Abstract
Global herbaria are the greatest repositories of information on the plant kingdom. Discoveries of trace element hyperaccumulator plants have historically required time-consuming destructive chemical analysis of fragments from herbarium specimens, which severely constrains the collection of large datasets. Recent advances in handheld X-Ray Fluorescence spectroscopy (XRF) systems have enabled non-destructive analysis of plant samples and here we propose a new method, which we term “Herbarium XRF Ionomics”, to extract elemental data from herbarium specimens. We present two case studies from major tropical herbaria where Herbarium XRF Ionomics has led to the discovery of new hyperaccumulator plants and provided valuable insights into phylogenetic patterns of trace element hyperaccumulation. Herbarium XRF Ionomics is a new value proposition for continued funding and retention of herbarium specimens globally.
Similar content being viewed by others
Introduction
Plants take up macronutrients (Ca, K, Mg, P, S), micronutrients (Fe, B, Cl, Cu, Mn, Mo, Ni, Zn), elements that are beneficial to some plants (Al, Na, Co, Se, Si), and also non-essential trace elements (As, Cd, Hg, Pb, Se, Tl)1. The supply of essential elements to a plant ranges from deficiency to optimum and eventual toxicity, and differs greatly between elements2. The elemental composition of plant tissues is controlled by genetically predisposed ecophysiological behaviour, and the availability of specific elements in the soil, as well as other environmental factors (climate, water supply, altitude, etc.). Taken together the concentrations of elements in plant tissue make up the “ionome3,4”. Ionomics has the ability to capture information about the physiology of a plant under different conditions, driven by genetic differences and environmental filters4,5,6. The ionome (or foliar elemental profile) can provide a wide range of information on: (i) phylogenetic patterns of trace elemental accumulation and nutrient acquisition; (ii) geographic and geological occurrence of minerals, including those of commercial value; and (iii) elemental homeostasis, including extremes of uptake, co-accumulation or exclusion of elements, in response to soil and habitat variation.
Plant species that accumulate trace elements to extreme concentrations are known as hyperaccumulators7,8,9. The phenomenon of hyperaccumulation is interesting from an evolutionary and physiological viewpoint, and also finds practical application in phytomining and related novel technologies10,11. Globally the discovery of hyperaccumulator plants has been hindered by the lack of systematic screening of plant species from different phylogenetic lineages, and is highly biased towards specific regions around the world, e.g. ultramafic regions8,9. Furthermore, most effort and focus has been on Ni hyperaccumulator plants, due in part to the existence of a simple reagent paper test (based on dimethylglyoxime), such that Ni accounts for over 500 of the approximately 700 known hyperaccumulator species12. However, this does not necessary mean that hyperaccumulators of other elements, such as Mn or Zn, are rare. It may merely mean that they have not yet been discovered.
The value of herbaria as references for taxonomic, genetic and biogeographic information is widely acknowledged. However, herbaria may also render other sources of information. Here we propose the “Herbarium XRF Ionomics” approach to extract new elemental data from herbarium specimens using X-ray fluorescence spectroscopy (XRF). Herbarium XRF Ionomics can provide insights into phylogenetic and biogeographic patterns of trace element accumulation by plants for a wide range of different chemical elements. New technology allows the process to be conducted rapidly, with no damage to herbarium specimens, and therefore gains access to this hitherto untapped resource of information.
In order to understand how foliar elements are regulated and how this relates to the ecophysiology of a plant species, it is necessary to measure as many elements as possible on a large number of specimens13. Therefore, ionomics requires high-throughput elemental analysis technologies and their integration with bioinformatics4. Recently, handheld XRF systems have been validated for measuring plant nutrients in agronomic samples, although this initially involved powdering and pelletisation of the plant material for analysis14,15,16. The advent of portable XRF instruments utilizing the latest type of fast detectors now enables non-destructive analysis of plant material and permits mass measurements of tens of thousands of samples in a relatively short time span at low cost. The latest generation of XRF instruments (equipped with Ag or Rh anode operating at 50 kV, 200 µA) can measure the concentrations of a wide range of different elements in a spot of ~6 mm in under two minutes with a detection limit of ~100 μg g−1 for most of the transition elements (such as Ni, Co, Zn). As such, a single operator can measure herbarium specimens at a rate of >300 specimens per day. For the first time, it is now feasible to determine elemental concentrations across entire phylogenetic lineages, including many replicate specimens of the same species orginating from different collection localities. Additionally, XRF screening (Fig. 1) may be combined with the (photographic) digitisation process of herbarium specimens, an effort already underway in many herbaria worldwide.
