Abstract
We previously demonstrated that cellular aging signals upregulated a secreted class 3 semaphorin E (Sema3E) and its receptor plexinD1 in the adipose tissue of a murine model of dietary obesity and that Sema3E was a chemoattractant, mediating its biological effects by inducing infiltration of plexinD1-positive inflammatory macrophages into the visceral white adipose tissue. This study was performed to develop a peptide vaccine for Sema3E and test its therapeutic potential in a murine model of dietary obesity. Two antigenic peptides were selected to generate neutralizing antibodies for a vaccine. These peptides were conjugated to keyhole limpet hemocyanin (KLH), and were administered with Freund’s adjuvant to obese wild-type male mice. The Sema3E antibody titer was analyzed by ELISA, and the biological effects of the peptides were tested in mice with dietary obesity. Among the two candidate peptides, the Sema3E antibody titer was significantly increased by injection of KLH-conjugated HKEGPEYHWS (Sema3E vaccine). Administration of Sema3E vaccine suppressed the infiltration of plexinD1-positive cells, ameliorated chronic inflammation in visceral white adipose tissue, and improved systemic glucose intolerance in mice with dietary obesity, suggesting that Sema3E vaccine has the potential to become a next generation therapy for obesity and diabetes.
Similar content being viewed by others
Introduction
The number of patients with diabetes continues to increase, so it is an urgent task to find better therapies for this disorder. Chronic sterile inflammation of visceral white adipose tissue (WAT) develops under metabolic stress, and is well accepted to have a causal role in the development and progression of systemic metabolic disorders1,2. We previously demonstrated that cellular aging signals were activated in visceral WAT by metabolic stress and contributed to adipose tissue inflammation3. We further found that cellular aging signals upregulated a secreted class 3 semaphorin E (Sema3E) and its receptor plexinD1 in visceral WAT of a murine model of dietary obesity4. Semaphorins and their receptors (plexins) are axon-guiding molecules that regulate development of the nervous system during embryogenesis. We found that Sema3E is a chemoattractant, which mediates its biological effects by promoting the infiltration of plexinD1-positive inflammatory macrophages into visceral WAT under metabolic stress. In addition to mice with dietary obesity, the circulating Sema3E level is also increased in patients with diabetes, suggesting that suppression of this secreted molecule could become a next generation therapy for diabetes by inhibiting chronic inflammation in visceral WAT4. Inhibiting Sema3E by administration of a neutralizing antibody is one option, but the diabetic population is huge and the expected medical cost burden is very large, so we considered other strategies for targeting this molecule. Accordingly, we tried to generate a peptide vaccine in order to utilize the endogenous immune system for production of Sema3E neutralizing antibody. In the present study, we demonstrated that a peptide vaccine targeting Sema3E could suppress inflammation in visceral WAT and improve glucose intolerance in mice with dietary obesity.
Results
Sema3E-vaccine inhibits adipose tissue inflammation in visceral fat
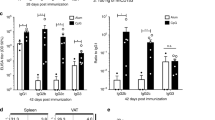
We tried to develop immunotherapy targeting Sema3E by injection of a Sema3E peptide vaccine to increase the level of circulating antibodies against Sema3E. Based on previous reports5, two antigenic peptides were selected to generate neutralizing antibodies targeting amino acids 385–394 (KVNGGKYGTT) or amino acids 359–368 (HKEGPEYHWS) of Sema3E. The N-terminus or lysine of each candidate peptide was conjugated to KLH via glutaraldehyde, and the synthetic peptides were purified by reverse-phase high-performance liquid chromatography (>99% purity)6. Then the KLH-conjugated peptides combined with Freund’s adjuvant were administered to wild-type male mice on a C57BL6/NCr background and the Sema3E antibody titer was analyzed by ELISA. In the chow fed mice, injection of the KLH-conjugated HKEGPEYHWS peptide led to a significant increase of the Sema3E antibody titer, while there was no marked increase after injection of the KLH-conjugated KVNGGKYGTT peptide (Supplementary Fig. 1A,B). Therefore, we further