOD), peroxidase (POX), catalase (CAT), and glutathione peroxidase (GPx) contents in seedlings and significantly decreased the stress-related malondialdehyde (MDA) content compared to ultrapure water. In conclusion, rice seedling germination and growth were promoted by priming with low selenate concentrations (15–75 mg kg−1) but inhibited by priming with high selenate concentrations (90–105 mg kg−1).
Similar content being viewed by others
Introduction
With the development of intensive agriculture worldwide, transplant cultivation has become a limiting factor. Direct-seeded cultivation is an alternative to transplanting. This technique accelerates soil preparation and reduces labour and water costs by 20% and 30%, respectively, compared to transplanting1. The seeds used in direct-seeded cultivation must have higher germination rates and uniformity than those used in transplanting. Seeds sown directly may be subjected to low temperatures, drought, flooding, and other adverse conditions. Embryonic cell membrane damage results in severe extracellular solute infiltration, lower seed moisture and vitality, and the accumulation of reactive oxygen species (ROS). All these factors reduce the seed germination rate. At low temperatures, however, antioxidants such as peroxidase (POD), catalase (CAT), and glutathione peroxidase (GPx) in the seeds scavenge free radicals. In this way, these enzymes protect the plants by alleviating low-temperature chilling effects. Seed initiation can increase the antioxidant enzyme content and vigour of seeds. Seed priming can also enhance antioxidant levels and the activity of oxidative defensive enzymes2,3. Certain trace elements are also known as seed initiators. When selenium (Se) is used as a seed initiator, it increases seed activity and vitality and serves as a human dietary source of this essential micronutrient4. Selenium is absorbed during rice germination and improves germination quality5,6, root activity7, and seedling growth and vitality8. This element also ensures high germination quality of seeds for direct rice seeding. Selenium also increases the antioxidant and defensive substance contents and activities in plants2, provides energy for germination and accelerates phosphorylation during membrane formation3. Selenium improves stress resistance and antioxidant and enzyme activity in plants. Plant selenium levels are closely related to human dietary selenium status9. Low selenium levels in humans are a global public health concern. Insufficient selenium intake can induce epilepsy, reduce fertility, and cause immunodeficiency10,11,12. Selenium has anti-ageing, anticancer, and immunity-boosting effects in humans. Artificial selenium supplementation is a basic biofortification strategy13. This strategy optimizes crop yield and quality and increases selenium levels in the human body14. Although selenium can improve seed germination and seedling quality, sodium selenate is rarely used as a seed initiator. Sodium selenite is known to initiate and prime seeds3. In the present study, we assume that sodium selenate can also ensure the uniformity of germination. Our objective was to investigate the application of sodium selenate as a seed germination initiator at different temperatures.
Results
Emergence attributes
The various concentrations of sodium selenate at the two temperatures significantly affected rice seed germination characteristics (P ≤ 0.05; Table 1) and the time to start of emergence (TSE) (P < 0.05). Nevertheless, the relative sensitivity of the TSE to the various sodium selenate treatments differed between the two temperatures. The TSE was advanced by 2 d after treatment with 60 mg kg−1 sodium selenate at 25 °C and 45 mg kg−1 sodium selenate at 30 °C. After treatment with > 60 mg kg−1 sodium selenate, the TSE was significantly delayed at both temperatures. Sodium selenate significantly affected the final emergence percentage (FEP) on day 7 (P ≤ 0.05). The TSE did not significantly differ between the 25 °C and 30 °C treatments. On day 7, the germination potential increased with an increase in sodium selenate concentration from 15–45 mg kg−1. At 45 mg kg−1 sodium selenate, the germination potential reached a maximum. When the concentration exceeded 75 mg kg−1, the germination potential tended to decline. The same pattern was observed for the FEP. Sodium selenate concentration significantly affected the FEP (P ≤ 0.05), but there were no significant differences between 25 and 30 °C. However, at both temperatures, the FEP reached a maximum at 60 mg kg−1 sodium selenate. Therefore, low-concentration selenate treatments promoted Changnongjing 1 rice seed germination, whereas high-concentration selenate treatments inhibited it.