Given that XRF devices are designed for measuring elemental concentrations in rock and soil matrices, validation for dried plant leaves (essentially a 200–500 micron thick cellulose low Z element matrix), is necessary. As detailed in the Methods section, Compton Normalisation in combination with element-specific emperical correction factors can be used for calibration of the X-ray fluorescence data. Correction factors must be determined empirically by measuring a selection of non-herbarium samples using both XRF and more traditional, destructive analytical methods such as inductively coupled plasma optical emission spectroscopy (ICP-OES) on acid-digested plant samples. Soil dust contamination adhering to leaves can confound measurements, but may be assessed from unusually high concomitant Cr, Fe, and Ti concentrations17. Another issue is the practise of some tropical collectors to use methylated spirit for temporarily preserving sample collections to prevent decomposition of the specimens during transport, which can potentially leach soluble elements from the plant material or contaminate other specimens; thus, such specimens should be avoided when possible. Finally, some (old) herbarium specimens may be treated with HgCl2 for long-term insect protection and therefore readings for Hg can be extremely high for such samples (i.e. > 500 μg g−1).
Results and Discussion
Brief summaries of two case studies of the application of Herbarium XRF Ionomics are summarized here, to provide an indication of the potential of this approach.
The Malaysian state of Sabah on the island of Borneo has high levels of plant diversity (>8000 species), occurring on a wide range of soil types18. Sabah has over 3500 km2 of ultramafic surface geology (~4.6% of the total landmass of the state), forming soils with high concentrations of Mg, Ni, Co, Cr, Cu, and Mn. More than 4250 plant species naturally occur on this substrate, including many known hyperaccumulators18,19. This makes it one of the most species-rich floras occupying ultramafic outcrops globally20. Using XRF scanning, ~7300 herbarium specimens were analysed at the Forest Research Centre (FRC) in Sabah, Malaysia. The measurements recorded 12 Co hyperaccumulator species (in 3 families, 7 genera), 51 Mn hyperaccumulator species (in 12 families, 24 genera) and 28 Ni hyperaccumulator species (in 10 families, 17 genera). Prior to the XRF scanning 3 Co hyperaccumulator species, 7 Mn hyperaccumulator species, 24 Ni hyperaccumulator species, and 2 Zn hyperaccumulator species, were known from Sabah18,21,22. The discovery of Zn hyperaccumulators here was unexpected, as previously-known Zn hyperaccumulators are from temperate climate regions and herbaceous species9. Our new approach also helps to explain the scientific oddity Dichapetalum gelanioides, recorded twenty-five years ago18 with a sub-species (subsp. tuberculatum) that hyperaccumulates Ni on ultramafic soils and other sub-species (subsp. pilosum and subsp. sumatranum) hyperaccumulating Zn on ‘normal’ non-Zn-rich soils. Extraordinarily this species appears to hyperaccumulate Zn from non-mineralised soils with only background concentrations of Zn23. This raises important questions about the evolution of both Ni and Zn hyperaccumulation: did Ni-hyperaccumulation evolve when species that were already Zn-hyperaccumulators on non-ultramafic soils colonised ultramafic soils? Targeted herbarium XRF screening has already yielded other fascinating discoveries, including Co hyperaccumulation in a tree from Borneo24, and Ni hyperaccumulation in a critically endangered species from a genus (Antidesma; Phyllanthaceae) previously unknown to contain hyperaccumulator plants25.