characterized the biological effects of the KLH-conjugated HKEGPEYHWS peptide (Sema3E vaccine) in mice with dietary obesity. Wild-type male mice were fed a high fat diet (HFD) from 4 weeks old and were administered 3 doses of Sema3E vaccine (at 6, 10, and 14 weeks old), followed by analysis at 20 weeks old (Supplementary Fig. 1C). A titer for sema3E increased mildly at 10 weeks of age after the initial injection. This increase became more significant after the second injection (at 14 weeks of age), and a robust increase was found at 20 weeks of age after the third injection (Fig. 1A). Antibodies in plasma from the Sema3E vaccine group bound to recombinant Sema3E protein and BSA-conjugated Sema3E peptide, unlike plasma from control mice (injected with KLH and Freund’s adjuvant) (Fig. 1B, left and middle panels). A commercially available anti-sema3E antibody detected recombinant Sema3E protein, but did not bind with BSA-conjugated Sema3E peptide (Fig. 1B, right panel). Infiltration of mononuclear-like cells and formation of crown-like structures in epididymal visceral WAT (eWAT) were note in mice with dietary obesity, while such changes were ameliorated by administration of Sema3E vaccine (Fig. 1C). There was a significant reduction in the expression of transcripts for Adgre1 (Emr1), a macrophage marker (Fig. 1D). We previously reported that Sema3E is a chemoattractant, which mediates inflammatory effects by promoting infiltration of plexinD1-positive inflammatory macrophages into eWAT. We also found that expression of transcripts for plexinD1 was reduced by Sema3E vaccine therapy, as shown by quantitative PCR (qPCR) (Fig. 1E). We also found that the number of plexinD1-positive cells was reduced in visceral adipose tissue of the Sema3E vaccine group compared with the control group (Supplementary Fig. 2A). Furthermore, expression of markers for inflammatory macrophages, including Itgam and Itgax (CD11c), was reduced by the vaccine (Fig. 1F). Under this condition, qPCR studies showed transcripts for other immune cell markers including Cd4, Cd8, Cd19, Cd335 and Ly6g, and a chemoattractant Ccl2 were comparable between the two groups (Supplementary Fig. 2B,C). It is now well recognized that cellular senescence associated with elevated production of reactive oxygen species (ROS) promotes inflammation in inflamed visceral WAT3,4,7,8. We found that injection of Sema3E vaccine suppressed ROS production (Fig. 1G, and Supplementary Fig. 2D), and also reduced expression of p53 (a key mediator of cellular senescence) and its downstream molecule Cdkn1a (p21) (Fig. 1H,I and Supplementary Fig. 2E,F) in eWAT under metabolic stress. These results suggested that suppressing the infiltration of plexinD1-positive cells by administration of Sema3E vaccine contributed to inhibition of the vicious cycle between ROS production/cellular senescence and inflammation.
Sema3E vaccine inhibits inflammation in visceral fat. (A) Mice fed a high fat diet (HFD) were immunized with KLH-conjugated HKEGPEYHWS peptide (Sema3E vaccine group) or KLH alone (KLH group), and plasma antibody titers were analyzed with ELISA at 6, 10, 14 and 20 weeks of age (n = 4, 4, 4, 4, 4, 4, 4, 4). (B) After the third dose, plasma was collected from the Sema3E vaccine group and the KLH group (Con). Recombinant Sema3E (r-Sema3E) protein or BSA-conjugated Sema3E peptide (Pep) was blotted with plasma samples or with a commercially available anti-Sema3E antibody (Sema3E-Ab). (C) Hematoxylin and eosin (HE) staining of epididymal WAT (eWAT) harvested from mice in the Sema3E vaccine group (Sema3E vaccine) or the KLH group (Con) maintained on a HFD. Nos. 1, 2, and 3 indicate that the eWAT originated from different mice. Scale bar = 50 μm. (D–F) Levels of transcripts for Adgre1 (Emr1)(D) (n = 6, 6), Plxnd1 (E) (n = 6, 6), Itgam (n = 6, 6), and Itgax (n = 6, 6)(F) in the indicated groups. (G) Evaluation of reactive oxygen species (ROS) performed by dihydroethidium (DHE) staining in the indicated groups. Scale bar = 50 μm. (H) Western blot analysis of p53 in the indicated groups. Full-length blots are presented in Supplementary Fig. 2F. (I) Cdkn1a expression was examined in the indicated groups (n = 6, 6). Outliers were excluded by boxplot analysis. All remaining data were analyzed by the two-tailed Student’s t-test. *P < 0.05, **P < 0.01. Values represent the mean ± s.e.m. NS = not significant.