The various sodium selenate concentrations significantly affected the Changnongjing 1 germination indices (P ≤ 0.05) at both temperatures. The effects of sodium selenate concentration on Changnongjing 1 priming at 25 °C and 30 °C are shown in Table 2. Clearly, the E50 varied significantly with sodium selenate concentration. Nevertheless, E50 did not significantly vary with temperature. At both 25 °C and 30 °C, the E50 decreased with increasing sodium selenate concentration from 15–75 mg kg−1, reaching a minimum at 45 mg kg−1. However, >75 mg kg−1 sodium selenate prolonged germination time by 50%. Temperature and sodium selenate concentration significantly affected the germination index. The germination index was highest at a temperature of 30 °C and a sodium selenate concentration of 45 mg kg−1. At both temperatures, the germination index increased with an increase in sodium selenate concentration from 15–75 mg kg−1 but decreased at >90 mg kg−1 sodium selenate. The germination index represents the speed and uniformity of germination. After receiving 45 mg kg−1 sodium selenate, the Changnongjing 1 rice seeds germinated rapidly and uniformly at both 25 °C and 30 °C (Table 2).
Seedling growth
Sodium selenate also had a strong positive effect on rice seedling growth. Seedlings developed from seeds germinated with sodium selenate were of higher quality than those primed with water alone (Table 3). Sodium selenate significantly affected rice plant height (Table 3). When the selenate concentration was >45 mg kg−1, the plant height began to decrease until 75 mg kg−1. At this high dose, the plants were shorter than those derived from seeds initiated with plain water. At all selenate concentrations, the plants subjected to 25 °C were taller than those exposed to 30 °C. Sodium selenate significantly affected the shoot fresh weight (P ≤ 0.05) (Table 3). Shoot fresh weights were greater in the 45, 60, and 75 mg kg−1 selenate treatments than in the water controls and lower in the 15, 90, and 105 mg kg−1 selenate treatments than in the controls. The maximum shoot fresh weight was obtained at 45 mg kg−1 sodium selenate. At 30 °C, the shoot fresh weights at all selenate concentrations except 105 mg kg−1 were greater than those in the water controls. The maximum shoot fresh weight was recorded at 45 mg kg−1 sodium selenate. The shoot fresh weights at 25 °C were significantly higher than those at 30 °C (P ≤ 0.05). Therefore, only certain sodium selenate concentrations and a temperature of 25 °C were conducive to rice seedling growth. Sodium selenate also significantly affected the shoot dry weight. Shoot dry matter accumulation in rice seedlings significantly varied with selenate concentration at 25 °C. Shoot dry matter accumulation increased with selenate concentration between 15 and 70 mg kg−1, peaked at 45 mg kg−1, and decreased from 75–105 mg kg−1. In contrast, at 30 °C, the shoot dry matter accumulation did not significantly differ between any selenate doses and the water control. Nevertheless, the shoot dry matter increased with an increase in sodium selenate concentration from 15–60 mg kg−1, peaked at 45 mg kg−1, and decreased between 7–105 mg kg−1. These patterns parallel those observed at 25 °C. Overall, the accumulation of shoot dry matter in rice seedlings did not significantly differ between 25 °C and 30 °C.
The effects of sodium selenate on rice seedling root development are shown in Table 4. Neither selenate concentration nor temperature significantly affected rice seedling root length. Selenate did, however, significantly affect root fresh weight (P ≤ 0.05). At 25 °C, the root fresh weights for all selenate treatments except 30 mg kg−1 were greater than those for the water control. The root fresh weight was highest in the 60 mg kg−1 treatment. At 30 °C, however, the root fresh weights for all selenate treatments except 15 mg kg−1 and 75 mg kg−1 surpassed those for the water control. At 30 °C, the maximum root fresh weight was measured in the 60 mg kg−1 sodium selenate treatment. The root fresh weights at 30 °C were significantly higher than those at 25 °C (P ≤ 0.05). Thus, rice seedling roots grew better at 30 °C. Sodium selenate significantly affected the rice root dry weight (Table 3). The greatest root dry weights at 25 °C and 30 °C were recorded for the 60 mg kg−1 and 75 mg kg−1 sodium selenate treatments, respectively. The root dry weights also significantly differed between 25 °C and 30 °C.