New Caledonia, in the southwest Pacific Ocean, is an island biodiversity hotspot harbouring over 3371 vascular plant species, of which nearly 75% are endemic26. The New Caledonian vegetation over ultramafic rocks contains 2145 species, including 1747 (80%) endemics27,28. The ultramafic flora and hyperaccumulators from New Caledonia are probably the best studied in the world27. New Caledonia is a global hotspot for hyperaccumulator plants, with 65 nickel and 11 manganese hyperaccumulator species recorded to date27,29. XRF analysis at the Institute for Research and Development (IRD) Herbarium in Nouméa, New Caledonia examined ~11 200 herbarium specimens (in 35 orders, 96 families, 281 genera, 1620 taxa). The sampling effert covered plant families known to contain numerous hyperaccumulators (e.g. Cunoniaceae, Phyllanthaceae, Salicaceae, Sapotaceae and Violaceae), as well as a systematic screening of 1–4 specimens (depending on availability in the herbarium) of all species known to occur on ultramafic soils in New Caledonia. The latter group of specimens (1372 species) represented 88.5% of the known taxa of vascular plants occurring on ultramafic outcrops in New Caledonia. Numerous marginal hyperaccumulator plants for Ni, Mn, Co, and Zn were noted; however, the results summarised here are restricted to exceptionally high records (i.e. Ni > 5000 µg g−1, Mn > 20 000 µg g−1, Co > 1000 µg g−1, and Zn > 10 000 µg g−1). Even based on these conservative criteria, the study found 92 Ni hyperaccumulator taxa (65 known previously), 70 Mn hyperaccumulator taxa (11 known previously), 8 Co hyperaccumulator taxa (none known previously), and 5 Zn hyperaccumulator taxa (none known previously). This demonstrates that XRF screening of herbarium specimens has the potential to discover vast numbers of ‘new’ hyperaccumulator species in short surveys (lasting only a few months), even in well-studied floras such that of New Caledonia.
Our team is also currently developing a “Herbarium X-ray Fluorescence Spectroscopy & Digitalisation Station”. This mobile (cabinet-sized) system will permit ultra-fast herbarium digitisation (high-resolution optical image) in tandem with X-ray fluorescence spectroscopy analysis. We envisage that the instrument will have a high-flux X-ray source (50 Watt, Mo-source) and fast large area (150 mm2) Silicon Drift Detector (SDD) to permit very high throughput (~1000 specimens per 8-hr day). In addition to faster measurements, such a system would have the ability to measure a much wider range of elements, including lighter elements, such as macronutrients (e.g. Ca, K, P, S) that cannot be accurately quantified using current handheld XRF technology, thereby acquiring true ionomic profiles of analysed herbarium specimens. The concept is for the system to be deployed for dedicated projects at major global herbaria, prioritizing tropical herbaria in the Asia-Pacific Region, Africa and Central and South America.
To date, the potential to unlock foliar elemental information from plant herbarium collections has not been fully realised, but Herbarium XRF Ionomics may be transformative in the emerging field of ionomics and the discovery of hyperaccumulator plants. The demonstrations summarized here represent two of the largest individual contributions to the global inventory of hyperaccumulator plants, and include a substantial expansion in phylogenetic records, with many genera not previously known to contain hyperaccumulators. They also highlight the potential to uncover novel evolutionary, ecological, and biogeographic phenomena. This information could then be used to target specific species for genetic and ecophysiological experiments under controlled conditions. Such investigations can be approached with various other X-ray techniques, including synchrotron X-ray fluorescence microscopy (XFM), proton-induced X-ray emission (PIXE) and scanning electron microscopy with energy dispersive X-ray spectroscopy (SEM-EDS) to answer questions at every level of metal(loid) homeostasis in plants, from the rhizosphere interface, to uptake pathways in the roots and shoots30. Finally, this approach demonstrates a new value proposition for continued funding and retention of herbarium specimens globally31.
Methods
XRF instrument
The Niton XL3t 950 analyser (Thermo-Fisher Scientific; Fig. 1) uses a miniaturised X-ray tube (Ag anode; 6–50 kV, 0–200 µA max.) as its main excitation source. The X-ray tube irradiates the sample with a stable source of high-energy X-rays in a ~6 mm spot. Fluorescent X-rays generated from excitation by the incident beam are continuously detected, identified and quantified by the in-built Silicon Drift Detector (SDD) with an energy-resolution of ~185 eV at up to 60 000 counts per second. The instrument incorporates Compton Normalisation, appropriate for the relatively low elemental concentrations found in the low Z element bulk composition of plant material (compared to the high Z element matrix of ores or metallurgical samples). The XRF instrument produces X-rays in the form of the high-energy incident beam, and lower energy X-rays from scattering and fluorescence. It is important to limit exposure to these X-rays by correctly operating the instrument, and in most jurisdictions a Radiation User License is required to own and operate an XRF device.