Sema3E-vaccine ameliorates systemic glucose intolerance
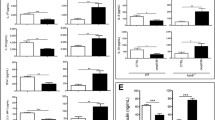
It is generally accepted that chronic sterile inflammation of visceral adipose tissue has a causal role in inducing systemic metabolic dysfunction1,2. Therefore, we tested the effects of Sema3E vaccine on systemic metabolism in mice with dietary obesity. We found that there were no differences between the control group and the Sema3E vaccine group with regard to food intake (Fig. 2A), body weight (Fig. 2B), eWAT weight (Fig. 2C), and visceral fat volume evaluated by CT (Fig. 2D). Metabolic cage studies showed that oxygen consumption (VO2), CO2 production (VCO2), the respiratory exchange ratio (RER), and energy expenditure (EE) were comparable between the control group and the Sema3E vaccine group (Fig. 2E). However, systemic glucose tolerance was better in the Sema3E vaccine group according to the glucose tolerance test (Fig. 2F), indicating that the vaccine improved systemic metabolic health as well as suppressing inflammation in visceral WAT.
Sema3E vaccine improves systemic glucose intolerance. (A–C) Food intake (A) (n = 7, 6), body weight (B) (n = 6, 6), and eWAT/BW ratio (mg/g) (C) (n = 14, 11) of mice administered KLH-conjugated Sema3E vaccine (Sema3E vaccine) or KLH alone (Con). (D) Visceral fat/BW ratio of the indicated groups determined by CT scanning (n = 8, 8). (E) Oxygen consumption (VO2) (n = 8, 8), CO2 production (VCO2) (n = 8, 8), respiratory exchange ratio (RER) (n = 8, 8), and energy expenditure (EE) (n = 8, 8) in the indicated groups. (F) Glucose tolerance test (GTT) in the indicated groups (n = 14, 15). Outliers were excluded by boxplot analysis. All remaining data were analyzed by the two-tailed Student’s t-test (A–E) or repeated measures (F). *P < 0.05, **P < 0.01. Values represent the mean ± s.e.m. NS = not significant.
Characterization of the immune response to Sema3E-vaccine
To investigate the potential immune response to Sema3E vaccine, we analyzed circulating immune cells, but found no differences between the groups (Fig. 3A,B). The enzyme-linked immunospot (ELISpot) test was performed using splenocytes from the Sema3E vaccine group to characterize T cell reactivity. Treatment with KLH led to an increase of IL-4 or IFN-γ production by the splenocytes, while recombinant Sema3E protein had no effect in this test (Fig. 3C,D). These results indicated that KLH contains epitopes for T cells, as reported previously5, while Sema3E protein did not elicit an immune response from T cells harvested from vaccinated mice. Taken together, these results suggest that a peptide vaccine targeting Sema3E could potentially become a therapeutic option for diabetes and/or unhealthy obesity in the future.
Characterization of immune response to Sema3E vaccine. (A) Peripheral white blood cell count (WBC) in mice administered KLH-conjugated Sema3E vaccine (Sema3E vaccine) or KLH alone (Con) (n = 6, 5) and maintained on a high fat diet (HFD). (B) Peripheral blood counts of lymphocytes (Lymph), neutrophils (Neut), monocytes (Mono), eosinophils (Eosino), and basophils (Baso) in the indicated groups (n = 6, 5). (C,D) ELISPOT assay showing production of IL-4 (C) or IFN-γ (D) by splenocytes harvested from mice fed an HFD and administered KLH-conjugated Sema3E vaccine (Sema3E vaccine). Splenocytes (106 cells) were stimulated with KLH, recombinant Sema3E protein (r-Sema3E), or PBS (Con). Right panels indicate the number of spots per 106 cells in the indicated groups (n = 6, 6, 6 for (C), n = 6, 6, 6 for (D)). No outliers or abnormal values were excluded by boxplot analysis. All data were analyzed by the two-tailed Student’s t-test (A, B) or 2-way ANOVA followed by Tukey’s multiple comparison test (C,D). *P < 0.05, **P < 0.01. Values represent the mean ± s.e.m. NS = not significant.