Biochemical attributes
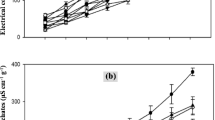
Sodium selenate concentration and temperature both significantly affected the total soluble carbohydrate content in rice seedlings (Fig. 1a). The total soluble carbohydrate content varied with selenate concentration (y25 = −0.0015x2 + 0.1072x + 15.746; y30 = −0.0013x2 + 0.0816x + 15.863). The maximum total soluble carbohydrate content was detected at 45 mg kg−1 selenate. The total soluble carbohydrate content was higher within the 15–60 mg kg−1 selenate concentration range and lower when the selenate concentration was >75 mg kg−1 than in the water control. Selenate also significantly affected the α-amylase activity in rice seedlings. The α-amylase activity varied quadratically with selenate concentration and temperature (y25 = −0.0004x2 + 0.0306x + 8.5172; y30 = −0.0005x2 + 0.0339x + 8.5543) (Fig. 1b). The maximum α-amylase activity was observed at 45 mg kg−1 selenate. The α-amylase activity was higher within the 15–60 mg kg−1 selenate concentration range and lower when the selenate concentration was >75 mg kg−1 than in the water control.
Sodium selenate significantly affected the total chlorophyll content in rice seedlings. The total chlorophyll content varied quadratically with temperature and selenate concentration (y25 = −0.0004x2 + 0.0303x + 3.5854; y30 = −0.0003x2 + 0.0269x + 3.5902) (Fig. 1c). The peak chlorophyll content was measured in the 45 mg kg−1 selenate treatment. The chlorophyll content was greater within the 15–60 mg kg−1 selenate concentration range and lower at 7 mg kg−1 selenate than in the water control. Selenate also significantly affected the water-soluble protein content of rice seedlings. The water-soluble protein content varied quadratically with selenate concentration and temperature (y25 = 5E-05x2 + 0.0053x + 0.1382; y30 = −4E-05x2 + 0.0041x + 0.1251) (Fig. 1d). The maximum water-soluble protein content was found in the 45 mg kg−1 selenate treatment.
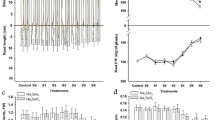
Sodium selenate significantly affected the total phenol content of the rice seedlings. The total phenol content varied quadratically with selenate concentration and temperature (y25 = y = 4E-07x2 + 9E-05x + 0.5204; y30 = y = 4E-08x2 + 0.0001x + 0.502) (Fig. 2a). The total phenol content increased with selenate concentration. Selenate also significantly affected the MDA levels in rice seedlings. The MDA content varied quadratically with selenate concentration and temperature (y25 = y = 0.0002x2 − 0.0168x + 7.7636; y30 = 0.0001x2 − 0.0045x + 7.7405) (Fig. 2b). The lowest MDA levels were detected for the 45 mg kg−1 selenate treatment. The MDA content was lower in the 15–75 mg kg−1 selenate concentration range and higher at >5 mg kg−1 selenate than in the water control.
Sodium selenate significantly affected the CAT activity in rice seedlings. CAT activity varied quadratically with selenate concentration and temperature (y25 = −0.0005x2 + 0.0482x + 0.7097; y30 = −0.0004x2 + 0.0407x + 0.6063) (Fig. 2c). The maximum CAT activity was observed for the 45 mg kg−1 selenate treatment. The CAT activity was higher within the 15–75 mg kg−1 selenate concentration range and lower at >75 mg kg−1 selenate than in the water control. GPx activity also varied quadratically with selenate concentration and temperature (y25 = −0.0421x2 + 3.5112x + 193.52; y30 = −0.0362x2 + 2.7853x + 163.19) (Fig. 2d). GPx activity peaked at 45 mg kg−1 selenate. The GPx activity was higher within the 15–75 mg kg−1 selenate concentration range and lower at >75 mg kg−1 selenate than in the water control.