XRF calibration
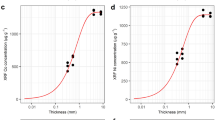
A total of 590 dried plant samples were used from the first author’s field collections originating from Sabah, Malaysia18. The plant samples included known Co, Ni, Mn and Zn hyperaccumulator plants that were selected such that the dataset would cover element-specific concentration ranges ranging from ‘normal’ to ‘abnormal’ (i.e. hyperaccumulation). From each sample, a 6-mm diameter leaf disc (to match the approximate incident X-ray beam width) was extracted using a paper punch. A square plate of ~99.7% pure titanium (2 mm thick × 10 cm × 10 cm; Sigma-Aldrich 369489-90 G) was used behind the specimens to provide a uniform background. Before XRF testing, a sheet of cardboard with a square hole made to fit the titanium plate at the centre was fixed on the XRF table, and a sheet of herbarium mounting paper was placed over the titanium plate. All testing was conducted on this platform, ensuring that the beam window of the XRF analyser completely covered the leaf sample. XRF testing was carried out in the ‘Soils Mode’ for 60 seconds (a sensitivity analysis showed that after 60 s no further improvement in signal is obtained). After scanning, the leaf fragment samples were weighed and acid digested using 5 mL HNO3 (70%) and 1 mL H2O2 (30%) in a microwave digestion system (Milestone Start D) on a 45-minute programme32 and diluted to 30 mL with ultrapure water (Millipore 18.2 MΩ·cm at 25 °C) before analysis with ICP-OES (Varian Vista Pro II) for Ni, Co, Mn, Fe and Zn. Correction factors were derived by linear regression of XRF data against corresponding ICP-OES measurements.
Herbarium XRF scanning
During six weeks, ~7300 herbarium specimens at the Forest Research Centre (FRC) in Sabah, Malaysia were measured with XRF, using the same settings and titanium backing plate described in the calibration procedure. Given that the FRC Herbarium contains ~350 000 specimens (~10 000 taxa) a selection was made and consisted of: all specimens in the families Phyllanthaceae, Salicaceae, Violaceae and Sapotaceae (totalling 5975 specimens), plus the genera Walsura (Sapindaceae – 98 specimens) and Mischocarpus (Meliaceae – 81 specimens), and a single specimen from all species (1183) known to occur on ultramafic soils in Sabah. Incompletely identified specimens were omitted. This selection was based on previous studies on hyperaccumulator plants in Sabah18,33 with the aim to target the most likely phylogenetic linages for hyperaccumulator plants.
In New Caledonia, herbarium XRF scanning was undertaken at the L’Institut de Recherche pour le Développement (IRD). The selection of specimens consisted of a number of families known to include hyperaccumulators, from which all available specimens (originating from ultramafic and non-ultramafic soils) were scanned. These were: Cunnoniaceae, Phyllanthaceae, Salicaceae, Sapotaceae and Violaceae. In addition, XRF scanning was undertaken on 1–4 (depending on availability) specimens selected from every dicot species known to occur on ultramafic soils in New Caledonia (on the basis of occurrence records and geological maps), totalling 1372 species34. Only dicotyledons were measured, and no gymnosperms or monocotyledons were considered in this study.
References
Marschner, H. Mineral Nutrition of Higher Plants. 2nd Ed. (Academic Press, London, 1995).
Chapin, F. S. The mineral nutrition of wild plants. Annu. Rev. Ecol. Syst. 11, 233–260 (1980).
Salt, D. E. Update on Plant Ionomics. Plant Physiol. 136, 2451–2456 (2004).
Salt, D. E., Baxter, I. & Lahner, B. Ionomics and the study of the plant ionome. Annu. Rev. Plant Biol. 59, 709–733 (2008).
Baxter, I. Ionomics: studying the social network of mineral nutrients. Curr. Opin. Plant Biol. 12, 381–386 (2009).