Discussion
There is accumulating evidence about the role of cellular senescence in promoting the pathology of various age-related disorders, including diabetes, heart failure, and atherosclerosis3,4,7,8,9,10. We previously reported that accumulation of ROS, DNA damage, and telomere dysfunction under metabolic stress promotes p53-induced cellular senescence in visceral WAT, resulting in chronic sterile inflammation of this tissue that leads to development of systemic metabolic dysfunction3. Thus, elimination of senescent cells is a therapeutic option to be considered, and modulation of various molecules involved in cellular senescence is also an important field for exploration9,11,12,13. It can be predicted that direct targeting of p53 will lead to tumorigenesis14, so we have instead tried to suppress the p53-induced inflammatory response by targeting downstream molecules. We previously demonstrated that a secreted semaphorin, Sema3E, was positively regulated by p53 at the transcriptional level and was a chemoattractant for inflammatory macrophages expressing its receptor (Plexin D1). Suppression of the Sema3E-Plexin D1 axis contributed to inhibiting the inflammatory response in visceral WAT, resulting in improvement of systemic metabolic health. In addition, the circulating level of Sema3E was increased in patients with diabetes, indicating that this molecule could be a target of next generation therapy for diabetes4. Generation of an antibody directed against secreted Sema3E might be a promising approach, but considering the huge population of patients with unhealthy obesity and/or diabetes, we considered it crucial to develop a therapy that was also reasonable in terms of medical economy. Accordingly, we investigated the development of a peptide vaccine for Sema3E. Peptide vaccine therapy has already been shown to be safe and useful in the fields of hypertension and diabetes, targeting angiotensin II (AngII) and dipeptidyl peptidase-4 (DPP4), respectively. Vaccination utilizes the endogenous immune system to generate neutralizing antibodies for target molecules. Antigen-presenting cells (APCs) phagocytose the antigen-KLH conjugate and present an epitope of KLH to T cells via the major histocompatibility complex (MHC), leading to T cell activation. Then B cells that specifically recognize the target antigen differentiate into plasmacytes and proliferate with the support of activated T cells, followed by initiation of antibody production against antigens such as AngII or DPP46,15. Importantly, this response can be achieved without induction of cell-mediated autoimmunity5. Using the same system, and this time Sema3E as an antigen, we tried to inhibit the Sema3E-PlexinD1 axis. We found that administration of a peptide vaccine based on amino acids 359–368 (HKEGPEYHWS) of Sema3E led to an increase of circulating antibodies and suppression of mononuclear-like cell infiltration into eWAT along with reduced Plexin D1 signaling. Our previous study revealed that Plexin D1 was expressed by inflammatory macrophages and was involved in systemic glucose intolerance. In the present study, we could show that administration of Sema3E vaccine suppressed the infiltration of these cells into eWAT and led to improvement of systemic metabolic health. Accordingly, inhibition of the Sema3E-Plexin D1 axis by vaccination may potentially be developed as a treatment for unhealthy obesity and diabetes.
Materials and Methods
Design and synthesis of the vaccine
To generate neutralizing antibodies for Sema3E, two antigenic peptide sequences (spanning amino acids 385–394 and amino acids 359–368 of Sema3E) were selected on the basis of previous reports (Pang Z, et al.5). The N-terminus or lysine of each candidate peptide was conjugated to keyhole limpet hemocyanin (KLH) via glutaraldehyde, and the synthetic peptides were purified by reverse-phase high-performance liquid chromatography (>99% purity) (Peptide Institute Inc., Osaka, Japan), as described previously6.
Animal models
All animal experiments were conducted in compliance with the protocol reviewed by the Institutional Animal Care and Use Committee of Niigata University and approved by the President of Niigata University. C57BL/6NCr mice were purchased from SLC Japan (Shizuoka, Japan), and were fed a high fat diet (HFD) (CLEA Japan, Tokyo, Japan) or normal chow from 4 to 20 weeks of age. Each KLH-conjugated antigenic peptide (20 µg) and an equal volume of complete or incomplete Freund’s adjuvant (Sigma-Aldrich: F5881 (complete) or F5506 (incomplete)) were emulsified by vortexing. For immunization, mice were injected subcutaneously with the KLH-conjugated peptide at the age of 6 weeks (with complete Freund’s adjuvant), 10 weeks (with incomplete Freund’s adjuvant), and 14 weeks (with incomplete Freund’s adjuvant). Control groups received an equal volume of KLH mixed with complete or incomplete Freund’s adjuvant. Plasma was collected from the tail vein of each mouse for analysis of antibody titers.