Sodium selenate significantly affected the SOD in rice seedlings. SOD activity varied quadratically with selenate concentration and temperature (y25 = −0.0865x2 + 8.2873x + 592.92; y30 = −0.0763x2 + 6.7787x + 496.53) (Fig. 3a). SOD activity reached a maximum at 45 mg kg−1 selenate. The SOD activity was higher within the 15–75 mg kg−1 selenate concentration range and lower at >75 mg kg−1 selenate than in the water control. Selenate also significantly affected the POX activity in rice seedlings. POX activity varied quadratically with selenate concentration and temperature (y25 = −0.0004x2 + 0.0498x + 0.9701; y30 = −0.0005x2 + 0.0519x + 0.9178) (Fig. 3b). The POX activity peaked at 45 mg kg−1 selenate. The POX activity was higher within the 15–75 mg kg−1 selenate concentration range and lower at >75 mg kg−1 selenate than in the water control.
Discussion
Selenium is not considered an essential trace element (micronutrient) for the growth and development of higher plants15. Nevertheless, this element has positive effects on many crops such as potato (Solanum tuberosum L.), ryegrass (Lolium perenne L.), lettuce (Lactuca sativa L.), and mustard (Brassica rapa L.)7,16,17. The present study showed that low concentrations of sodium selenate act as seed priming initiators and are beneficial for plants. Khaliq et al.6 studied the effect of sodium selenite on Basmati rice germination and seedling growth. Moulick et al.18 investigated the effects of selenium on the germination of rice seeds under heavy metal stress. In both cases, however, selenium was supplied in the form of sodium selenite instead of sodium selenate. In the present study, sodium selenate was used for seed priming. Low-concentration sodium selenate treatments had positive effects on seed germination mainly by optimizing germination characteristics such as the TSE, E50, MET, EI, and FEP. Selenate at 45–60 mg kg−1 significantly outperformed water and higher selenate concentrations as a seed germination initiator. In contrast, very high selenate levels inhibited germination. This finding corroborated those reported by Khaliq et al.6. Compared to the water control and the high-dose selenate treatments, low selenate concentrations significantly increased plant height, root length, and shoot and root biomass accumulation. Therefore, low selenate concentrations significantly affect plant growth ability. However, high selenate doses inhibited seedling growth and development, corroborating the data of Moulick et al.18. Low selenate concentrations are thus preferable for seed germination and seedling development19. The use of low levels of sodium selenate can increase the germination rate and uniformity in direct-seeded rice. Such levels of sodium selenate can also improve overall seedling quality. Relative to the water control, low selenate doses improved dry matter accumulation in rice seedlings. In contrast, high selenate concentrations actually inhibited seedling dry matter accumulation. This negative effect may be explained by the inhibitory influence of selenate on chlorophyll content and α-amylase activity. There is no evidence that sodium selenate directly participates in higher plant photosynthesis. Nevertheless, photosynthetic activity in rice is closely correlated with sodium selenate application20. Low selenate concentrations may promote photosynthesis21,22 and increase the chlorophyll content18 and α-amylase activity3 in rice. High sodium selenate concentrations have the opposite effects. The present study shows that there is a quadratic relationship between soluble sugar/soluble protein content and selenate concentration. Similarly, Lyons et al.16 found that low selenate concentrations increased photosynthesis. The total phenol content increased with selenate concentration, but the correlation was not significant at the early stages of seedling development. At >75 mg kg−1 selenate, the seedling phenol content significantly increased. The rice seedlings were abiotically stressed at these very high selenate concentrations. MDA is another important biochemical indicator of plant stress. MDA levels increase quadratically with selenate concentration. MDA was lowest at 45 mg kg−1 selenate. The MDA content was lower within the 15–75 mg kg−1 selenate concentration range and higher at >75 mg kg−1 selenate than in the water control. Since sodium selenate is also an antioxidant23, the reduced MDA content at low selenate concentrations may be correlated with selenate antioxidant activity. Relative to the water control, however, >75 mg kg−1 selenate increased the MDA content. Therefore, very high selenate levels may be phytotoxic, as the high seedling phenol content at these selenate doses can have negative impacts on rice seedlings; this finding is consistent with the result for phenol content. The changes in CAT, GPx, SOD, and POX activity were also consistent with selenate antioxidant activity. Peroxide and free radicals formed during seed germination and seedling development are scavenged and quenched by a series of antioxidant enzymes24,25. Khaliq et al.6 reported similar observations using sodium selenite as a seed initiator.