Baxter, I. Ionomics: The functional genomics of elements. Brief. Funct. Genomics. 9, 149–156 (2010).
Jaffré, T., Brooks, R. R., Lee, J. & Reeves, R. D. Sebertia acuminata: A hyperaccumulator of nickel from New Caledonia. Science. 193, 579–580 (1976).
Reeves, R. D. Tropical hyperaccumulators of metals and their potential for phytoextraction. Plant Soil. 249, 57–65 (2003).
van der Ent, A., Alan, J. M., Reeves, R. D., Pollard, A. J. & Schat, H. Hyperaccumulators of metal and metalloid trace elements: facts and fiction. Plant Soil 362, 319–334 (2013).
Chaney, R. L. et al. Improved understanding of hyperaccumulation yields commercial phytoextraction and phytomining technologies. J. Environ. Qual. 36, 1429–1443 (2007).
van der Ent, A. et al. Agromining: farming for metals in the future? Environ. Sci. Technol. 49, 4773–4780 (2015b).
Reeves, R. D. et al. A global database for hyperaccumulator plants of metal and metalloid trace elements. New Phytol. 218, 407–411 (2017).
Djingova, R., Mihaylova, V., Lyubomirova, V. & Tsalev, D. L. Multielement analytical spectroscopy in plant ionomics research. Appl. Spectrosc. Rev. 48, 384–424 (2013).
McLaren, T. I., Guppy, C. N. & Tighe, M. K. A rapid and nondestructive plant nutrient analysis using portable X-Ray fluorescence. Soil Sci. Am. J. 76, 1446–1449 (2012).
Reidinger, S., Ramsey, M. H. & Hartley, S. E. Rapid and accurate analyses of silicon and phosphorus in plants using a portable X-ray fluorescence spectrometer. New Phytol. 195, 699–706 (2012).
Guerra, M. B. B. et al. Comparison of analytical performance of benchtop and handheld energy dispersive X-ray fluorescence systems for the direct analysis of plant materials. J. Anal. At. Spectrom 29, 1667–1674 (2014).
Cary, E. E. & Kubota, J. Chromium concentration plants: effects of soil chromium concentration and tissue contamination by soil. J. Agric. Food Chem. 38, 108–114 (1990).
van der Ent, A., Erskine, P. D. & Sumail, S. Ecology of nickel hyperaccumulator plants from ultramafic soils in Sabah (Malaysia). Chemoecol. 25, 243–259 (2015).
van der Ent, A., Erskine, P. D., Mulligan, D. R., Repin, R. & Karim, R. Vegetation on ultramafic edaphic islands in Kinabalu Park (Sabah, Malaysia) in relation to soil chemistry and altitude. Plant Soil 403, 77–101 (2016).
Galey, M. C. et al. Ultramafic geoecology of South and Southeast Asia. Bot. Studies 58, 18 (2017).
van der Ent, A., Mulligan, D. R., Repin, R. & Erskine, P. D. Foliar elemental profiles in the ultramafic flora of Kinabalu Park (Sabah, Malaysia). Ecol. Res. 33, 659–674 (2018).
Baker, A. J. M., Proctor, J., Van Balgooy, M. M. J. & Reeves, R. D. Hyperaccumulation of nickel by the flora of the ultramafics of Palawan, Republic of the Philippines. In: The vegetation of ultramafic (serpentine) soils. Baker A. J. M, Proctor J., Reeves, R. D. (editors), Intercept: Andover, UK 291–304 (1992).
Nkrumah, P. N, Echevarria, G., Erskine, P. D. & van der Ent, A. Contrasting nickel and zinc hyperaccumulation in subspecies of Dichapetalum gelonioides from Southeast Asia. Sci. Rep. 8, 9659 (2018).
van der Ent, A., Mak, R., de Jong, M. D. & Harris, H. H. Simultaneous hyperaccumulation of nickel and cobalt in Glochidion cf. sericeum (Phyllanthaceae): elemental distribution and speciation. Sci. Rep. 8, 9683 (2018).
Nkrumah, P. N, Echevarria, G., Erskine, P. D. & van der Ent, A. Nickel hyperaccumulation in Antidesma montis-silam: from herbarium discovery to collection in the native habitat. Ecol. Res. 33, 675–685 (2018).