Antibody titer measurement
The antibody titer generated by injection of each peptide was measured by ELISA. A 96-well microtiter plate (Nunc-Immuno MicroWell 96 well solid plate, Thermo Scientific) was coated with one of the antigenic peptides conjugated to bovine serum albumin (BSA) in carbonate buffer. After coating, plasma collected from immunized mice was added to the wells and incubated overnight. Then the plate was incubated with the secondary antibody (ECL Anti-mouse IgG, Horseradish Peroxidase linked whole sheep antibody, GE Healthcare). After reaction with the TMB substrate (Sigma-Aldrich, T0440), the optical density was measured at 450 nm (OD450) by using a microtiter plate reader (iMark microplate reader, Bio-Rad).
Physiological analyses
Mice were housed individually for monitoring of their body weight and food intake. Adiposity was examined by CT scanning (LaTheta, Aloca) according to the manufacturer’s protocol. CT scans were obtained at 2-mm intervals from the diaphragm to the base of the abdominal cavity. Oxygen consumption was measured in 20-week-old mice by using an O2/CO2 metabolic measurement system (Columbus Instruments) according to the manufacturer’s instruction.
Laboratory tests
The intraperitoneal glucose tolerance test (IGTT) was performed as described previously with slight modifications, and glucose was given intraperitoneally at a dose of 1 g kg−1 (body weight)4. Peripheral white blood cells from immunized mice were counted by Oriental Yeast Co.(Tokyo, Japan).
Histological analyses and immunostaining
Samples of epididymal white adipose tissue (WAT) were harvested, fixed overnight in 10% formalin, embedded in paraffin, and sectioned for immunofluorescence or hematoxylin-eosin (HE) staining. Staining for reactive oxygen species (ROS) was done, as described previously4,7. For immunostaining, deparaffinized sections were retrieved with citrate buffer and incubated with anti-PlexinD1 antibody (Abcam, ab28762) at 1:50 dilution. Anti-Goat IgG Cy5-conjugated (Abcam, ab6566) was used as a secondary antibody. The sections were stained with Wheat Germ Agglutinin, Alexa Fluor 488 Conjugate (Invitrogen, W11261, 1:10) and Hoechst (Life Technologies, 33258, 1:1000), and photographed with a Biorevo (Keyence Co., Osaka, Japan).
RNA analysis
Extraction of RNA and real-time PCR (qPCR) was performed, as described previously4, using the following primers. Rplp0 was used as the internal control.
Adgre1: GGAGGACTTCTCCAAGCCTATT, AGGCCTCTCAGACTTCTGCTT
Ccl2: CATCCACGTGTTGGCTCA, GATCATCTTGCTGGTGAATGAGT
Cd4: ACACACCTGTGCAAGAAGCA, GCTCTTGTTGGTTGGGAATC
Cd8: CTCACCTGTGCACCCTACC, ATCCGGTCCCCTTCACTG
Cd19: AAGGTCATTGCAAGGTCAGC, CTGGGACTATCCATCCACCA
Cd335: ACACTACTCATCACAGGAGGTGTT, GTTGAAAGGTCAAACTCCCAAT
Cdkn1a: TCCACAGCGATATCCAGACA, GGACATCACCAGGATTGGAC
Itgam: CAATAGCCAGCCTCAGTGC, GAGCCCAGGGGAGAAGTG
Itgax: ATGGAGCCTCAAGACAGGAC, GGATCTGGGATGCTGAAATC
Ly6g: GGCTCAGAAAAGTGCACCA, CGTACGTGGAAGCGAACAG
Plxnd1: CTGGATGTCCATCTGCATGT, CAGGAAGAACGGCTCACCTA
Rplp0: GATGCCCAGGGAAGACAG, ACAATGAAGCATTTTGGATAA.