As a seed germination initiator, 15–75 mg kg−1 sodium selenate can increase rice seed germination and enhance seedling growth. Low selenate doses may also increase starch, protein and sugar accumulation in the seedlings and improve their antioxidant capacity. The accumulation of sodium selenate in rice should also be assessed as a biofortification mechanism.
Methods
In this study, the rice (Oryza sativa L.) variety Changnongjing 1 (Yangtze University, Hubei, China) was used. The initial seed moisture content was 9.22%. Dry rice seeds were primed at two different temperatures (25 °C or 30 °C) in a solution of sodium selenate (Na2O4Se) at a 0, 15, 30, 45, 75, 90, or 105 mg kg−1 concentration. A two-factor, completely randomized design was used. In each treatment, 25 g of seeds was placed in a 200-mL conical flask containing 125 mL initiator solution26. The flasks were placed in an incubator [darkness, 25 ± 1 °C or 30 ± 1 °C, and 80% relative humidity]. The flasks were shaken once every 6 h27. After priming, the seeds were filtered through gauze, placed in distilled water for 20 min, rinsed five times with ultrapure water and set aside. Autoclavable glass Petri dishes were lined with double layers of filter paper, placed on a laboratory bench and left to air dry for 24 h3.
The Petri dishes were sterilized by autoclaving and left to cool. The seeds were surface sterilized by soaking for 15 Min in 5% sodium hypochlorite and washed with ultrapure water 3–5 times. Thereafter, 10 mL sterile water was aseptically pipetted into each Petri dish; then, 100 seeds were placed in each Petri dish, and each seed-soaking treatment was performed in triplicate. The Petri dishes were transferred into a phytotron (KRG-400BP, China) and incubated under 8,000 lx illumination for 14 h d−1. No light was used for the remaining 10 h. The temperature was either 25 ± 1 °C or 30 ± 1 °C, and the relative humidity was 80%28. The Petri dishes were weighed daily and replenished with the required amount of selenate solution.
Seed bioassay
Seedling emergence was counted daily using the method prescribed by the Association of Official Seed Analysts (AOSA)29 until a constant count was reached. A seedling was scored as emerged when the hypocotyl length was ≥2 mm. The time to 50% emergence (E50) was calculated according to the modified equation of Farooq et al.30:
where N is the final number of emerged seeds; ni and nj are the cumulative number of emerged seeds counted at time ti and tj, respectively, where ni < N/2 < nj.
The mean emergence time (MET) was calculated following Ellis and Roberts31:
where n is the number of emerged seeds on day D, and D is the number of days counted from the beginning of emergence.
The emergence index (EI) was calculated as described by the AOSA32:
The root and shoot lengths of five seedlings randomly selected from each experimental unit were measured 18 d after sowing (DAS). The roots and shoots were excised separately and oven dried at 70 °C for 48 h to obtain the dry biomass of each component. The two measurements were summed to derive the total seedling biomass.