Morat, P. et al. The taxonomic reference base Florical and characteristics of the native vascular flora of New Caledonia. Adansonia. 34, 177–221 (2012).
Jaffré, T., Pillon, Y., Thomine, S. & Merlot, S. The metal hyperaccumulators from New Caledonia can broaden our understanding of nickel accumulation in plants. Front. Plant Sci. 4, 1–10 (2013).
Jaffré, T. et al. Input of the different vegetation units to the richness and endemicity of the New Caledonian flora. Flora 162, 54–59 (2009).
van der Ent, A., Jaffré, T., L’Huillier, L., Gibson, N. & Reeves, R. D. The flora of ultramafic soils in the Australia-Pacific Region: state of knowledge and research priorities. Aust. J. Bot. 63, 173–190 (2015).
van der Ent, A. et al. X-ray elemental mapping techniques for elucidating the ecophysiology of hyperaccumulator plants. New Phyt. 218, 432–452 (2017).
Deng, B. Plant collections left in the cold by cuts. Nature. 523, 16 (2015).
Huang, L., Bell, R. W., Dell, B. & Woodward, J. Rapid nitric acid digestion of plant material with an open-vessel microwave system. Comm. Soil Sci. Plant Anal. 35, 427–440 (2007).
van der Ent, A. & Mulligan, D. Multi-element concentrations in plant parts and fluids of Malaysian nickel hyperaccumulator plants and some economic and ecological considerations. J. Chem. Ecol. 41, 396–408 (2015).
Isnard, S., L’Huillier, L., Rigault, F. & Jaffré, T. How did the ultramafic soils shape the flora of the New Caledonian hotspot? Plant Soil 403, 53–76 (2016).
Acknowledgements
We would like to express our gratitude to John Sugau (Forest Research Centre Herbarium, Sepilok, Sabah, Malaysia) and Sandrine Isnard and Tanguy Jaffré (IRD Herbarium, Nouméa, New Caledonia) for their support and enabling access to these herbaria. We thank Ana Ocenar and Vidiro Gei for undertaking XRF measurements on herbarium specimens. The French National Research Agency through the national “Investissements d’avenir” program (ANR-10-LABX-21 - LABEX RESSOURCES21) is acknowledged for funding to A. van der Ent. A. van der Ent was the recipient of a Discovery Early Career Researcher Award (DE160100429) from the Australian Research Council.
Author information
Authors and Affiliations
Contributions
Research concept and design: A.V.D.E. and P.D.E.; XRF data collection: Ana Ocenar and Vidiro Gei; Data Analysis: A.V.D.E. and P.D.E.; Manuscript Writing: A.V.D.E., P.D.E., G.E. and A.J.P.; Figure Preparation: A.V.D.E. and P.D.E.; Review & Editing: A.J.P.; All authors participated in discussion of the research.
Corresponding author
Ethics declarations
Competing Interests
The authors declare no competing interests.
Additional information
Publisher’s note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made. The images or other third party material in this article are included in the article’s Creative Commons license, unless indicated otherwise in a credit line to the material. If material is not included in the article’s Creative Commons license and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this license, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
van der Ent, A., Echevarria, G., Pollard, A.J. et al. X-Ray Fluorescence Ionomics of Herbarium Collections. Sci Rep 9, 4746 (2019). https://doi.org/10.1038/s41598-019-40050-6
Received:
Accepted:
Published:
DOI: https://doi.org/10.1038/s41598-019-40050-6
- Springer Nature Limited
This article is cited by
-
Revegetation and ecosystem reclamation of post-mined land: toward sustainable mining
International Journal of Environmental Science and Technology (2024)
-
Unravelling the fate of foliar-applied nickel in soybean: a comprehensive investigation
Plant and Soil (2024)
-
The use of a portable X-ray fluorescence spectrometer for measuring nickel in plants: sample preparation and validation
Environmental Monitoring and Assessment (2024)
-
Rare earth elements (REEs) in soils and plants of Bangka Island (Indonesia) focussing on (hyper)accumulation
Plant and Soil (2024)
-
Rare earth element (hyper)accumulation in some Proteaceae from Queensland, Australia
Plant and Soil (2023)