Western blot analysis
Western blot analysis was performed as described previously4. The primary antibodies were anti-p53 (1C12) antibody (Cell Signaling, #2524) and anti-actin antibody (Cell Signaling, #4970), which were used at a dilution of 1:1000. For western blot analysis of mouse plasma, recombinant full-length mouse Sema3E protein (R&D, 3238-S3) and the antigenic peptide conjugated with BSA were resolved in the buffer (10 mM Tris-HCl, pH 7.8, 150 mM NaCl, 1 mM EDTA) and subjected to SDS-PAGE. The membrane was incubated with plasma collected from mice immunized with the antigenic peptide or KLH (1:200 dilution) or with anti-Sema3E antibody (Santa Cruz, sc-49733) (1:1000 dilution) as the primary antibody, followed by the incubation with horseradish peroxidase-conjugated anti-mouse immunoglobulin G (Jackson Immunoresearch, #115-035-003) or anti-goat immunoglobulin G (Jackson Immunoresearch, #705-035-003).
ELISPOT assay
ELISPOT assays for INF-γ and IL-4 were performed using Mouse IFN-γ ELISpotPLUS and Mouse IL-4 ELISpotPLUS (MABTECH Inc., 3321-4HST-2 for IFN-γand 3311-4HPW-2 for IL-4) according to the manufacturer’s instruction. Briefly, splenocyte suspensions from immunized mice were added to the 96-well ELISpot assay plates (106 cells per well) and stimulated with 10 μg/mL recombinant mouse Sema3E protein (R&D, 3238-S3), 10 μg/mL KLH (WAKO, 080-07666), or PBS (Con) at 37 °C for 48 hrs. The plates were washed with PBS and incubated with biotin-conjugated detection antibodies for 2 hrs at room temperature. After washing, the plates were incubated with HRP-conjugated streptoavidin for 1 hr at room temperature, followed by the incubation with TMB substrate. Colored spots were photographed using a dissecting microscope and counted using ImageJ software.
Statistical analysis
Statistical analysis was done with SPSS software (version 20). Results are shown as the mean ± SEM. Outliers were excluded by boxplot analysis. Differences between groups were examined by the two-tailed Student’s t-test, by repeated measures for temporal studies (glucose tolerance test), or 2-way ANOVA followed by Tukey’s multiple comparison test. In all analyses, P < 0.05 was considered statistically significant.
References
Hotamisligil, G. S., Shargill, N. S. & Spiegelman, B. M. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science 259, 87–91 (1993).
Ouchi, N., Parker, J. L., Lugus, J. J. & Walsh, K. Adipokines in inflammation and metabolic disease. Nat Rev Immunol 11, 85–97 (2011).
Minamino, T. et al. A crucial role for adipose tissue p53 in the regulation of insulin resistance. Nat Med 15, 1082–1087 (2009).
Shimizu, I. et al. Semaphorin3E-induced inflammation contributes to insulin resistance in dietary obesity. Cell Metab 18, 491–504 (2013).
Pang, Z. et al. Therapeutic vaccine against DPP4 improves glucose metabolism in mice. Proc Natl Acad Sci USA 111, E1256–1263 (2014).
Nakagami, F. et al. Decrease in blood pressure and regression of cardiovascular complications by angiotensin II vaccine in mice. PLoS One 8, e60493 (2013).
Shimizu, I. et al. p53-induced adipose tissue inflammation is critically involved in the development of insulin resistance in heart failure. Cell Metab 15, 51–64 (2012).
Shimizu, I., Yoshida, Y., Suda, M. & Minamino, T. DNA damage response and metabolic disease. Cell Metab 20, 967–977 (2014).
Xu, M. et al. Targeting senescent cells enhances adipogenesis and metabolic function in old age. Elife 4 (2015).
Zhu, Y. et al. The Achilles’ heel of senescent cells: from transcriptome to senolytic drugs. Aging Cell 14, 644–658 (2015).
Tchkonia, T., Zhu, Y., van Deursen, J., Campisi, J. & Kirkland, J. L. Cellular senescence and the senescent secretory phenotype: therapeutic opportunities. J Clin Invest 123, 966–972 (2013).
Chang, J. H. et al. Clearance of senescent cells by ABT263 rejuvenates aged hematopoietic stem cells in mice. Nature Medicine 22, 78–+ (2016).
Baker, D. J. et al. Naturally occurring p16(Ink4a)-positive cells shorten healthy lifespan. Nature 530, 184–+ (2016).