Biochemical analyses
Lipid peroxidation in the treated and untreated rice seeds was determined from the malondialdehyde (MDA) content using the thiobarbituric acid method33. The α-amylase activity in ground rice seeds (1 g) was measured according to the technique of Suksoon and Jaehyeun34. The rate of maltose liberation from starch was calculated from the reduction in 3,5-dinitrosalicylic acid. Total soluble sugars and soluble proteins were quantified according to Dubois et al.35 and Bradford36, respectively. The activities of superoxide dismutase (SOD) at 560 nm, CAT at 240 nm, POD at 470 nm, and GPx at 340 nm were determined according to Giannopolitis and Ries37, Dhindsa et al.38, Egley et al.39 and Drotar et al.40, respectively. One unit of enzyme activity was defined as that required to reduce 50% of nitro blue tetrazolium chloride per minute (SOD), oxidize 1 μmol H2O2 per minute (CAT), oxidize 1 μmol guaiacol per minute (POD), or catalyse the H2O2-induced oxidation of 1 μmol reduced glutathione at a pH of 7.0 and temperature of 25 °C (GPx). At 18 DAS, three randomly selected rice seedlings were harvested from each experimental unit. The total foliar soluble phenol content was determined from absorbance readings at 663 nm and 645 nm41. Photosynthetic pigments (chlorophyll a and b) were extracted in 80% v/v ice-cold acetone42 and read at 663 and 645 nm wavelengths.
Statistical analyses
Data were analysed using the statistical software Statistix 8.1 (Analytical Software, Tallahassee, FL, USA), while differences among means were separated by using a least significant difference (LSD) test at the 5% probability level. Origin 8.1 (OriginLab Co., Northampton, MA, USA) was used for graphical representation.
Data Availability
The data used to support the findings of this study are available from the corresponding author upon request.
References
Lee, M. H., Kim, J. K., Kim, S. S. & Park, S. T. Status of dry-seeding technologies for rice in Korea. In Direct seeding: research strategies and opportunities (eds Pandey, S.et al.) 161–176 (International Rice Research Institute, 2002).
Roqueiro, G., Maldonado, S., Mdel, C. R. & Maroder, H. Fluctuation of oxidative stress indicators in Salix nigra seeds during priming. J. Exp. Bot. 63, 3631–3642 (2012).
Kaklewski, K., Nowak, J. & Ligocki, M. Effects of selenium content in green parts of plants on the amount of ATP and ascorbate-glutathione cycle enzyme activity at various growth stages of wheat and oilseed rape. J. Plant Physiol. 165, 1011–1022 (2008).
Tapiero, H., Townsend, D. M. & Tew, K. D. The antioxidant role of selenium and seleno-compounds. Biomed. Pharmacother. 57, 134–144 (2003).
Lazo-Vélez, M. A., Chávez-Santoscoy, A. & Serna-Saldivar, S. O. Selenium-enriched breads and their benefits in human nutrition and health as affected by agronomic, milling, and baking factors. Cereal Chem. 92, 134–144 (2015).
Khaliq, A. et al. Seed priming with selenium: consequences for emergence, seedling growth, and biochemical attributes of rice. Biol. Trace Elem. Res. 166, 236–244 (2015).
Hartikainen, H., Xue, T. & Piironen, V. Selenium as an anti-oxidant and pro-oxidant in ryegrass. Plant Soil 225, 193–200 (2000).
Teklić, T., Lončarić, Z., Kovačević, V. & Singh, B. R. Metallic trace elements in cereal grain – a review: how much metal do we eat? Food Ener. Secur. 2, 81–95 (2013).
Feng, R., Wei, C. & Tu, S. The roles of selenium in protecting plants against abiotic stresses. Environ. Exper. Bot. 87, 58–68 (2013).
Wang, Y. et al. Progress in research on selenium-enriched land-crops and horticultural plants in China. J. Henan Agric. Sci. 44, 7–12 (2015).
Naziroglu, M. Role of selenium on calcium signaling and oxidative stress-induced molecular pathways in epilepsy. Neurochem. Res. 34, 2181–2191 (2009).