Lane, D. P. Cancer. p53, guardian of the genome. Nature 358, 15–16 (1992).
Nakagami, H., Koriyama, H. & Morishita, R. Peptide Vaccines for Hypertension and Diabetes Mellitus. Vaccines (Basel) 2, 832–840 (2014).
Acknowledgements
We thank Kaori Yoshida, Keiko Uchiyama, Satomi Kawai, Naomi Hatanaka, Yoko Sawaguchi, Runa Washio, Takako Ichihashi (Niigata University) for their excellent technical assistance. This work was supported by a Grant-in-Aid for Scientific Research (Grant number 17H04172), a Grant-in-Aid for Scientific Research on Innovative Areas (Stem Cell Aging and Disease, Grant number 26115008), and a Grant-in-Aid for Exploratory Research from the Ministry of Education, Culture, Sports, Science and Technology (MEXT, Grant number 18K19536) of Japan and grants from the Takeda Medical Research Foundation, the Japan Foundation for Applied Enzymology, the Takeda Science Foundation, the SENSHIN Medical Research Foundation, the Terumo Foundation, the Manpei Suzuki Diabetes Foundation, the Naito Foundation, and the NOVARITIS foundation (to T.M.) as well as by a Grants-in-Aid for Young Scientists (Start-up) (JSPS KAKENHI, Grant Number 26893080), and grants from the Uehara Memorial Foundation, Takeda Science Foundation, Kowa Life Science Foundation, Manpei Suzuki Diabetes Foundation, Kanae Foundation, Japan Heart Foundation Research Grant, The Senri Life Science Foundation, SENSHIN Medical Research Foundation, ONO Medical Research Foundation, Tsukada Grant for Niigata University Medical Research, The Nakajima Foundation, SUZUKEN memorial foundation, HOKUTO Corporation, Inamori Foundation, Mochida Memorial Foundation for Medical & Pharmaceutical Research, MSD Life Science Foundation, Public Interest Incorporated Foundation, Grant for Basic Science Research Projects from The Sumitomo Foundation, Grants-in-Aid for Encouragement of Young Scientists (A) (JSPS KAKENHI Grant Number 16H06244), Grant From Japan Cardiovascular Research Foundation, Japan Diabetes Foundation, Kimura Memorial Heart Foundation, TERUMO FOUNDATION for LIFE SCIENCES and ARTS, Daiichi Sankyo Foundation of Life Science, Grant-in-Aid for Challenging Exploratory Research (JSPS KAKENHI Grant Number 17K19648), Astellas Foundation for Research on Metabolic Disorders (to I.S.); by a Grants-in-Aid for Encouragement of Young Scientists (B) (JSPS KAKENHI Grant Number 16K19531), a Japan Heart Foundation Dr. Hiroshi Irisawa & Dr. Aya Irisawa Memorial Research Grant, Senshin Medical Research Foundation grant, SUZUKEN memorial foundation, Takeda Science Foundation, ONO Medical Research Foundation, Uehara Memorial Foundation, Research Foundation for Community Medicine, Kanae Foundation, MSD Life Science Foundation, HOKUTO Corporation, Japan Heart Foundation Research Grant, Yujin Memorial Grant, The Tokyo Biochemical Research Foundation (to Y.Y.) and by a grant from Bourbon (to T.M., I.S. and Y.Y.).
Author information
Authors and Affiliations
Contributions
Professor Tohru Minamino is the guarantor of this work and had full access to all data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Y.Y. and I.S. wrote the manuscript and researched data. Y.H., R.I., M.S., G.K., T.W., M.N., H.N. and R.M. researched data T.M. reviewed/edited the manuscript and supervised the whole project.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yoshida, Y., Shimizu, I., Hayashi, Y. et al. Peptide vaccine for semaphorin3E ameliorates systemic glucose intolerance in mice with dietary obesity. Sci Rep 9, 3858 (2019). https://doi.org/10.1038/s41598-019-40325-y
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-40325-y
- Springer Nature Limited
This article is cited by
-
PCPE-1, a brown adipose tissue-derived cytokine, promotes obesity-induced liver fibrosis
The EMBO Journal (2024)
-
Axon guidance molecules in immunometabolic diseases
Inflammation and Regeneration (2022)