Listed, N. Selenium and human health. Nutr. Rev. 34, 347 (1976).
Zeng, H. & Combs, G. F. Selenium as an anticancer nutrient: roles in cell proliferation and tumor cell invasion. J. Nutr. Biochem. 19, 1–7 (2008).
Broadley, M. R. et al. Selenium biofortification of high-yielding winter wheat (Triticum aestivum L.) by liquid or granular Se fertilisation. Plant Soil 332, 5–18 (2010).
Sors, T. G., Ellis, D. R. & Salt, D. E. Selenium uptake, translocation, assimilation and metabolic fate in plants. Photosynth. Res. 86, 373–389 (2005).
Lyons, G. H. et al. Selenium in Australia: selenium status and biofortification of wheat for better health. J. Trace Elem. Med. Biol. 19, 75–82 (2005).
Turakainen, M., Hartikainen, H. & Seppanen, M. M. Effects of selenium treatments on potato (Solanum tuberosum L.) growth and concentrations of soluble sugars and starch. J. Agric. Food Chem. 52, 5378–5382 (2004).
Moulick, D., Ghosh, D. & Santra, S. C. Evaluation of effectiveness of seed priming with selenium in rice during germination under arsenic stress. Plant Physiol. Biochem. 109, 571–578 (2016).
Malik, J. A. et al. Selenium antagonises the toxic effects of arsenic on mungbean (Phaseolus aureus Roxb.) plants by restricting its uptake and enhancing the antioxidative and detoxification mechanisms. Environ. Exper. Bot. 77, 242–248 (2012).
Zhang, M. et al. Selenium uptake, dynamic changes in selenium content and its influence on photosynthesis and chlorophyll fluorescence in rice (Oryza sativa L.). Environ. Exper. Bot. 107, 39–45 (2014).
Liu, H. et al. Interactive effects of molybdenum and phosphorus fertilizers on photosynthetic characteristics of seedlings and grain yield of Brassica napus. Plant Soil 326, 345–353 (2010).
Xia, Y. X., Liu, S. Q., He, L. I. & Chen, X. W. Effects of selenium on physiological characteristics, selenium content and quality of garlic. Plant Nutr. Fert. Sci. 18, 733–741 (2012).
Heath, R. L. & Packer, L. Photoperoxidation in isolated chloroplasts. I. Kinetics and stoichiometry of fatty acid peroxidation. Arch. Biochem. Biophys. 125, 189–198 (1968).
McDonald, B. & Seed, M. Deterioration: physiology, repair and assessment. Seed Sci. Technol. 27, 177–237 (1999).
Bailly, C. Active oxygen species and antioxidants in seed biology. Seed Sci. Res. 14, 93–107 (2007).
Ruan, S., Xue, Q. & Tylkowska, K. Effect of priming on germination and health of rice (Oryza sativa L.) seeds. Seed Sci. Technol. 30, 451–458 (2002).
Lu, C. Emergence of kentucky bluegrass (Poa pratensis varietes). Doctoral dissertation, (Lanzhou University, Lanzhou, 2006).
Wang, H., Guo, T. & Yang, Y. Effects of phosphate concentration on germination and seedling growth of perennial ryegrass. Chin. Agron. Bull. 22, 203–207 (2013).
Association of Official Seed Analysts (AOSA). Rules for testing seeds. J. Seed Technol. 12, 1–112 (1990).
Farooq, M., Basra, S. M. A., Ahmad, N. & Hafeez, K. Thermal hardening: a new seed vigor enhancement tool in rice. J. Integr. Plant Biol. 47, 187–193 (2005).
Ellis, R. A. & Roberts, E. H. The quantification of aging and survival in orthodox seeds. Seed Sci. Technol. 9, 373–409 (1981).
AOSA. Seed vigor hand testing book. Contribution no. 32 to the handbook on seed testing. (Association of official seed analysts (AOSA), Springfield, 1983).
Bailly, C., Benamar, A., Corbineau, F. & Come, D. Changes in malondialdehyde content and in superoxide dismutase, catalase and glutathione reductase activities in sunflower seeds as related to deterioration during accelerated aging. Physiol. Plantar. 97, 104–110 (2012).
Suksoon, L. & Jaehyeun, K. Total sugars, α-amylase activity, and germination after priming of normal and aged rice seeds. Kor. J. Crop. Sci. 45, 108–111 (2000).
Dubois, M., Gilles, K. A., Hamilton, J. K., Rebers, P. A. & Smith, F. Colorimetric method for determination of sugar and related substances. Anal. Chem. 28, 350–356 (1956).
Bradford, M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 (1976).
Giannopolitis, C. N. & Ries, S. K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 59, 309–314 (1977).
Dhindsa, R. S., Plumb-Dhindsa, P. & Thorpe, T. A. Leaf senescence: correlated with increased levels of membrane permeability and lipid peroxidation, and decreased levels of superoxide dismutase and catalase. J. Exp. Bot. 32, 93–101 (1981).
Egley, G. H., Paul, R. N., Vaughn, K. C. & Duke, S. O. Role of peroxidase in the development of water-impermeable seed coats in Sida spinosa L. Planta 157, 224–232 (1983).
Drotar, A., Phelps, P. & Fall, R. Evidence for glutathione peroxidase activities in cultured plant cells. Plant Sci. 42, 35–40 (1985).
Randhir, R. & Shetty, K. Developmental stimulation of total phenolics and related antioxidant activity in light- and dark-germinated corn by natural elicitors. Proc. Biochem. 40, 1721–1732 (2005).
Lichtenthaler, H. K. Chlorophyll and carotenoids: pigments of photosynthetic bio-membranes. In Methods in enzymology (eds Packer, L. & Douce, R.) 350–382 (Academic Press, 1987).
Acknowledgements
The authors would like to express their appreciation to Dr. Huoyun Chen and Danyin Xing for their technical assistance and valuable suggestions. The research was supported by the National Natural Science Foundation of China (31471442 and 31271646), the Special Fund for Agro-scientific Research in the Public Interest of China (201203059), and the fund for the National Key Technology R&D Program of China (2011-G18(2)).
Author information
Authors and Affiliations
Contributions
Bin Du and Haowen Luo were the primary principal investigators who designed and implemented the research. Longxin He, Yangfang Liu and Lihe Zhang oversaw the study and performed statistical analysis. Zhaowen Mo wrote the English version of the manuscript. Hua Tian, Meiyang Duan and Xiangru Tang provided guidance during experimentation. Shenggang Pan and Hua Tian reviewed the final version of the manuscript prior to submission for peer review.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Du, B., Luo, H., He, L. et al. Rice seed priming with sodium selenate: Effects on germination, seedling growth, and biochemical attributes. Sci Rep 9, 4311 (2019). https://doi.org/10.1038/s41598-019-40849-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-40849-3
- Springer Nature Limited
This article is cited by
-
Alleviation potential of green-synthesized selenium nanoparticles for cadmium stress in Solanum lycopersicum L: modulation of secondary metabolites and physiochemical attributes
Plant Cell Reports (2024)
-
Effect of selenium nanoparticles on biological and morphofunctional parameters of barley seeds (Hordéum vulgáre L.)
Scientific Reports (2023)
-
The Efficiency of Humic Acid for Improving Salinity Tolerance in Salt Sensitive Rice (Oryza sativa): Growth Responses and Physiological Mechanisms
Gesunde Pflanzen (2023)
-
Modulation of Antioxidant Attributes and Grain Yield in Fragrant Rice by Exogenous Cu Application
Journal of Plant Growth Regulation (2023)
-
Promoting Effect of Pectic-Oligosaccharides Produced from Pomelo Peel on Rice Seed Germination and Early Seedling Growth
Journal of Plant Growth Regulation (2023)